Hypothermic Oxygenated Perfusion Improves Extended Criteria Donor Liver Graft Function and Reduces Duration of Hospitalization Without Extra Cost: The PERPHO Study
Abstract
Few studies have evaluated the efficacy or the cost of hypothermic oxygenated perfusion (HOPE) in the conservation of extended criteria donor (ECD) grafts from donation after brain death (DBD) donors during liver transplantation (LT). We performed a prospective, monocentric study (NCT03376074) designed to evaluate the interest of HOPE for ECD-DBD grafts. For comparison, a control group was selected after propensity score matching among patients who received transplants between 2010 and 2017. Between February and November 2018, the HOPE procedure was used in 25 LTs. Immediately after LT, the median aspartate aminotransferase (AST) level was significantly lower in the HOPE group (724UI versus 1284UI; P = 0.046) as were the alanine aminotransferase (ALT; 392UI versus 720UI; P = 0.01), lactate (2.2 versus 2.7; P = 0.01) There was a significant reduction in intensive care unit stay (3 versus 5 days; P = 0.01) and hospitalization (15 versus 20 days; P = 0.01). The incidence of early allograft dysfunction (EAD; 28% versus 42%; P = 0.22) was similar . A level of AST or ALT in perfusate >800UI was found to be highly predictive of EAD occurrence (areas under the curve, 0.92 and 0.91, respectively). The 12-month graft (88% versus 89.5%; P = 1.00) and patient survival rates (91% versus 91.3%; P = 1.00) were similar. The additional cost of HOPE was estimated at € 5298 per patient. The difference between costs and revenues, from the hospital's perspective, was not different between the HOPE and control groups (respectively, € 3023 versus € 4059]; IC, –€ 5470 and € 8652). HOPE may improve ECD graft function and reduce hospitalization stay without extra cost. These results must be confirmed in a randomized trial.
Abbreviations
-
- ALD
-
- alcohol-related liver disease
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- AUC
-
- area under the curve
-
- BMI
-
- body mass index
-
- CTP
-
- Child-Turcotte-Pugh
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- DRG
-
- diagnosis-related group
-
- EAD
-
- early allograft dysfunction
-
- ECD
-
- extended criteria donor
-
- GGT
-
- gamma-glutamyltransferase
-
- GHM
-
- Groupe Homogène de Malade
-
- HA
-
- hepatic artery
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HOPE
-
- hypothermic oxygenated perfusion
-
- HV
-
- hepatic vein
-
- ICU
-
- intensive care unit
-
- INR
-
- international normalized ratio
-
- IRI
-
- ischemia/reperfusion injury
-
- KDIGO
-
- Kidney Disease: Improving Global Outcomes
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NA
-
- not available
-
- PERPHO
-
- Hypothermic Oxygenated Perfusion in Preservation of Hepatic Grafts From Expanded Criteria Donors
-
- PMSI
-
- Programme de Médicalisation des Systèmes d’Information
-
- PNF
-
- primary nonfunction
-
- POD
-
- postoperative day
-
- PRS
-
- postreperfusion syndrome
-
- ROC
-
- receiver operating characteristic
Liver transplantation (LT) is the best treatment for end-stage liver disease and primary liver malignancies. Because of the growing gap between the number of candidates and transplant recipients, transplantation teams have been forced to increase the donor pool by using more and more grafts from so-called extended criteria donors (ECDs).
Although they may currently represent more than 50% of the grafts used,(1) there is still no consensual definition of an ECD graft. However, they are mostly represented by elderly donors, donation after circulatory death (DCD) donors, or fatty liver grafts and are well known to be more vulnerable to ischemia/reperfusion injuries (IRIs)(2) created during conventional static cold storage. These grafts are therefore associated with higher rates of graft dysfunction.(3-6)
As a result, there has been increasing interest in perfusion machines to improve the quality of conservation and reduce the consequences of IRI. Among the different techniques of perfusion, hypothermic oxygenated perfusion (HOPE) is simple and has already proved its efficacy in preserving DCD grafts by reducing biliary complications and improving graft and patient survival.(7) However, few studies have evaluated the interest of HOPE in preserving ECD grafts procured from donation after brain death (DBD) donors or the cost from a hospital’s perspective because the procedure is not covered by health insurance in most countries.
We hypothesized that HOPE could benefit LTs performed with ECD grafts and implemented a prospective trial, the Oxygenated Hypothermic Perfusion in Preservation of Hepatic Grafts From Expanded Criteria Donors (PERPHO) Study, to confirm it.
Patients and Methods
Study Design and Purpose
The PERPHO Study (clinical trial number NCT03376074) was a prospective, monocentric, single-arm, pilot study designed to evaluate the interest of HOPE in the preservation and functional recovery of ECD grafts during LT. For comparison, a control group was selected after propensity score matching among patients who received ECD grafts in our institution between 2010 and 2017.
Ethics Statement
Informed and written consent was obtained from all included patients in the HOPE group, and the absence of opposition was obtained from patients in the control group. The study was approved by the National Ethics Committee and received authorization from the French National Drug Safety Agency.
Inclusion Criteria
HOPE Group
All adult patients with cirrhosis (regardless of cause), who were candidates for a first LT between February and November 2018 without the need for combined organ transplantation, were eligible to participate in the study and were included if they received ECD grafts from DBD donors. Patients who required emergency transplantation for acute liver failure or retransplantation without cirrhosis or who received split grafts were not eligible. Patients who received grafts from DCD donors were also not eligible because in France those grafts systematically receive in situ normothermic regional perfusion.
An ECD graft was defined by the presence of at least 1 of the following criteria as previously reported(8): age >65 years; body mass index (BMI) >30 kg/m2; intensive care unit (ICU) stay prior to procurement >7 days; natremia >155 mmol/L; liver enzymes 3 times higher than the normal value (ie, aspartate aminotransferase [AST] blood level >150 IU/mL, alanine aminotransferase [ALT] blood level >170 IU/mL); occurrence of cardiac arrest before procurement; and biopsy-proven macrovesicular steatosis >30%. Patients were recruited during pre-LT consultations or just before the procedure.
Control Group
The control group was selected after propensity score matching (1:3 ratio) among LTs with ECD grafts performed in our institution between 2010 and 2017.
- Recipients: age, sex, BMI, Model for End-Stage Liver Disease (MELD) score, Child-Turcotte-Pugh (CTP) grade, indication for LT, location at time of LT (ie, home, hospital, or ICU), intubation.
- Donors: age, sex, BMI, ICU stay prior to procurement >7 days, natremia >155 mmol/L, AST blood level >150 IU/mL, ALT blood level >170 IU/mL, occurrence of cardiac arrest before procurement.
- Duration of cold ischemia time.
All variables were attributed the same weight in the propensity score calculation.
Study Protocol
Procurement and Machine Perfusion Settings
After graft acceptance and standard procurement, the graft was initially preserved in a static cold ischemia phase using CUSTODIOL (SERB®, Luxembourg). After arrival at our center, the liver graft was prepared during the back-table procedure and then flushed with 1 L of machine perfusion solution (UWMP) (Bridge to life®, Ilinois) before connection to the machine perfusion device (Liver Assist (Organ Assist, Netherland)).
According to the Karangwa et al. classification,(9) perfusion was performed through the portal vein at a target pressure of 3 to 5 mm Hg with 2 L of machine perfusion solution (UWMP) oxygenated with 1 L/minute at a temperature of 11°C. The duration of perfusion was ideally between 2 and 4 hours with a minimum of 1 hour and a maximum of 6 hours. Our aim was to achieve the shortest ischemia time (ideally <8 hours) and not to test HOPE to increase ischemia time. Perfusion was therefore started immediately after the back-table procedure and during native liver hepatectomy and then stopped just before graft implantation.
LT and Postoperative Care
All patients had orthotopic LT with inferior vena cava preservation. Briefly, after standard wound incision and exposition, the liver pedicle was first dissected. The native liver was removed, and careful hemostasis was performed. The graft was removed from the perfusion machine, flushed with 500 mL of 5% albumin, and its implantation was started with side-to-side caval anastomosis followed by end-to-end portal vein anastomosis. The graft was then vascularized prior to artery and biliary anastomosis.
After the procedure, standardized immunosuppression (associating calcineurin inhibitor [usually tacrolimus], mycophenolate mofetil, and a short course of corticosteroids) was systematically administered. Systematic Doppler ultrasonography was performed on postoperative days (PODs) 1 and 7 and if hepatic dysfunction or vascular complication was suspected. When suspected, computed tomography was systematically performed to confirm vascular complications, and treatment (medical, radiological, or surgical) was systematically discussed in multidisciplinary meetings.
Patients were discharged from the hospital only when they presented with normal liver graft function, had immunosuppressive treatment within the therapeutic range, and demonstrated sufficient autonomy (eating, physical movement, able to correctly take their medications).
Study Endpoint
Primary Endpoints
The primary endpoint was the incidence of early allograft dysfunction (EAD) as described by Olthoff et al.,(10) with the presence of 1 or more of the following criteria: bilirubin ≥171 µmol/L on POD 7 after LT, international normalized ratio (INR) ≥1.6 on POD 7 after LT, or peak ALT >2000 U/L within the first 7 days after LT, and/or occurrence of primary nonfunction (PNF) defined as liver failure requiring retransplantation or leading to death within 7 days after transplantation.
Secondary Endpoints
- Intraoperative parameters: number of intraoperative transfusions, incidence of postreperfusion syndrome (PRS; defined as a decrease of more than 30% of the mean arterial pressure value for at least 1 minute and occurring within 5 minutes after revascularization(11)), and duration of procedure.
- Biological parameters:
- Graft function: prothrombin level, serum bilirubin, serum AST and ALT, factor V, INR during day 7 after LT, and arterial lactate (dosed until normalization).
- Renal function: serum creatinine, clearance estimated by the Chronic Kidney Disease Epidemiology Collaboration formula(12) and acute kidney injuries (with Kidney Disease: Improving Global Outcomes [KDIGO] classification) observed at 48 hours after LT.(13)
- Postoperative parameters: incidence of severe postoperative complications assessed by Clavien-Dindo classification ≥3 during initial hospitalization, duration of hospital and ICU stays, biliary complications at 12 months, and patient and graft survival at 12 months.
- Economic impact from the hospital’s perspective: evaluation of the additional cost of the machine perfusion procedure and estimation of the costs and incomes of the hospital stay.
Cost Analysis
In France, the hospital income is paid by French national health insurance and is mainly determined by the characteristics of each inpatient’s stay (taking into consideration the patient’s age and comorbidities, diagnosis, duration of stay, medical and surgical procedures, ICU stays, treatments, etc.). These inpatient stays are then classified in a medically and economically homogenous group (Groupe Homogène de Malade [GHM]). A standard national tariff is applied to each group. For each French hospital, all the hospital stays, their characteristics, the GHM, and associated tariffs are recorded in the Programme de Médicalisation des Systèmes d’Information (PMSI) database. Data on hospital revenues for hospital stays of the patients included in the study were identified from the Rennes hospital PMSI database.
The tariffs of each GHM are defined by the national authorities. They correspond to an estimate of the average costs of hospital stays for this GHM (eg, for an average length of stay). Data on hospital costs for hospital stays of the patients included in the study were retrieved from the French National Cost Database Echelle Nationale des Coûts, which collects average real-world costs for each GHM from a French hospital representative sample. We adjusted the costs for each GHM with the actual length of stay of patients included in the study. In addition, for each hospital stay in the HOPE group, cost data included amortization of the machine perfusion device, perfusion kits, machine perfusion solution, and maintenance of the machine.
Cost and revenue were expressed as mean value ± standard deviation and compared using bootstrap confidence intervals of the difference of mean cost between the 2 groups. Because of the sampling fluctuations, the distribution of diagnosis-related group (DRG) was not necessarily the same in both groups. We therefore estimated the average costs of hospital stays in the HOPE group by performing standardization (ie, by applying the distribution of DRG of the control group to the HOPE group).
Statistical Analysis
Comparative Cohort Matching
After the calculation of propensity scores, matching was performed with a 1:3 ratio between patients in the HOPE and control group. Exact match was prioritized followed by selection of the closest control, and the maximum distance allowed between 2 matched patients was set at 0.2 (ie, caliper restriction).
Quantitative variables were expressed as mean value ± standard deviation or by median with extreme values and compared using the Student t test or Wilcoxon test as appropriate. Qualitative variables were expressed as number and percentage and compared using chi-square or Fisher’s exact tests as appropriate.
All statistical analyses were performed with R software version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria), the medico-economic analysis with SAS software version 9.4 (SAS Institute, Cary, NC), the propensity score analysis with MatchIt R package version 3.0.2., and receiver operating characteristic (ROC) curve analysis with ROCR R package version 1.0-7. A P value <0.05 was considered statistically significant.
Results
Recipient and Graft Characteristics
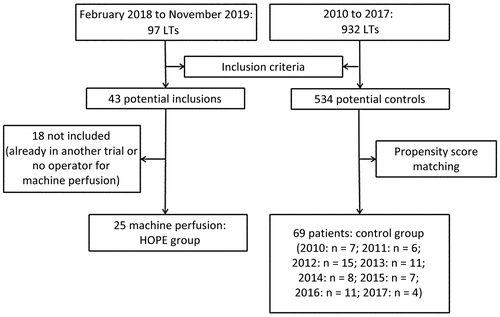
Between February and November 2018, 97 LTs were performed in our institution. An ECD graft was used in 54 (56%) cases. After taking into consideration exclusion criteria, 43 LTs could have been potentially included in the PERPHO Study; among them, 25 patients were finally included and constituted the HOPE group. The remaining 18 LTs were already included in another study(14) or were not included because of operator availability for the machine perfusion (Fig. 1).
Patients were men in 80% (n = 20) of the cases, with a median age of 63 (range, 43-69) years and a median MELD score before LT of 18.3 (range, 7-37). Of the patients, 72% (n = 18) were at home prior to LT, and 28% (n = 7) were hospitalized, including 16% (n = 4) in the ICU with intubation. Of the donors, 54% (n = 14) were male, with a median age of 70 (range, 45-87) years, and 19 (76%) were >65 years of age (Table 1).
| recipient characteristics | donor characteristics | intraoperative | postoperative outcome | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patient number | Age, Years | Sex | CTP Grade | MELD Score at LT | Indication for LT | Age, Years | BMI | Cardiac Arrest | Steatosis | Perfusion Duration (Minutes) | CIT (Minutes) | PRS | EAD | PNF | Clavien-Dindo | Hospital Stay (Days) | 3-Month Graft Survival | 3-Month Patient Survival | Cause of Graft Loss and/or Death |
| 1 | 64 | Male | 5 | 7 | HCC + ALD | 80 | 23.8 | No | 5 | 93 | 642 | Yes | Yes | No | 1 | 8 | Yes | Yes | – |
| 2 | 63 | Female | 9 | 15 | ALD | 68 | 43 | No | 30 | 109 | 536 | No | No | No | 3 | 24 | Yes | Yes | – |
| 3 | 63 | Male | 7 | 9 | HCC + ALD | 83 | 28.2 | No | 0 | 75 | 425 | No | No | No | 2 | 12 | Yes | Yes | – |
| 4 | 61 | Male | 6 | 10 | HCC + ALD | 69 | 25.2 | No | 15 | 125 | 491 | No | Yes | Yes | 4 | 97 | No | Yes | HV thrombosis |
| 5 | 62 | Male | 12 | 17 | HCC + ALD | 47 | 25 | Yes | 0 | 115 | 630 | No | No | No | 2 | 21 | Yes | Yes | – |
| 6 | 64 | Male | 6 | 22 | HCC + ALD | 83 | 29 | No | 0 | 105 | 616 | Yes | No | No | 1 | 10 | Yes | Yes | – |
| 7 | 58 | Male | 13 | 34 | ALD | 87 | 18 | No | 10 | 95 | 511 | Yes | No | No | 2 | 13 | Yes | Yes | – |
| 8 | 66 | Female | 12 | 18 | ALD | 75 | 26.2 | No | 10 | 97 | 414 | Yes | No | No | 3 | 13 | Yes | Yes | – |
| 9 | 49 | Male | 15 | 34 | ALD | 76 | 22.6 | No | 0 | 90 | 526 | Yes | Yes | No | 2 | 33 | Yes | Yes | – |
| 10 | 43 | Male | 12 | 25 | ALD | 64 | 32.4 | No | 5 | 117 | 554 | No | No | No | 2 | 13 | Yes | Yes | – |
| 11 | 50 | Male | 5 | 16 | HCC + ALD | 53 | 21.6 | Yes | 5 | 226 | 692 | No | No | No | 2 | 19 | Yes | Yes | – |
| 12 | 63 | Female | 11 | 37 | ALD | 68 | 25.3 | No | 5 | 130 | 379 | Yes | No | No | 2 | 15 | Yes | Yes | – |
| 13 | 63 | Male | 9 | 12 | HCC + ALD | 70 | 29.4 | No | 0 | 252 | 816 | Yes | Yes | No | 3 | 19 | Yes | Yes | – |
| 14 | 57 | Male | 8 | 19 | ALD + HCV | 84 | 29 | No | 15 | 222 | 547 | Yes | Yes | No | 2 | 24 | Yes | Yes | – |
| 15 | 62 | Male | 12 | 21 | ALD | 70 | 20.7 | No | 0 | 178 | 525 | Yes | No | Yes | 3 | 23 | No | Yes | HA thrombosis |
| 16 | 66 | Male | 5 | 12 | HCC + ALD | 70 | 27.3 | No | 0 | 130 | 615 | Yes | No | No | 2 | 13 | Yes | Yes | – |
| 17 | 57 | Male | 12 | 22 | HCC + ALD | 68 | 23.4 | Yes | 0 | 116 | 512 | No | No | No | 2 | 15 | Yes | Yes | – |
| 18 | 68 | Male | 9 | 17 | HCC + ALD | 82 | 23.7 | No | 5 | 195 | 530 | No | No | No | 2 | 12 | Yes | Yes | – |
| 19 | 59 | Male | 10 | 22 | Other | 71 | 22.1 | No | 0 | 124 | 444 | No | No | No | 2 | 10 | No | No | Multivisceral failure |
| 20 | 65 | Male | 5 | 18 | HCC + ALD | 51 | 25.1 | Yes | NA | 107 | 495 | No | Yes | No | 2 | 20 | Yes | Yes | – |
| 21 | 62 | Male | 6 | 14 | HCC + ALD | 82 | 20.3 | No | 0 | 144 | 505 | Yes | No | No | 2 | 8 | Yes | Yes | – |
| 22 | 56 | Female | 12 | 32 | NASH | 79 | 24.5 | No | 0 | 153 | 665 | No | No | No | 2 | 15 | Yes | Yes | – |
| 23 | 66 | Male | 11 | 18 | ALD | 78 | 22.9 | Yes | 10 | 100 | 519 | Yes | No | No | 3 | 13 | Yes | Yes | – |
| 24 | 69 | Female | 11 | 20 | ALD | 48 | 25.4 | No | 0 | 124 | 458 | No | No | No | 2 | 17 | Yes | Yes | – |
| 25 | 66 | Male | 5 | 8 | HCC + ALD | 45 | 40.9 | No | NA | 90 | 445 | Yes | Yes | No | 2 | 11 | Yes | Yes | – |
Procedure and Perfusion Characteristics
Median ischemia time was 525 (range, 379-824) minutes, with a median perfusion time of 117 (range, 75-252) minutes. Median perfusion pressure was 5 (range, 2-6) mm Hg, and the median flow rate was 448 (range, 205-624) mL/minute. No adverse event attributed to machine perfusion was observed.
Perfusion time was more than 3 hours in 5 cases. In 2 cases (patients 11 and 13), surgery had to be delayed because of another LT at the same time (patient 11) or another emergency surgical procedure (patient 13). In the 3 other cases (patients 14, 15, and 18), prolonged perfusion time was related to prolonged hepatectomy as a result of extended portal thrombosis (patients 14 and 18) requiring extensive portal thrombectomy or because of recipient obesity (patient 15; Table 2).
| HOPE Group (n = 25) | Control Group (n = 69) | P Value | |
|---|---|---|---|
| Recipient characteristics | |||
| Sex, male | 20 (80) | 57 (82.6) | 0.77 |
| Age (years) | 63 (43-69) | 62 (36-70) | 0.32 |
| BMI (kg/m2) | 26.7 (19.7-44.8) | 27.4 (18.1-39.2) | 0.88 |
| MELD score | 18.3 (7-37) | 18.3 (5-40) | 0.63 |
| CTP | 9 (5-15) | 9 (5-15) | 0.84 |
| Creatinine level, day before LT (umol/L) | 75 (44-197) | 76 (43-486) | 0.96 |
| Total bilirubin level, day before LT (umol/L) | 35 (11-625) | 43.5 (5-749) | 0.83 |
| Indication of LT | 0.96 | ||
| HCC | 13 (52) | 33 (47.8) | |
| Alcoholic cirrhosis | 9 (36) | 24 (34.8%) | |
| HCV | 1 (4) | 6 (8.7) | |
| Other | 2 (8) | 6 (8.6) | |
| Donor characteristics | |||
| Sex, male | 14 (56) | 43 (62.3) | 0.58 |
| Age (years) | 70 (45-87) | 72 (25-88) | 0.88 |
| BMI (kg/m2) | 25.1 (18-43) | 24.7 (15.2-37.2) | 0.61 |
| ICU stay, days | 2 (0-13) | 2 (1-10) | 0.91 |
| Cardiac arrest before procurement | 5 (20) | 14 (20.3) | 1.00 |
| CIT, minutes | 525 (379-824) | 555 (207-722) | 0.55 |
| Steatosis (%) | 0 (0-30) | 0 (0-50) | 0.45 |
| Graft weight (grammes) | 1340 (900-2000) | 1355 (650-2270) | 0.85 |
| Primary outcomes | |||
| EAD | 7 (28) | 29 (42) | 0.22 |
| PNF | 2 (8) | 2 (2.9) | 0.29 |
| Secondary outcomes | |||
| Intraoperative parameters | |||
| Surgical time, minutes | 300 (206-387) | 395 (169-582) | <0.001 |
| Red blood cell transfusion | 5 (0-10) | 5 (0-23) | 0.64 |
| Fresh frozen plasma transfusion | 4 (0-14) | 5 (0-20) | 0.07 |
| Platelet count transfusion | 0 (0-2) | 1 (0-3) | 0.06 |
| PRS | 13 (52) | NA | NA |
| Postoperative parameters | |||
| Acute kidney injury | 11 (44) | 20 (28.9) | 0.17 |
| KDIGO stage 1 | 7 (28) | 11 (15.9) | 0.86 |
| KDIGO stage 2 | 3 (12) | 6 (8.7) | — |
| KDIGO stage 3 | 1 (4) | 3 (4.3) | — |
| Need for renal replacement | 1 (4) | 2 (2.9) | 1.00 |
| Peak AST level within 24 hours (UI) | 722 (184-6673) | 1301 (23610,979) | 0.07 |
| Peak ALT level within 24 hours (UI) | 493 (132-4353) | 722 (169-5754) | 0.02 |
| Clavien-Dindo ≥3 | 6 (24) | 31 (44.9) | 0.07 |
| ICU stay, days | 3 (1-72) | 5 (1-43) | 0.01 |
| Hospital stay, days | 15 (8-92) | 20 (9-92) | 0.01 |
| Biliary complication at 12 months | 2 (8) | 8 (11.6) | 1.00 |
| Anastomotic, leak or stenosis | 2 (8) | 7 (10.1) | |
| Nonanastomotic stricture | 0 | 0 | |
| Ischemic necrosis | 0 | 1 (1.4) | |
| 12-month graft survival | 22 (88) | 59 (89.5) | 1.00 |
| 12-month patient survival | 23 (91) | 63 (91.3) | 1.00 |
NOTE:
- Data are presented as median (range) or n (%).
Comparison After Propensity Score Matching
Propensity Score Matching
Between 2010 and 2017, 932 LTs were performed in our institution. Among them, 534 LTs were performed with an ECD graft and constituted the pool of potential controls. After propensity score matching, only 69 patients were included in the control group instead of 75 because of caliper restriction (Fig. 1). In particular, there were no controls found for patient 13 (probably as a result of the prolonged ischemia time).
To avoid giving an advantage to a group, we decided to keep the unmatched patients in the HOPE group in the comparative analysis because the 2 groups were still comparable.
Endpoints and Outcomes
As for the primary outcome, EAD was present in 28% (n = 7) of patients in the HOPE group and 42% (n = 29) in the control group without significant difference (P = 0.22). Retransplantation by day 7 was observed in 8% (n = 2) of the patients in the HOPE group (patient 4 on POD 4 for hepatic vein [HV] thrombosis with liver failure and patient 15 on POD 7 for hepatic artery [HA] thrombosis with normal liver function, discovered on systematic Doppler ultrasound) and 2.9% (n = 2) of the patients in the control group without significant difference (P = 0.29). The primary outcome was therefore present in 32% (n = 8) of the patients in the HOPE group and 42% (n = 29) of the patients in the control group without significant difference (P = 0.38; Table 1).
Predictive Value of Liver Enzyme Levels Measured on Perfusate at the End of Perfusion
Liver enzyme dosage on the perfusion solution was analyzed for 20 patients in the HOPE group (dosage not performed or failed in 3 cases and excluded in 2 cases [patients 4 and 15] because of the presence of vascular complications, which could impact postoperative biological parameters). The median AST level in the perfusion solution was 248.5 (range, 48-2076) U/L, and the median ALT level was 285.5 (range, 38-1397) U/L.
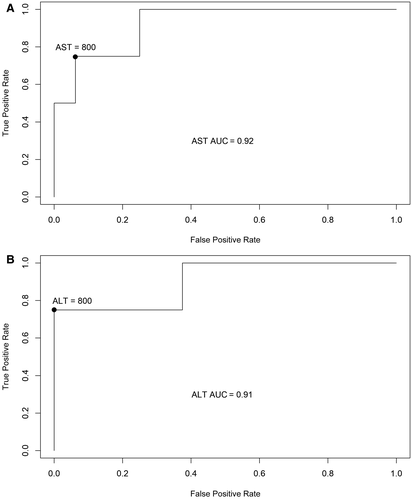
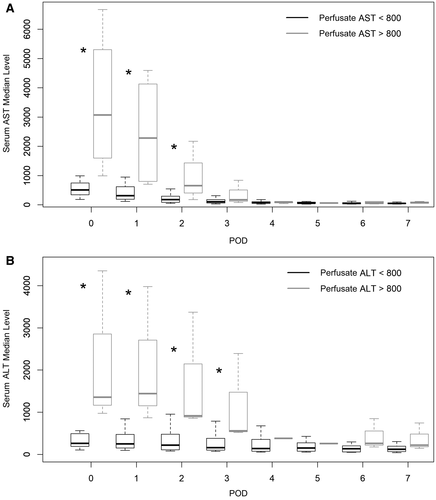
Among the 20 patients, 4 patients presented with EAD and had a significantly higher AST level (980.5 U/L versus 170 U/L; P = 0.01) and ALT level (1073.5 U/L versus 143.5 U/L; P = 0.01) in the perfusion solution compared with the patients who did not present EAD. The ROC curve analysis (Fig. 2) revealed that AST and ALT levels in perfusate were excellent predictors of EAD occurrence with areas under the curve (AUCs), respectively, of 0.92 and 0.91. The best predictive cutoff was 800 U/L for AST and ALT. As a consequence, postoperative blood liver enzymes were significantly increased in the early PODs when liver enzyme levels were increased in the perfusate (Fig. 3).
Intraoperative Parameters
Surgical time was significantly reduced in the HOPE group compared with the control group (300 versus 395 minutes; P < 0.001). There was no difference regarding the median number of packed red blood cells transfused (5 versus 5; P = 0.29), whereas the difference was close to being significant for the median number of fresh frozen plasma transfused (4 versus 5; P = 0.07) or the median platelet count (0 versus 1; P = 0.06). PRS was present in 52% (n = 13) of the patients in the HOPE group and could not be evaluated retrospectively in the control group.
Postoperative Parameters
The median AST level was significantly lower in the HOPE group on POD 0 (724 U/L versus 1284 U/L; P = 0.046), as were the ALT (392 U/L versus 720 U/L; P = 0.01), lactate (2.2 versus 2.7; P = 0.01), and creatinine level (73 versus 89 µmol/L; P = 0.01). On POD 1, the lactate level was still lower in the HOPE group (1.5 versus 2; P = 0.03), whereas the difference was no more significant regarding the AST (599 U/L versus 870 U/L; P = 0.09) and ALT (454 U/L versus 668 U/L; P = 0.06; Fig. 4).
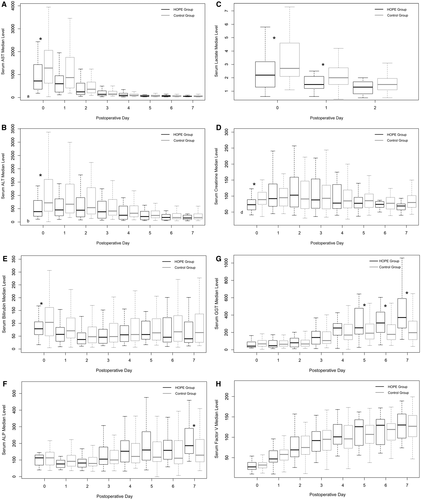
The acute kidney injury incidence (calculated at 48 hours after LT) was no different between groups (44% [n = 11] versus 28.9% [n = 20]; P = 0.17). As for cholestasis enzymes, the gamma-glutamyltransferase (GGT) level was significantly increased in the HOPE group from POD 5 (253 versus 191; P = 0.04) as in the alkaline phosphatase (ALP) level on POD 7 (186 versus 129; P = 0.024), whereas the bilirubin levels were not different.
Median ICU stay was significantly reduced in the HOPE group (3 versus 5 days; P = 0.01), as was hospital stay (15 versus 20 days; P = 0.01). Of the patients, 6 (24%) in the HOPE group and 31 (44.9%) in the control group had severe complications (ie, Clavien-Dindo score ≥3), without significant difference (P = 0.07).
The occurrence of biliary complications during the first 12 months was similar between groups (8% [n = 2] versus 11.6% [n = 8]; P = 1). In the HOPE group, 2 patients presented with anastomotic complications requiring surgical revision for bile leakage in 1 case and endoscopic treatment for anastomotic stricture in the other. In the control group, 7 patients presented with anastomotic complications represented by 4 bile leakages (requiring surgery in 2 cases and medical treatment only in 2 cases) and 3 anastomotic strictures treated by surgery in 1 case and endoscopic treatment in 2 cases. One patient in the control group presented with biliary necrosis requiring retransplantation. No patient presented with nonanastomotic stricture.
At 12 months, graft survival was similar between the 2 groups (88% in the HOPE group versus 89.5% in the control group; P = 1.00), as was patient survival (91% in the HOPE group versus 91.3% in the control group; P = 1.00).
Cost Analysis
In the HOPE group, machine perfusion device cost was estimated at €429 (US $509) per patient according to an amortization time of 7 years and an annual number of LT procedures in 25 patients. The costs of perfusion kits and machine perfusion solution were estimated at €4195 (US $4977) and €338 (US $401) per patient, respectively. Maintenance cost was estimated at €336 (US $399) per patient, and total additional cost for the procedure was estimated at €5298 (US $6286) per patient.
The average cost of a hospital stay was €46,136 ± €39,994 (US $54,737 ± $47,450) in the HOPE group and €42,756 ± €27,943 (US $50,727 ± $33,152) in the control group. Average hospital income was estimated at €49,159 ± €24,740 (US $58,324 ± $29,352) in the HOPE group and €46,815 ± €17,886 (US $55,543 ± $21,220) in the control group.
The average difference between cost and revenue for a hospital stay from a hospital perspective was not statistically significant between the HOPE and the control groups (respectively, €3023 ± €16,537 [US $3587 ± $19,620] and €4059 ± €16,266 [US $4816 ± $19,298]; IC = 95% confidence interval, −€5470 to €8652 [US −$6490 to $10,265]).
Discussion
LT is the best treatment for end-stage liver disease, but it is limited by the scarcity of grafts. Expanding the donor pool with DCD or ECD grafts appears to be the most effective solution despite their higher risk of dysfunction. To decrease this risk and improve outcome, machine perfusion has been evaluated and shown promising results.(15, 16)
The PERPHO Study is the first prospective trial evaluating the interest of HOPE in the preservation of ECD grafts procured from DBD donors. The study was not designed to test an increase of the total conservation time or to increase the number of LTs performed by reconditioning discarded grafts.
We found a significant reduction in AST and ALT levels in the early postoperative period, attesting to a reduction in hepatocyte damage, as well as a significant decrease in lactate levels, reflecting faster graft function recovery. However, we did not note a significant decrease in EAD incidence (28% in the HOPE group versus 42% in the control group), which was probably attributed to a lack of power. Interestingly, liver enzyme levels in the perfusion liquid, measured at the end of the HOPE procedure, were highly predictive of EAD incidence and correlated to postoperative serum liver enzymes.
In addition, we observed that the serum level of ALP and GGT (as well as the bilirubin level) started to increase from POD 2 in both groups. However, this increase was higher in the HOPE group and became significant at POD 5 for GGT and POD 7 for ALP before spontaneously resolving. We believe this phenomenon may represent the liver regeneration, which was described as positively correlated with the serum level of a biliary marker(17) instead of a marker of biliary damage.
We also observed a significant reduction in ICU (3 versus 5 days; P = 0.01) and total hospital stays (15 versus 20 days; P = 0.01). This could be explained by better graft function recovery as well as the reduced incidence of severe complications, which was close to being significant (P = 0.07). Consequently, from a hospital’s perspective, the additional cost of the machine perfusion procedure (estimated at €5298 [US $6286] per patient) was compensated by a reduction of hospitalization length, resulting in a nonsignificant difference between cost and revenue between the 2 groups. This analysis was made from the hospital’s perspective (and not the health care system’s perspective) because the decision to invest or not into the machine perfusion program, with the extra costs, which is the main obstacle to its implementation, is up to the hospitals with no participation of the national health care systems in most countries.
Moreover, even if the aim of the study was not to increase the number of LTs performed, we believe that 1 procedure was only allowed by the HOPE procedure. Indeed, in the case of patient 13, an unpredictable weather event led to the graft arriving with more than 10 hours of cold ischemia time and without immediate access to an operating room (because of another urgent procedure). In this case, the graft would have probably been considered unfit for transplantation without the potential benefit of HOPE because we would not have risked using an ECD graft with such prolonged ischemia. Instead, we decided to put the liver on machine perfusion and wait until another operating room was available. As a consequence, we did not find a suitable control for this case, highlighting its exceptional nature. In the same way, when the hepatectomy is complicated, we believe that HOPE makes it possible to partially mitigate the stress and the pressure of cold ischemia.
Another change of paradigm was represented by recipient-graft matching. Indeed, ECD grafts are usually allocated to “good” recipients (mostly hepatocellular carcinoma [HCC] patients with compensated cirrhosis), whereas most “high-MELD” recipients receive non-ECD grafts. However, in our study, 3 patients who received a transplant had a MELD score >30 with grafts from donors >75 years of age. Because the postoperative outcomes were uneventful, we believe that HOPE makes it possible to safely allocate ECD grafts to “high-MELD” recipients.
Similar to kidney transplantation, liver perfusion started in the beginning of this decade with the first clinical series in 2010 reported by Guarrera et al.(15) In their pilot study, the authors reported the use of hypothermic machine perfusion without oxygenation for conservation of DBD “standard” donors and found an improvement in biological markers and shorter hospital stays in the machine group. Thereafter, the same team reported their experience with 31 liver grafts considered as “orphan”(18) and reported higher 1-year patient survival and significantly shorter hospital stays as well as fewer biliary complications and acute kidney injuries. However, Schlegel et al.(19, 20) showed that HOPE recharged depleted cellular energy stores (ie, ATP level), restored the mitochondrial redox state by reversible suppression of oxidative metabolism, and subsequently decreased the production of oxygen radical species, resulting in less IRI and improved liver function.(21) Thereafter, machine perfusion proved its utility in LT with DCD grafts by improving graft function as well as survival compared with conventional static conservation.(7, 22) Recently, Nasrala et al.,(23) in a prospective randomized trial using normothermic perfusion for preservation of all sorts of grafts, reported a significant decrease in postoperative liver enzyme levels as well as in the incidence of EAD and the rate of discarded grafts. However, despite the fact that normothermic machine perfusion is supposed to be more efficient than HOPE (but also more complex), they failed to show any clinical improvement, as the duration of hospital stays as well as the graft and patient survival were no different. One could therefore think that machine perfusion is not required for all LTs, especially those with “non-ECD grafts.” Recently, Patrono et al.(24) reported their results of 25 patients who received ECD grafts using dual HOPE. They noted a significant reduction in postoperative AST and ALT blood levels, resulting in a decrease in EAD incidence and a significant reduction in stages 2 and 3 acute kidney injuries. However, they did not report a significant difference regarding hospital stay and survival, which could be partly explained by the significantly higher duration of preservation in the perfusion group (499 versus 371 minutes; P < 0.001).
Our results are in line with studies relating the benefits of the HOPE procedure, as we noted a reduction in liver enzyme levels(23, 24) and better graft function recovery. However, we found that HOPE significantly reduced hospital stays, resulting in equivalent hospitalization costs. Therefore, as with transplantations with DCD grafts,(7, 22) we believe that use of machine perfusion is justified and economically acceptable for LT with DBD-ECD grafts.
However, our results must be interpreted with caution. First, because this was a pilot study, we had a limited number of included patients (n = 25). Moreover, we only found 69 patients for the control group (as a result of caliper restriction) instead of 75, which decreased the power of our study. However, because the unmatched patients of the HOPE group (which could be considered as having a poorer prognosis) were kept in the analysis, we believe that our results may underestimate the potential benefit of HOPE. Second, our control group was not prospective, which could induce a methodological bias in both the clinical and cost analysis. However, we voluntarily decided to not create a prospective control group because we believe that a prospective randomized trial with only 25 patients in both the HOPE and control groups has a high risk of leading to unbalanced randomization and then to noncomparable groups. Third, our definition of ECD graft could be questionable and may not reflect the real quality of the graft. However, there is still no consensual definition of an ECD graft, and we previously reported those criteria, which are now used in prospective trials in our country.(14, 25) In any case, our promising results must be confirmed in a prospective randomized trial, which is currently ongoing.(25) Fourth, the wide range of the HOPE duration in our study may impact the liver enzyme level in the perfusate. However, because the plateau level is quickly reached and stays mostly stable until the end of perfusion(18), we believe that the HOPE procedure duration does not represent a major bias. Finally, our cost analysis finding may not be directly applicable to other health care systems because of the specificities of the French hospital funding. However, because our estimation of the extra cost of the HOPE procedure will be the same whatever the country or the health care system, we believe that our results will provide informative data for further studies.
In conclusion, we believe that HOPE is a promising method to improve the preservation of DBD-ECD grafts because it may provide better graft function. In our study, the additional cost of the procedure was compensated by better outcomes. These promising results must be confirmed in a prospective, multicentric, randomized trial.