In situ normothermic regional perfusion versus ex situ normothermic machine perfusion in liver transplantation from donation after circulatory death
Kayvan Mohkam, David Nasralla, Hynek Mergental, Darius F. Mirza, and Mickaël Lesurtel contributed equally to this work.
SEE EDITORIAL ON PAGE 1701
Abstract
In situ normothermic regional perfusion (NRP) and ex situ normothermic machine perfusion (NMP) aim to improve the outcomes of liver transplantation (LT) using controlled donation after circulatory death (cDCD). NRP and NMP have not yet been compared directly. In this international observational study, outcomes of LT performed between 2015 and 2019 for organs procured from cDCD donors subjected to NRP or NMP commenced at the donor center were compared using propensity score matching (PSM). Of the 224 cDCD donations in the NRP cohort that proceeded to asystole, 193 livers were procured, resulting in 157 transplants. In the NMP cohort, perfusion was commenced in all 40 cases and resulted in 34 transplants (use rates: 70% vs. 85% [p = 0.052], respectively). After PSM, 34 NMP liver recipients were matched with 68 NRP liver recipients. The two cohorts were similar for donor functional warm ischemia time (21 min after NRP vs. 20 min after NMP; p = 0.17), UK–Donation After Circulatory Death risk score (5 vs. 5 points; p = 0.38), and laboratory Model for End-Stage Liver Disease scores (12 vs. 12 points; p = 0.83). The incidence of nonanastomotic biliary strictures (1.5% vs. 2.9%; p > 0.99), early allograft dysfunction (20.6% vs. 8.8%; p = 0.13), and 30-day graft loss (4.4% vs. 8.8%; p = 0.40) were similar, although peak posttransplant aspartate aminotransferase levels were higher in the NRP cohort (872 vs. 344 IU/L; p < 0.001). NRP livers were more frequently allocated to recipients suffering from hepatocellular carcinoma (HCC; 60.3% vs. 20.6%; p < 0.001). HCC-censored 2-year graft and patient survival rates were 91.5% versus 88.2% (p = 0.52) and 97.9% versus 94.1% (p = 0.25) after NRP and NMP, respectively. Both perfusion techniques achieved similar outcomes and appeared to match benchmarks expected for donation after brain death livers. This study may inform the design of a definitive trial.
Abbreviations
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate aminotransferase
-
- cDCD
-
- controlled donation after circulatory death
-
- CI
-
- confidence interval
-
- COPE
-
- Consortium for Organ Preservation in Europe
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- DWI
-
- donor warm ischemia
-
- HAT
-
- hepatic artery thrombosis
-
- HCC
-
- hepatocellular carcinoma
-
- HOPE
-
- hypothermic oxygenated machine perfusion
-
- HR
-
- hazard ratio
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- ITU
-
- intensive therapy unit
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- NAS
-
- nonanastomotic biliary strictures
-
- NMP
-
- ex vivo normothermic machine perfusion
-
- NRP
-
- in situ normothermic regional perfusion
-
- PSM
-
- propensity score matching
-
- SCS
-
- static cold storage
-
- SMD
-
- standardized mean difference (absolute value)
-
- UK-DCD
-
- UK–Donation After Circulatory Death
INTRODUCTION
Liver transplantation (LT) from controlled donation after circulatory death (cDCD) donors has been developed during the past two decades in an attempt to address the organ shortage but at the cost of inferior outcomes.[1] Several studies have reported higher rates of early graft loss and increased rates of biliary complications in cDCD LT recipients compared with those receiving a donation after brain death (DBD) allograft.[1-3] These inferior outcomes are attributed to allograft injury caused by prolonged donor warm ischemia (DWI) occurring after the withdrawal of life-sustaining therapies and before organ retrieval. Various dynamic preservation strategies have been developed to ameliorate this damage, including in situ normothermic regional perfusion (NRP) and ex situ normothermic machine perfusion (NMP).[4, 5]
The NRP approach consists of cannulating donor iliac vessels and commencing perfusion of the abdominal compartment organs shortly after donor circulatory arrest. This strategy has been adopted by countries including France and Spain.[4, 6] Large retrospective studies from these programs suggested that NRP was associated with superior posttransplant outcomes in cDCD allografts compared with super-rapid recovery with static cold storage (SCS), achieving results similar to DBD livers.[4, 7]
In contrast, NMP involves the ex situ perfusion of livers with oxygenated blood and medications at body temperature to preserve the liver in a physiological, functioning state. This preservation strategy can be commenced at the donor hospital or at the recipient center after a period of SCS. NMP commenced at the donor hospital was compared with SCS in a randomized controlled trial conducted by the Consortium for Organ Preservation in Europe (COPE). The study demonstrated lower posttransplant peak aspartate aminotransferase (AST) levels and lower rates of early allograft dysfunction in the NMP group, with these benefits being greatest in the cDCD subgroup.[8]
NRP and NMP have not yet been compared directly; this study is the first to compare the results of LT using these two different strategies in the context of LT from cDCD.
PATIENTS AND METHODS
Study population
The study enrolled 264 donation after circulatory death (DCD) donors, which comprised 224 NRP procedures performed according to French national guidelines between February 2015 and December 2019 and 40 NMP procedures performed between August 2014 and March 2016 in the COPE trial. The NRP procedures were performed in six French centers, whereas the NMP procedures were performed in six European centers from the United Kingdom, Germany, Spain, and Belgium. Graft acceptance criteria in the NRP French program were donor age ranging from 18 to 75 years, no circulatory arrest before organ procurement, and cold ischemia time less than 9 h. Although graft acceptance criteria in the NMP cohort were broader in theory, all grafts that were considered in the present NMP cohort conformed to the same criteria. The details of particular protocols were published elsewhere.[5-8] In short, the NRP procedure was commenced in the intensive care unit using an oxygenated normothermic in situ perfusion through arterial and venous femoral cannula. The perfusion was limited to abdominal organs only by using an endo-aortic balloon clamp positioned in the supraceliac aorta. Following a 1–4 h duration of NRP, the graft was procured in a standard fashion and transported to the recipient site using SCS. The NMP procedure was also commenced at the donor hospital following a short period of SCS during preparation of the liver for perfusion. NMP perfusate comprised packed red blood cells and gelofusine, with infusions of heparin, insulin, sodium taurocholate, and prostacyclin. NMP was maintained during transportation to the recipient center, where the graft was flushed with standard preservation fluid before being implanted.
Endpoints and definitions
Study endpoints included liver use rate; 30-day and 12- and 24-month patient and graft survival rates; incidence of clinically manifest biliary complications; early allograft dysfunction; and peak transaminase levels. Clinically manifest biliary strictures were divided into the following three categories: anastomotic strictures, nonanastomotic biliary strictures (NAS) that were unrelated to any hepatic artery complications, and ischemic biliary lesions related to hepatic artery thrombosis (HAT).
The use rate was calculated as the number of allografts that were transplanted divided by the total number of cDCD donors that proceeded to asystole within the time frame that allows procurement. Early allograft dysfunction was assessed using Olthoff's criteria.[9]
Because of differences in terms of recipient selection—for example, the French program prioritizing recipients with hepatocellular carcinoma (HCC) for DCD graft allocation—patient and graft survival rates were calculated both with and without censoring for death from HCC recurrence. Clinically manifest biliary complications were defined as any biliary problem diagnosed on magnetic resonance cholangiopancreatiography (MRCP) resulting in the need for invasive specific treatment and/or resulting in graft loss.
Variables related to graft ischemia were defined as follows: total DWI was defined as the time between therapeutic withdrawal in the donor and start of cold aortic perfusion in the NMP group or initiation of the perfusion in the NRP group. In the NMP group, functional DWI was defined as the time from systolic pressure below 50 mm Hg until start of aortic cold perfusion, whereas in the NRP group, it was defined as the time from mean arterial blood pressure below 45 mm Hg until initiation of NRP. Asystolic DWI started from the onset of cardiac arrest. Total ex vivo preservation time was defined as the time from donor aortic cross-clamp until graft reperfusion in the recipient. Cold ischemia time was defined as the duration of graft SCS. Donors were graded using Feng's donor risk index[10] and the UK-DCD risk score.[11]
Statistical analysis
Categorical variables were presented as number with percentages and compared using chi-square or Fisher's exact test, as appropriate. Continuous variables were presented as median values with interquartile ranges (IQRs) and compared using the Mann–Whitney U test. The survival rates were estimated using the Kaplan–Meier curve and compared with a log-rank test.
Because of differences in terms of organ allocation and recipient selection between the COPE trial and the French cDCD LT program, a few differences could be expected between the two groups. To overcome potential baseline covariate imbalances, we performed propensity score matching (PSM). The propensity score was calculated using a multivariable logistic regression model with group allocation as the outcome and included all variables involved in the UK-DCD risk score, a robust tool that predicts cDCD-LT outcome (namely, donor age, donor body mass index, functional DWI, recipient age, laboratory Model for End-Stage Liver Disease [MELD] score), with the exception of cold ischemia time (which is considered as an inherent benefit of the NMP strategy) and retransplantation (because only primary LT cases were included in both treatment groups). Matching was performed with a 1:2 ratio using the nearest neighbor method, without replacement and with a caliper of 0.20. After PSM, balance assessment was performed using the absolute value of the standardized mean difference (SMD), and values less than 0.10 were considered well balanced. Outcome variables were only reported for the propensity score–matched cohorts.
All calculations were performed with SPSS software Version 23.0 (IBM Corp., Armonk, NY) and R software Version 3.1.3 (R Foundation for Statistical Computing). All tests were two-tailed, and statistical significance was established for p < 0.05.
RESULTS
Graft use rates
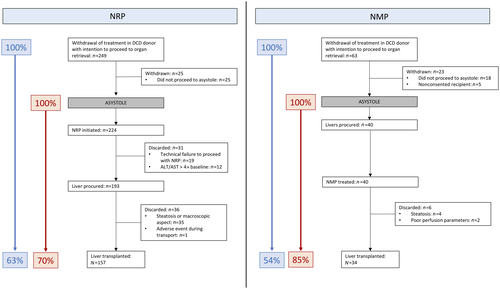
Of the 249 cDCD donors in the NRP cohort that were considered for procurement, 224 proceeded to asystole. Of these, 31 livers were not procured because of technical failure or dramatic postperfusion peak AST, whereas 193 livers were eventually procured, resulting in 157 transplants and 36 discarded livers, mainly as a result of steatosis or poor macroscopic aspect (Figure 1). In the NMP cohort, of the 63 cDCD donations that were considered for procurement, 40 proceeded to asystole. Normothermic perfusion was commenced in all 40 cases and resulted in 34 transplants. The corresponding organ use rates were 70% and 85% (p = 0.052) in the NRP and NMP cohort, respectively (Figure 1).
Donor and recipient characteristics
Before PSM, the donor demographics of both study groups were comparable, including age, weight, body mass index, cause of death, functional DWI, and donor risk index. The donor intensive therapy unit (ITU) stay (9 vs. 4 days; p < 0.001) was longer in the NRP group. Because of differences in the donor cannulation and organ procurement technique between the two groups, the total DWI (31 [IQR, 27–37] vs. 25 [IQR, 22–29] min; p < 0.001) and asystolic DWI (18 [IQR, 15–20] vs. 12 [IQR, 10–13] min; p < 0.001) were longer in the NRP group. As expected, cold ischemia time (346 vs. 138 min; p < 0.001) was longer in the NRP group. The total dynamic perfusion time (184 vs. 525 min; p < 0.001) and total ex situ preservation times (516 vs. 651 min; p < 0.001) were longer in the NMP group. Details are provided in Table 1.
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Variables | NMP | NRP | p value | NMP | NRP | p value |
| n | 34 | 157 | 34 | 68 | ||
| Donor age, year | 48 (33–62) | 50 (39–59) | 0.970 | 48 (33–62) | 49 (37–60) | 0.673 |
| Donor sex, male/female | 20/14 | 113/44 | 0.680 | 20/14 | 48/20 | 0.235 |
| Donor body mass index, kg/m2 | 25 (23–29) | 25 (22–29) | 0.109 | 25 (23–29) | 25 (23–29) | 0.845 |
| Donor intensive care unit stay, days | 4 (2–5) | 9 (6–15) | <0.001 | 4 (2–5) | 9 (6–15) | <0.001 |
| Cause of donor death | 0.183 | 0.056 | ||||
| Trauma | 3 (8.8) | 41 (26.1) | 3 (8.8) | 23 (33.8) | ||
| Anoxia | 15 (44.1) | 65 (41.4) | 15 (44.1) | 27 (39.7) | ||
| Cerebrovascular accident | 11 (32.4) | 51 (32.5) | 11 (32.4) | 18 (26.5) | ||
| Donor risk index | 2.13 (1.90–2.42) | 2.01 (1.69–2.35) | 0.148 | 2.13 (1.90–2.42) | 1.98 (1.68–2.43) | 0.234 |
| Functional DWI time, min | 20 (17–25) | 22 (19–26) | 0.179 | 20 (17–25) | 21 (18–25) | 0.603 |
| Cold ischemia time, min | 138 (118–144) | 346 (291–395) | <0.001 | 138 (118–144) | 346 (285–397) | <0.001 |
| Recipient age, year | 56 (45–61) | 59 (54–63) | 0.052 | 56 (45–61) | 57 (50–62) | 0.949 |
| Recipient sex, male/female | 18/16 | 141/16 | <0.001 | 18/16 | 58/10 | <0.001 |
| Recipient body mass index, kg/m2 | 26 (22–33) | 27 (24–30) | 0.609 | 26 (22–33) | 27 (24–30) | 0.771 |
| Main transplant indication | <0.001 | <0.001 | ||||
| HCC | 7 (20.6) | 103 (65.6) | 7 (20.6) | 41 (60.3) | ||
| End-stage liver disease | 27 (79.4) | 54 (34.4) | 27 (79.4) | 27 (39.7) | ||
| Alcohol-associated liver disease | 12 (35.3) | 68 (43.3) | 12 (35.3) | 29 (42.6) | ||
| Nonalcoholic fatty liver disease | 2 (5.9) | 21 (13.3) | 2 (5.9) | 10 (14.7) | ||
| Viral hepatitis | 1 (33.3) | 54 (34.4) | 1 (2.9) | 25 (36.8) | ||
| Primary sclerosing cholangitis | 4 (11.7) | 0 (0) | 4 (11.8) | 0 (0) | ||
| Other | 8 (23.5) | 14 (8.9) | 8 | 8 | ||
| Pretransplant renal replacement therapy | 0 (0.0) | 3 (1.9) | 1.000 | 0 (0.0) | 0 (0.0) | 1.000 |
| Laboratory MELD score | 12 (9–13) | 11 (8–15) | 0.759 | 12 (9–13) | 12 (9–13) | 0.831 |
| UK-DCD score | 5 (3–6) | 6 (3–8) | 0.238 | 5 (3–6) | 5 (3–7) | 0.383 |
| UK-DCD risk group | 0.882 | 0.742 | ||||
| Low risk | 18 (52.9) | 77 (49.0) | 18 (52.9) | 34 (50.0) | ||
| High risk | 14 (41.2) | 68 (43.3) | 14 (41.2) | 31 (45.6) | ||
| Futile | 2 (5.9) | 14 (8.9) | 2 (5.9) | 5 (7.4) | ||
- Note: Data are provided as n, n (%), or median (IQR).
- Abbreviations: DWI, donor warm ischemia; HCC, hepatocellular carcinoma; IQR, interquartile range; MELD, Model for End-Stage Liver Disease; NMP, ex vivo normothermic machine perfusion; NRP, in situ normothermic regional perfusion; PSM, propensity score matching; UK-DCD, UK–Donation After Circulatory Death.
The NRP recipients were predominantly males (91% vs. 53%; p < 0.001) and older, although the difference was not statistically significant (59 vs. 56 years; p = 0.052). The main underlying liver disease in the NRP group was alcohol-associated liver disease while a majority of patients receiving LT for HCC (65.6% vs. 20.6%; p < 0.001), respectively. The laboratory MELD score, recipient body mass index, and UK-DCD score distribution were similar in both groups. The study median follow-up was 22 months (IQR, 14–32) in the NRP group versus 24 months (IQR, 23–24) in the NMP group (p = 0.75), and each study patient still alive completed at least 12 months of follow-up.
After PSM, the two groups became well balanced for all variables included in the UK-DCD risk score except for cold ischemia time (Tables 1 and 2).
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| NMP | NRP | SMD | NMP | NRP | SMD | |
| n | 34 | 157 | 34 | 68 | ||
| Donor age, year | 48 (33–62) | 50 (39–59) | 0.040 | 48 (33–62) | 49 (37–60) | 0.077 |
| Donor body mass index, kg/m2 | 23 (25–29) | 25 (22–29) | 0.313 | 23 (25–29) | 25 (23–29) | 0.007 |
| Functional DWI, min | 20 (17–25) | 22 (19–26) | 0.272 | 20 (17–25) | 21 (18–25) | 0.091 |
| Recipient age | 56 (45–61) | 59 (54–63) | 0.559 | 56 (45–61) | 57 (50–62) | 0.019 |
| Retransplant | 0 | 0 | – | 0 | 0 | – |
| Laboratory MELD score | 12 (9–13) | 11 (8–15) | 0.051 | 12 (9–13) | 12 (9–13) | 0.011 |
| UK-DCD score | 5 (3–6) | 6 (3–8) | 0.210 | 5 (3–6) | 5 (3–7) | 0.087 |
| UK-DCD risk group | ||||||
| Low risk | 18 (52.9) | 77 (49.0) | 0.078 | 18 (52.9) | 34 (50.0) | 0.058 |
| High risk | 14 (41.2) | 68 (43.3) | 0.042 | 14 (41.2) | 31 (45.6) | 0.089 |
| Futile | 2 (5.9) | 12 (7.6) | 0.065 | 2 (5.9) | 5 (7.4) | 0.059 |
- Note: Data are provided as n, n (%), or median (IQR).
- Abbreviations: DWI, donor warm ischemia; IQR, interquartile range; MELD, Model for End-Stage Liver Disease; NMP, ex vivo normothermic machine perfusion; NRP, in situ normothermic regional perfusion; PSM, propensity score matching; SMD, standardized mean difference (absolute value); UK-DCD, UK–Donation After Circulatory Death.
- a All UK-DCD score variables were included except cold ischemia time because the latter is expected to be decreased in the NMP group as part of its inherent benefits.
Early postoperative outcomes
There was no difference between the groups in 30-day graft (97% [n = 65] after NRP vs. 91% [n = 31] after NMP; p = 0.39) and patient (100% [n = 68] after NRP vs. 94% [n = 32] after NMP; p = 0.11) survival rates. In the NRP group, the three cases of 30-day graft loss were attributed to primary nonfunction (n = 1), complete hepatic vein thrombosis (n = 1), and hyperacute rejection related to ABO incompatibility (n = 1), whereas in the NMP group, the three cases were attributed to inferior vena cava thrombosis at reperfusion (n = 1), multiple organ failure caused by HAT (n = 1), and nonthrombotic graft infarction (n = 1). The NRP grafts showed higher peak AST and alanine transaminase (ALT) values within 7 days after LT, but there was no difference between the groups in early allograft dysfunction (Table 3). Recipients in both groups experienced similar rates of HAT (2.9% [n = 2] after NRP vs. 2.9% [n = 1] after NMP; p > 0.99).
| Variables | NMP | NRP | p value |
|---|---|---|---|
| n | 34 | 68 | |
| ITU stay, days | 3 (2–5) | 5 (4–7) | <0.001 |
| Hospital stay, days | 14 (8–17) | 16 (13–20) | 0.018 |
| 30-day graft loss | 3 (8.8) | 3 (4.4) | 0.398 |
| 30-day patient death | 2 (5.9) | 0 (0.0) | 0.109 |
| Early allograft dysfunction | 3 (8.8) | 14 (20.6) | 0.133 |
| AST peak 7 days, IU/L | 344 (216–701) | 872 (538–1281) | <0.001 |
| ALT peak 7 days, IU/L | 311 (186–590) | 725 (400–1304) | 0.001 |
| INR level Day 7 | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 0.566 |
| Total bilirubin Day 7, μmol/L | 29 (16–57) | 20 (12–43) | 0.217 |
| Serum creatinine Day 7, μmol/L | 73 (51–97) | 66 (55–83) | 0.826 |
| HAT | 1 (2.9) | 2 (2.9) | p > 0.99 |
- Note: Data are provided as n, n (%), or median (IQR).
- Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HAT, hepatic artery thrombosis; INR, international normalized ratio; ITU, intensive therapy unit; NMP, ex vivo normothermic machine perfusion; NRP, in situ normothermic regional perfusion.
Biliary complications
A total of eight (11.8%) versus seven (20.6%) recipients developed a clinically manifest biliary complication in the NRP and NMP groups, respectively (p = 0.249). Overall, 44 (64.7) and 25 (73.5%) patients underwent MRCP protocol (p = 0.369), showing that the majority of clinically manifest biliary complications consisted of anastomotic strictures (8.8% [n = 6] after NRP vs. 17.6% [n = 6] after NMP; p = 0.208). The incidence of symptomatic NAS was similar and low in both groups (1.5% [n = 1] after NRP vs. 2.9% [n = 1] after NMP; p = 1.000), leading to a single graft loss in each group (1.5% vs. 2.9%; p = 1.000) within the median 23-month follow-up period. Details regarding biliary complications are provided in Table 4.
| Variables | NMP | NRP | p value |
|---|---|---|---|
| n | 34 | 68 | |
| Clinically manifest biliary stricturesa | |||
| Any type | 7 (20.6) | 8 (11.8) | 0.249 |
| NASb | 1 (2.9) | 1 (1.5) | 1.000 |
| HAT-related ischemic-type biliary lesionsc | 0 (0.0) | 1 (1.5) | 1.000 |
| Anastomotic stricture | 6 (17.6) | 6 (8.8) | 0.208 |
| MRCP performed | 25 (73.5) | 44 (64.7) | 0.369 |
| Total biliary strictures (clinical and/or on imaging) | |||
| Anastomotic stricture | 13 (38.2) | 7 (10.3) | <0.001 |
| Nonanastomotic stricture | 3 (8.8) | 2 (2.9) | 0.330 |
- Abbreviations: HAT, hepatic artery thrombosis; MRCP, magnetic resonance cholangiopancreatography; NAS, nonanastomotic biliary strictures; NMP, ex situ normothermic machine perfusion; NRP, in situ normothermic regional machine perfusion.
- a Refers to biliary stricture requiring a specific treatment or resulting to graft loss and/or patient death.
- b Refers to NAS with patent hepatic artery.
- c Refers to NAS related to HAT.
Survival rates
The 1- and 2-year graft survival rates were 93.9% versus 88.2% and 89.4% versus 88.2% (hazard ratio [HR], 0.67; 95% confidence interval [CI], 0.20–2.23; p = 0.516) for the NRP and NMP groups, respectively (Table 5). The 1- and 2-year patient survival rates were 98.5% versus 94.1% and 96.4% versus 90.9% (HR, 0.38 [95% CI, 0.06–2.30]; p = 0.275) for the NRP and NMP groups, respectively. Because of the high incidence of the HCC in the NRP group, we also calculated survival rates with censoring of recipients with HCC recurrence and found similar graft and patient survival rates (Table 5, Figures 2 and 3).
| Variables | NMP, % | NRP, % | p value |
|---|---|---|---|
| Graft survival rates | 0.516 | ||
| 1 year | 88.2 | 93.9 | |
| 2 years | 88.2 | 89.4 | |
| Tumor-censored graft survival rates | 0.523 | ||
| 1 year | 88.2 | 94.1 | |
| 2 years | 88.2 | 91.5 | |
| Patient survival rates | 0.275 | ||
| 1 year | 94.1 | 98.5 | |
| 2 years | 90.9 | 96.4 | |
| Tumor-censored patient survival rates | 0.255 | ||
| 1 year | 94.1 | 100 | |
| 2 years | 94.1 | 97.9 |
- Abbreviations: NMP, ex situ normothermic machine perfusion; NRP, in situ normothermic regional machine perfusion.
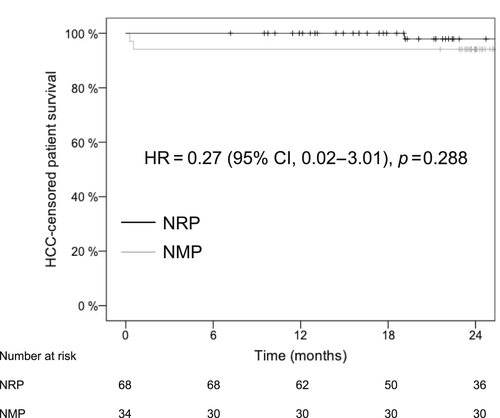
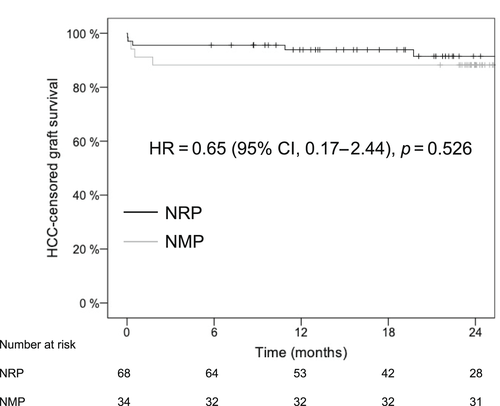
DISCUSSION
This is the first study comparing NRP and NMP strategies in cDCD LT. The results show patient and graft outcomes that are comparable with benchmarks established for DBD and much lower rates of NAS than contemporary cDCD series preserved by SCS.[2, 11-13] Our findings suggest that the NMP procedure allows longer total preservation times and may achieve higher organ use rates. The NRP intervention achieved similar posttransplant results and also potentially improves the quality of other donated abdominal organs.[14]
As a result of the historically poorer outcomes of cDCD livers, the selection criteria are usually more restrictive, resulting in lower organ use and higher discard rates compared with DBD donors. An important objective of machine perfusion is to recover graft quality and increase use rates. In the randomized COPE trial, the organ discard rate was 50% lower for the NMP compared with the SCS group and yet, despite the increased use of suboptimal cDCD livers, the results were superior to the SCS group.[8] This suggests that NMP allows increased organ use without compromising outcomes. Conversely, studies comparing the use of NRP with the standard cDCD super-rapid recovery have not yet demonstrated improvement in terms of cDCD graft use rates. In the study by Hessheimer et al., the liver use rates from withdrawal of life-sustaining therapies were 34% and 38% after NRP and super-rapid recovery, respectively.[4] These findings seem to be confirmed by our results, which suggest that the use rate might be higher after super-rapid recovery followed by NMP commenced at the donor center than after NRP.
Although open-abdomen cannulation is preferred in some countries, as it appears to be technically less demanding and results in very low rates of technical failure,[15] other countries advocate the use of percutaneous femoral performed in the ITU. Because open-abdominal aortic cannulation for cDCD donation is not allowed in France,16] the NRP cannulas needs to be inserted percutaneously by ITU doctors, which represents a demanding procedure associated with a steep learning curve.[7] Technical failure to commence NRP was encountered in 19 of 224 donors in the NRP group, contributing to the reported use rates. The rates of technical success may be improved when cannulation is performed by skilled and well-trained operators[17] or by using premortem cannulation, the latter of which is not allowed in several countries, including France.
Posttransplant AST or ALT are surrogate markers of hepatocyte injury used in definitions for early allograft dysfunction[9, 18, 19] and also may predict the development of NAS in cDCD grafts.[20] In comparison with SCS, both NMP and NRP are associated with a decreased AST peak after the LT of cDCD grafts. In the COPE trial, the peak AST was the primary endpoint that was found to be significantly lower in both the cDCD and DBD subgroups.[8] In the first comparative study of NRP versus ex situ hypothermic oxygenated machine perfusion (HOPE) LT from cDCD donors, the peak AST in the NRP group was significantly lower than in the HOPE group.[21] This might suggest that commencing perfusion early in the cDCD liver preservation pathway is more efficient in preventing the graft damage compared with perfusion initiated at the recipient center. However, such an explanation must be taken with caution because of the hazardous interpretation of transaminase level variations after machine perfusion, the retrospective nature of these comparisons, and other differences between the studies. The present study also suggests that clinicians feel more confident in extending the preservation time during NMP than with NRP. Indeed, NRP protocol applied herein required not to exceed a 4-h perfusion time while there is no data suggesting any deleterious effects of prolonged NRP (>4 h).
The biliary tract represents tissue that is most vulnerable to prolonged ischemia/reperfusion injury. The majority of studies assessing the outcome of cDCD versus DBD LT have reported increased rates of biliary complications in cDCD livers[2] resulting either in decreased graft and patient survival 22] or in more stringent selection criteria resulting in lower use rates.[23] One of the main objectives of the use of machine perfusion in cDCD LT is to decrease the rate of NAS.[24] The COPE trial was not powered to demonstrate any difference between SCS and NMP in terms of biliary complications; although it showed lower rates of radiologically diagnosed NAS in the NMP arm (11% vs. 26%), this difference did not achieve statistical significance, and the majority of these cases were clinically asymptomatic.[8] In the Spanish cDCD transplant experience comparing super-rapid recovery versus NRP, the rates of both NAS and overall biliary complications were significantly reduced in the NRP group.[4] All of these findings suggest that dynamic liver preservation strategies decrease the incidence of NAS in cDCD LT. In the present study, the rate of clinically manifest NAS was similar among the two groups (1.5% in the NRP group vs. 2.9% in the NMP group). This is considerably lower compared with the cDCD preserved by cold storage only,24] which suggests that both perfusion technologies are clinically efficacious in reducing biliary complications in cDCD LT.
DCD livers represent a valuable source of allografts that has helped to address the shortage of organs in several countries albeit at the cost of the inferior outcomes. In addition to the NAS that may lead to graft loss, the cDCD livers have also been associated with increased rates of posttransplant acute kidney failure and inferior patient survival rates.[2, 22, 24, 25] In the present study, recipients in both groups achieved a 90-day graft loss of less than 10% (4.4% after NRP, 8.8% after NMP), and 2-year graft and patient survival rates higher than 85%, thus achieving outcomes similar to those expected after LT from DBD nonmarginal grafts.[12]
There are clear limitations to this study in relation to its retrospective nature and nonrandomized design. The two compared perfusion strategies were performed in different trials and in the context of different organ allocation policies, graft selection criteria, and health care systems. Use rates are also affected by waitlist pressure, which may vary across countries and centers. Despite some differences in recipient and donor baseline characteristics, the two groups were similar with respect to variables that could have an influence on the analyzed outcomes, and we performed an inverse probability treatment weighting adjustment, which allowed us to obtain two cohorts that were comparable for the most relevant variables, such as donor age, donor risk index, UK-DCD score, and recipient laboratory MELD score. The main difference between the groups was a higher proportion of recipients who received transplants for HCC in the NRP group; we avoided the risk of a tumor-related survival bias by presenting both overall and tumor-censored graft and patient survival rates. The other shortcoming is the relatively small size of the NMP cohort.
In conclusion, the present study is the first to compare two different machine perfusion strategies applied to cDCD livers at a donor hospital. The results suggest that both interventions, NRP and continuous NMP, improve outcomes to a level expected for DBD livers in terms of 1- and 2-year graft and patient survival rates. On the other hand, we have produced no good evidence that either technology is superior and therefore propose that this state of equipoise might be the basis for a formal prospective, randomized, controlled clinical trial to compare not only the effectiveness of these two approaches to perfusion of DCD livers but also the logistic and cost elements.
ACKNOWLEDGMENTS
The authors acknowledge the support of the project by all local teams, the Consortium for Organ Preservation in Europe, and the Agence de la Biomédecine.
CONFLICT OF INTEREST
Darius F. Mirza owns stock in OrganOx. M. Thamara P. R. Perera is on the speakers’ bureau for OrganOx. Xavier Muller received grants from Intitut Georges Lopez. Peter J. Friend is the cofounder of, owns stock in, is employed by, consults for, advises, received grants from, and is an inventor on a licensed patent for OrganOx.
ETHICS APPROVAL
The COPE study approval was obtained from the London–Dulwich National Research Ethics Committee and the Medicines and Healthcare Regulatory Agency in the United Kingdom and research ethics committees and medical device regulatory bodies in Belgium, Spain, and Germany. The NRP program was approved by the French institutional local and national ethics committee. The presented study was approved by the COPE steering committee and by the Hospices Civils de Lyon ethics committee (approval number: CSE-HCL_21_360).