Sustainable Utilization of Industrial Waste in Metakaolin-Based Geopolymer Systems
Abstract
The global demand for cement significantly contributes to greenhouse gas emissions and the unsustainable consumption of natural resources. Simultaneously, industrial waste poses disposal challenges and serious environmental and public health risks. This study addresses both issues by exploring the use of metakaolin-based geopolymers to incorporate 20% wt. of three types of industrial wastes: suction dust, red mud, and electro-filter dust. pH and conductivity measurements, along with FT-IR analysis, confirmed successful geo-polymerization. The leaching test revealed that only the geopolymer containing suction dust did not fully immobilize the waste. These analyses also provided insights into the compounds responsible for antibacterial properties, tested against E. coli and S. aureus. Finally, mechanical tests indicated their suitability for construction purposes, with compressive strength values ranging from 37–58 MPa, depending on the waste type.
1 Introduction
One of the European Union's goals is to reduce CO2 emission levels, which contributes to the greenhouse effect, by 2030.[1] The cement industry is currently a major concern as it is known for significant CO2 emissions, as well as high water and energy consumption. In this context, geopolymers represent a more sustainable alternative to Portland cement because they not only reduce CO2 emissions but can also incorporate industrial waste into their matrix, making these materials even more environmentally friendly.[2, 3]
In this study, three types of industrial wastes were incorporated into metakaolin-based geopolymers activated with NaOH and sodium silicate. The stability of these materials was assessed through pH and conductivity measurements, as well as FT-IR analysis. The safety of the materials was evaluated through leaching and Kirby–Bauer tests. Finally, compressive strength was measured to determine their suitability as cement substitutes.
2 Results and Discussion
2.1 Stability Evaluation of Samples
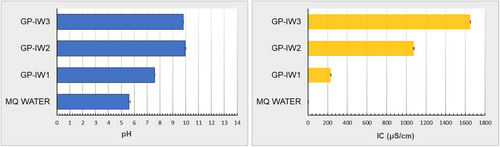
Once removed from the molds, the samples appeared well-hardened. Their stability was evaluated by pH and conductivity measurements, as well as FT-IR analysis. The pH and conductivity results are shown in Figure 1. As can be observed, all samples caused a slight alkalinization of the water. This indicates that the samples released ionic species, reaching pH values of 7.5 for the GP-IW1 sample, while GP-IW2 and GP-IW3 showed pH values of 10. Regarding conductivity, the sample with the highest conductivity was GP-IW3, followed by GP-IW2 and GP-IW1. This trend is partly related to the ions released into the water and partly due to the presence of soluble salts that, while not altering the pH, can affect the conductivity.
FT-IR analysis was used to investigate the occurrence of geo-polymerization. By analyzing the shift of the density of state peak maximum (DOSPM), associated with the Si─O─Si(Al) signal in the range of 1250–800 cm−1, toward lower wavenumbers, it is possible to evaluate the substitution of Si atoms with Al atoms, and thus assess the formation of new Si─O─Al bonds within the three-dimensional geopolymer network.[4]
The starting metakaolin shows absorption bands at 1090, 830, 671, and 460 cm−1. The band at 1090 cm−1 is related to the asymmetric stretching of the Si─O─Si bond. In the control geopolymer, this signal shifts to 1013 cm−1, while in samples containing waste, it varies from 1034 to 1007 cm−1. The reason for the slight shifts of this peak in the waste-containing samples compared to the control is attributed to the presence of metal oxides in the starting waste. The other signals of the starting precursor, namely those at 830 and 671 cm−1, derived from Al(VI)─OH or Al(VI)─O vibrations, shift to higher wavenumbers in the geo-polymeric samples, further indicating the incorporation of aluminum into the geopolymer matrix and the formation of new Si─O─Al bonds. The Si─O bending signal at 460 cm−1 remains present even after geo-polymerization in both the control and the waste-containing samples.
From the IR spectra, a difference between the control and the waste-containing geopolymers is observed in the carbonate absorption band (1440–1380 cm−1). This band is present only in the waste-containing geopolymers, suggesting interactions between the cations on the geopolymer surfaces and ambient carbon dioxide, leading to the phenomenon of efflorescence.
2.2 Evaluation of the Environmental Safety of the Samples
To assess the environmental risk and potential use of the samples in construction, a leaching test was conducted. The results revealed that aluminum (Al) concentrations vary among the samples, with the control showing the highest release. Boron (B) was released in trace amounts by all geo-polymeric samples. Calcium (Ca) was released in higher amounts by the MK precursor and the control, while potassium (K) was significantly higher in the GPIW3 sample. Magnesium (Mg) was uniformly released in low amounts exclusively by the waste-containing geopolymers. Nickel (Ni) was released in trace amounts only by GPIW2 and GPIW3. Antimony (Sb) was released in large quantities by the GPIW1 sample, whereas vanadium (V) was released in low concentrations in the geo-polymeric samples and in higher amounts by the control.
Most of the samples released Al, Ca, Mg, and K—elements that are not considered environmental contaminants.[5] However, the release of Al and V is linked to the presence of the geopolymer precursor, metakaolin (MK). Although MK does not release Al and V by itself, after alkaline activation—a key process in geopolymer synthesis—it facilitates the release of these elements, as evidenced by the higher concentrations in the control compared to samples containing 80% MK. This explains the absence of Al and V release in MK and their more pronounced presence in the control, with reduced release in waste-containing geopolymer samples.
The concentrations of Al, V, B, Ni, and Sb are generally below 10 ppm, indicating limited presence. A significant exception is the GPIW1 sample, which released 25 ppm of Sb, presumably due to the Sb content in IW1, making this material unsuitable for construction due to the environmental risk associated with antimony.
Table 1 shows that all samples exhibited antibacterial activity against the tested bacteria. The starting metakaolin (MK) had an inhibition zone of 1.3 cm for both bacteria. The control demonstrated antibacterial activity comparable to MK against S. aureus but was more effective against E. coli. This increased activity against E. coli is likely due to the elevated pH from alkaline activation, which creates a less favorable environment for E. coli growth.[6] Geopolymers containing waste as filler exhibited inhibition zones equal to or greater than the control, suggesting that waste materials interact with the bacteria and inhibit their growth. In some cases, the waste materials alone showed greater antibacterial activity than the samples containing them as filler. IW1 and IW3 waste materials showed higher efficacy against both E. coli and S. aureus. GPIW2 and GPIW3 exhibited the highest antibacterial activity against E. coli, with GPIW2 also being more effective against S. aureus.
| Bacterium | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibition Halos | MK | Control | IW1 | GPIW1 | IW2 | GPIW2 | IW3 | GPIW3 | |
| E. coli | MEAN | 1.3 | 1.71 | 3.84 | 2.2 | 1.86 | 2.53 | 3.76 | 2.59 |
| DEV.ST. | 0.05 | 0.03 | 0.09 | 0.02 | 0.05 | 0.05 | 0.17 | 0.03 | |
| S. aureus | MEAN | 1.3 | 1.3 | 2.56 | 1.41 | 2.35 | 1.65 | 2.75 | 1.44 |
| DEV.ST. | 0.05 | 0.01 | 0.05 | 0.06 | 0.04 | 0.05 | 0.06 | 0.08 | |
Variations in antibacterial activity among the samples containing 20% waste materials can be partially attributed to alkaline activation and the structural differences between the tested bacterial strains. S. aureus is a Gram-positive bacterium with a thick peptidoglycan cell wall, while E. coli is a Gram-negative bacterium with a more complex cell wall and an outer membrane that acts as a protective barrier against many antimicrobial agents.
The active compounds in the precursors and waste materials can influence antibacterial activity in various ways. For instance, potassium release can disrupt bacterial osmotic balance, leading to increased osmotic pressure and membrane potential. High potassium concentrations can cause water influx into bacterial cells, resulting in swelling and disruption of enzymatic and metabolic processes, potentially leading to cell death. Aluminum ions can bind to nucleic acids, interfering with replication processes, and to cell wall components, destabilizing the membrane, and altering its permeability. Additionally, they can catalyze the formation of reactive oxygen species (ROS), causing irreversible oxidative damage to cellular structures.[1]
2.3 Mechanical Strength
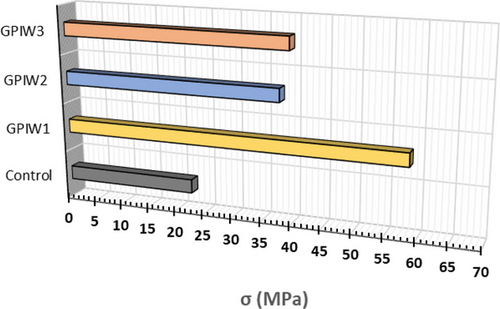
The compressive strength results are shown in Figure 2. The sample with the highest compressive strength was GPIW1, reaching 58.6 ± 1.3 MPa, followed by GPIW2 and GPIW3 with 37.8 ± 2.9 MPa and 39.4 ± 3.4 MPa, respectively. Notably, all waste-containing samples demonstrated greater compressive strength compared to the control geopolymer, which achieved only 22.5 MPa. This increased strength is attributed to the presence of various metals in the waste materials. For example, antimony (Sb) in GPIW1 forms compounds in an alkaline environment that enhances geo-polymerization by substituting aluminum (Al) atoms, thereby strengthening the material. Similarly, in GPIW2, the high iron content enhances compressive strength by replacing Al in the geopolymer network. For GPIW3, the combined presence of zinc (Zn), copper (Cu), and antimony (Sb) contributes to the observed strength increase compared to the control.[1]
3 Conclusions
This study demonstrates the feasibility of incorporating industrial waste into metakaolin-based geopolymers. pH and conductivity measurements, along with FT-IR analysis, confirmed the stability and successful geo-polymerization of the samples. The leaching test revealed that only the geopolymer containing suction dust did not fully immobilize the waste. Antibacterial tests showed activity against E. coli and S. aureus across all samples. Finally, mechanical tests indicated that these geopolymers have sufficient compressive strength, making them suitable for general construction purposes.
4 Experimental Section
Synthesis Procedures and Raw Materials
The synthesis of the samples was carried out using an electric mixer (AUCMA model SM-1815Z). The metakaolin (MetaMax – BASF) was mixed with a solution of NaOH and sodium silicate, purchased from Prochin S.r.l, Italy. The wastes used in the synthesis (20wt.%), namely suction dust (Industrial Waste 1, IW1), red mud (Industrial Waste 2, IW2), and electro-filter dust (Industrial Waste 3, IW3) were sourced from local industry. The synthesis steps followed the protocol reported by Viola et al.[1]
pH and Ionic Conductivity Measurements
The IC and PC were measured with Crison GLP31 and Crison GLP21 (Hach Lange Spain, S.L.U, Barcelona, Spain) using MQ water purchased from Sigma Aldrich.
FT-IR Analysis
Transmittance spectra were collected in the range of 400–4000 cm−1 with a resolution of 2 cm−1 (60 scans) using the Prestige21 Shimadzu system, which is equipped with a DTGS KBr sensor (deuterated triglycine sulfate with potassium bromide windows). For the analysis, KBr disks were prepared by mixing 2 mg of the sample with 200 mg of KBr (Sigma Aldrich). The spectra were processed using IR Solution and Origin 8 software.
Leaching Test
The concentration of released heavy metals was measured following the EN ISO 11885:2009 standard, using inductively coupled plasma optical emission spectrometry (ICP-OES) with an Agilent system (Santa Clara, CA, USA).
Antimicrobial Properties
The antibacterial analysis of the samples was conducted against Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) using the Kirby–Bauer method. The whole procedure is reported by D'Angelo et al.[7]
Compressive Strength
Compressive strength was assessed in accordance with European standard BS EN 12390—testing hardened concrete. Five cubic specimens (5 cm × 5 cm × 5 cm) from each formulation were tested using an Instron 5967 electromechanical testing machine (Instron, Torino, Italy) with a maximum load capacity of 10 kN, at a constant displacement rate of 5 mm/min.
Acknowledgements
The authors have nothing to report.
Open access publishing facilitated by Universita degli Studi della Campania Luigi Vanvitelli, as part of the Wiley - CRUI-CARE agreement.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data presented in this study are available on request from the corresponding author




