Utility of Mac-2 Binding Protein Glycosylation Isomer to Evaluate Graft Status After Liver Transplantation
Potential conflict of interest: Nothing to report.
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
SEE EDITORIAL ON PAGE 325
Abstract
Mac-2 binding protein glycosylation isomer (M2BPGi) is a novel liver fibrosis biomarker, but there are few studies on M2BPGi in liver transplantation (LT) recipients. This study aimed to evaluate the utility of M2BPGi measurement in LT recipients. We collected the clinicopathological data of 233 patients who underwent a liver biopsy at Kyoto University Hospital after LT between August 2015 and June 2019. The median values of M2BPGi in patients with METAVIR fibrosis stages F0, F1, F2, and ≥F3 were 0.61, 0.76, 1.16, and 1.47, respectively, whereas those in patients with METAVIR necroinflammatory indexes A0, A1, and ≥A2 were 0.53, 1.145, and 2.24, respectively. Spearman rank correlation test suggested that the necroinflammatory index had a stronger correlation to the M2BPGi value than the fibrosis stage. The area under the receiver operating characteristic curve of M2BPGi to predict ≥A1 was 0.75, which was significantly higher than that of any other liver fibrosis and inflammation marker. Patients with a rejection activity index (RAI) of ≥3 had a higher M2BPGi value than those with RAI ≤ 2 (P = 0.001). Patients with hepatitis C virus viremia had a higher M2BPGi value than sustained virological responders or those with other etiologies. In conclusion, the present study demonstrated that M2BPGi values are more strongly influenced by necroinflammatory activity and revealed M2BPGi, which has been thought to be a so-called fibrosis marker, as a disease activity marker in transplant recipients. M2BPGi measurement may be useful to detect early stage liver inflammation that cannot be detected by routine blood examination of LT recipients.
Abbreviations
-
- ALB
-
- albumin
-
- ALT
-
- alanine aminotransferase
-
- APRI
-
- aspartate aminotransferase-to-platelet ratio index
-
- ARFI
-
- acoustic radiation force impulse
-
- AST
-
- aspartate aminotransferase
-
- AUROC
-
- area under the receiver operating characteristic curve
-
- CI
-
- confidence interval
-
- COI
-
- cutoff index
-
- DAPI
-
- 4′,6-diamidino-2-phenylindole
-
- FIB-4
-
- fibrosis-4
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HCV-SVR
-
- hepatitis C virus patients who had achieved sustained virological response
-
- HCV-viremia
-
- hepatitis C virus patients with viremia
-
- LDH
-
- lactate dehydrogenase
-
- LT
-
- liver transplantation
-
- M2BP
-
- Mac-2 binding protein
-
- M2BPGi
-
- Mac-2 binding protein glycosylation isomer
-
- NASH
-
- nonalcoholic steatohepatitis
-
- NLR
-
- negative likelihood ratio
-
- NPV
-
- negative predictive value
-
- ns
-
- not significant
-
- PBC
-
- primary biliary cirrhosis
-
- PLR
-
- positive likelihood ratio
-
- PPV
-
- positive predictive value
-
- PSC
-
- primary sclerosing cholangitis
-
- PT-INR
-
- prothrombin time–international normalized ratio
-
- RAI
-
- rejection activity index
-
- ROC
-
- receiver operating characteristic
-
- SD
-
- standard deviation
-
- SVR
-
- sustained virological response
-
- T-Bil
-
- total bilirubin
-
- WFA
-
- Wisteria floribunda agglutinin
Liver transplantation (LT) is the only therapeutic option for end-stage liver disease and acute liver failure.(1) After LT, we encounter a broad spectrum of pathological abnormalities in the liver allograft, such as allograft rejection, de novo or recurrent autoimmune hepatitis, and recurrence of the primary disease. All of them can eventually lead to graft fibrosis that potentially results in graft failure and affects the longterm prognosis.(2)
A liver biopsy is the gold standard to assess the graft status.(3, 4) However, there is no universal guideline on how often a liver biopsy should be performed in the longterm follow-up of recipients. Graft abnormalities are frequently found in liver biopsies of recipients showing no abnormalities in blood tests, which is why Neuberger et al. advocate a protocol biopsy.(5) On the other hand, Rosenthal et al. do not recommend a protocol biopsy because it is associated with complications and its contribution to improvement of prognosis has not been proven.(6) If less invasive methods can be used to detect potential graft abnormalities with high sensitivity, the frequency of unnecessary liver biopsies can be reduced without decreasing the chance of early detection of abnormalities.
Blood test–based evaluation is one of the less invasive approaches. Several methods based on a blood test are reported to be useful to evaluate liver fibrosis in patients with chronic liver disease (eg, aspartate aminotransferase-to-platelet ratio index [APRI],(7, 8) fibrosis-4 [FIB-4] index,(9) hyaluronic acid,(10, 11) and type 4 collagen(12)). Liver stiffness measurement is another promising method to diagnose liver fibrosis in a noninvasive manner, eg, FibroScan(13) and acoustic radiation force impulse (ARFI)(14).
However, fewer studies have evaluated noninvasive assessments of liver fibrosis in LT recipients. Liver fibrosis indices based on laboratory tests, such as APRI(15) and FIB-4 index,(16-18) have been reported to be useful to evaluate graft fibrosis. Yoshino et al. reported that liver stiffness measurements by ARFI were more useful to predict graft fibrosis after LT than blood test–based indices that are profoundly affected by clinical factors other than the extent of liver fibrosis.(19)
Recently, the utility of serum Mac-2 binding protein glycosylation isomer (M2BPGi) has been reported as a novel liver fibrosis marker of chronic liver diseases, including hepatitis C virus (HCV),(20, 21) hepatitis B virus (HBV),(22) nonalcoholic steatohepatitis (NASH),(23) and primary biliary cirrhosis (PBC).(24) We have also reported its utility to assess the operative risk of hepatocellular carcinoma (HCC) patients who undergo a liver resection.(25) However, only 1 study on pediatric patients has investigated the utility of M2BPGi in LT recipients, which prompted us to evaluate the clinical utility of the M2BPGi value to assess graft liver fibrosis and inflammation in LT recipients including adult recipients. Our aim was to determine whether M2BPGi is effective to evaluate the graft status.
Patients and Methods
Patients
We collected the clinicopathological data of 233 patients who underwent liver biopsy at Kyoto University Hospital after an LT between August 2015 and June 2019. In these patients, no organs from executed prisoners were used. With regard to patients who underwent more than 2 liver biopsies in this period, we included only the data of the first biopsy to avoid duplicates of the same patients. We obtained informed consent from all patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and it was approved by the ethics committee of Kyoto University Graduate School and Faculty of Medicine (approval code E2477).
Clinical Data Collection
We collected clinical data including sex, age, primary disease, the date of the transplantation from the operative note of the LT, and blood examination. Blood examinations were performed on the same day or the day before the biopsies. Blood examinations included the complete blood count, coagulation test, and biochemistry including measurements of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), and albumin (ALB) levels. We also examined the M2BPGi values and other serum markers of liver fibrosis, ie, hyaluronic acid(10, 11) and type 4 collagen.(12) The M2BPGi value was measured using the HISCL M2BPGi Assay Kit (Sysmex, Kobe, Japan). The cutoff index (COI) of serum M2BPGi was calculated according to the equation reported previously.(26) The COI of M2BPGi was calculated as follows: COI = ([M2BPGi]sample – [M2BPGi]negative control)/([M2BPGi]positive control – [M2BPGi]negative control), where [M2BPGi]sample represents the M2BPGi value of the serum sample. The positive control was supplied as a calibration solution that was preliminarily standardized to yield COI = 1.0.(27) M2BPGi value ranges from 0.25 to 20.0 in previous studies on HBV, HCV, and biliary atresia patients.(21, 28) Simultaneously, we calculated liver fibrosis indicators, ie, APRI(7, 8) and FIB-4 index,(9) according to previously reported equations. We also performed ultrasound-based elastography, ie, ARFI,(29, 30) for liver stiffness measurement on the day of the biopsy, as reported previously.(19, 31)
Liver Biopsy and Pathological Data Collection
Liver biopsy examination was undertaken when clinically indicated (eg, when the liver function test showed abnormal findings) or at designated intervals (a so-called nonepisode biopsy) with informed consent. Generally, a nonepisode biopsy is performed in pediatric recipients, recipients whose primary disease can recur (HCV, primary sclerosing cholangitis [PSC], PBC, or NASH), and recipients with abnormal findings in the previous liver biopsy, with an interval of 2 or 3 years depending on the discretion of attending physicians.
We used an 18-gauge needle for the liver biopsy. Liver specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin and Masson’s trichrome.
All histological assessments were performed by expert pathologists at Kyoto University Hospital, and we referred to the histopathological reports. Pathological assessment was based on METAVIR scores of the fibrosis stage (F0-F4) and necroinflammatory activity (A0-A3).(32) The severity of transplant rejection was classified by the rejection activity index (RAI).(33, 34)
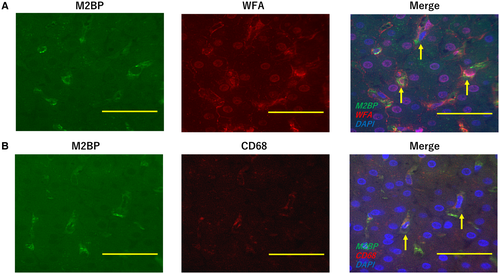
We performed immunostaining of some representative liver biopsy samples, 3 samples from HCV patients, 2 samples from PSC patients, 2 samples from acute liver failure patients, and 1 sample from a biliary atresia patient. Liver biopsy samples were stained with biotinylated Wisteria floribunda agglutinin (WFA; Vector Laboratories, Burlingame, CA), anti-Mac-2 binding protein (M2BP; R&D Systems, Minneapolis, MN), and anti-CD68 [KP1] (Abcam, Cambridge, MA) antibodies, followed by staining with Streptavidin DyLight–conjugated (Vector Laboratories) or Alexa Fluor–conjugated antibodies (Thermo Fisher Scientific, Waltham, MA). Images were obtained using a BZ-9000 microscope (KEYENCE Co., Osaka, Japan).
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median (range). They were compared by the Mann-Whitney U test or Kruskal-Wallis test. Statistical associations of each index were evaluated by the Spearman rank correlation coefficients. We calculated the 95% confidence interval (CI) and P value for the difference between 2 correlation coefficients by the bootstrap method with 10,000 repetitions. The predictive values of liver fibrosis were assessed by receiver operating characteristic (ROC) analysis, and the area under the receiver operating characteristic curve (AUROC) was calculated. The ROC curves were compared using the DeLong test. A P value of <0.05 was considered as statistically significant.
Statistical analyses were carried out using SAS software (JMP 14.0; SAS Institute, Cary, NC). Analysis by the bootstrap method was carried out using R, version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
Patient characteristics and other clinicopathological data are shown in Table 1. The median age of patients in this study was 33 years (range, 7-75 years). Primary liver diseases were HBV (n = 18, 7.7%), HCV (n = 29, 12.4%), PBC (n = 30, 12.9%), PSC (n = 10, 4.3%), alcohol (n = 5, 2.1%), NASH (n = 2, 0.9%), biliary atresia (n = 103, 44.2%), acute liver failure (n = 14, 6.0%), and others including cryptogenic causes (n = 22, 9.4%). Among these 233 patients, 222 patients were recipients from living donors, and 11 patients were recipients from brain dead donors. Among the 233 patients, 44 patients were pediatric patients (under 20 years of age). A total of 17 patients had HCC before LT. Among the 233 liver biopsies, 24 patients underwent liver biopsies for a clinical indication (a concern of disease recurrence or rejection), whereas 209 patients underwent so-called nonepisode biopsies.
| Total (n = 233) | |
|---|---|
| Age, years | 33 (7-75) |
| Sex | |
| Male | 92 (39.5) |
| Female | 141 (60.5) |
| Primary liver disease | |
| HBV | 18 (7.7) |
| HCV | 29 (12.4) |
| PBC | 30 (12.9) |
| PSC | 10 (4.3) |
| Alcohol | 5 (2.1) |
| NASH | 2 (0.9) |
| Biliary atresia | 103 (44.2) |
| Acute liver failure | 14 (6.0) |
| Others or cryptogenic | 22 (9.4) |
| Coexistence with HCC | 17 (7.3) |
| Immunosuppressive agents | |
| Tacrolimus | 202 |
| Cyclosporine | 16 |
| Steroid | 67 |
| Mycophenolate mofetil | 102 |
| Rapamycin | 3 |
| Blood examination | |
| Platelet count, ×1000/μL | 182 ± 90 |
| PT-INR | 1.06 ± 0.14 |
| LDH, U/L | 201 ± 54 |
| AST, U/L | 39 ± 34 |
| ALT, U/L | 36 ± 37 |
| T-Bil, mg/dL | 1.1 ± 1.6 |
| ALB, g/dL | 3.9 ± 0.5 |
| Fibrosis marker | |
| Hyaluronic acid, ng/mL | 79 ± 140 |
| Type 4 collagen, ng/mL | 5.4 ± 2.2 |
| M2BPGi (COI) | 1.3 ± 1.7 |
| Fibrosis indicators | |
| APRI | 0.98 ± 1.42 |
| FIB-4 index | 2.1 ± 3.1 |
| Liver stiffness (ARFI), m/second | 1.4 ± 0.4 |
| Duration from the transplantation, days | 5382 (87-11105) |
| Histological evaluation | |
| METAVIR score | |
| Fibrosis stage | |
| F0 | 49 (21.0) |
| F1 | 131 (56.2) |
| F2 | 39 (16.7) |
| F3 | 12 (5.1) |
| F4 | 2 (0.9) |
| Necroinflammatory index | |
| A0 | 120 (51.5) |
| A1 | 106 (45.5) |
| A2 | 6 (2.6) |
| A3 | 1 (0.4) |
| RAI | 0 (0-5) |
NOTE:
- Data are given as mean ± SD, medians (ranges), or n (%).
Diagnostic Performance of M2BPGi for Liver Fibrosis and Inflammation
The number of patients with METAVIR scores of F0, F1, F2, F3, and F4 were 49, 131, 39, 12, and 2, respectively. (We regrouped F3 and F4 together because the numbers of patients in F3 and F4 were too low for statistical analysis.) The median and quartile values of M2BPGi in patients with F0, F1, F2, and ≥F3 were 0.61 (0.425, 0.965), 0.76 (0.48, 1.34), 1.16 (0.52, 3.09), and 1.47 (0.51, 4.64), respectively. The M2BPGi value in patients with F2 was significantly higher than that in patients with F1 (P = 0.01) and F0 (P = 0.001). Moreover, the M2BPGi value in patients with ≥F3 was significantly higher than that in patients with F1 (P = 0.04) and F0 (P = 0.01). However, there was no statistically significant difference between F0 and F1, and between F2 and ≥F3 (Fig. 1A).
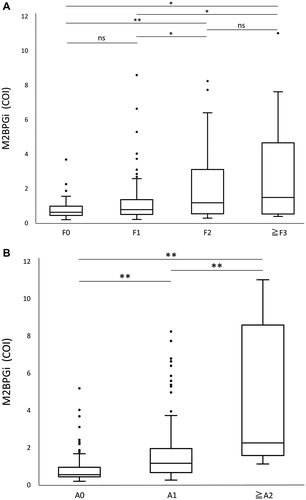
The numbers of patients with METAVIR scores of A0, A1, A2, and A3 were 120, 106, 6, and 1, respectively. (We regrouped A2 and A3 together because the number of patients in A2 and A3 was too low for statistical analysis.) The median and interquartile range values of M2BPGi in patients with A0, A1, and ≥A2 were 0.53 (0.42, 0.9325), 1.145 (0.6475, 1.9375), and 2.24 (1.56, 8.57), respectively. There were significant differences in the M2BPGi values between A0 and A1 (P < 0.001) and between A1 and ≥A2 (P = 0.006; Fig. 1B).
Spearman correlation analysis indicated a significant positive correlation between the fibrosis stage and M2BPGi value (r = 0.25, P < 0.001). Similarly, there was a significant positive correlation between the necroinflammatory index and M2BPGi value (r = 0.46, P < 0.001). The difference calculated by subtracting the Spearman rank correlation coefficient between the necroinflammatory index and the M2BPGi value from the value between the fibrosis stage and M2BPGi value was 0.21 (95% CI, 0.095-0.39; P = 0.002), indicating that the necroinflammatory index had a stronger correlation to the M2BPGi value than the fibrosis stage.
The AUROC to predict liver fibrosis is shown in Table 2. The AUROC of M2BPGi to predict ≥F1, ≥F2, and ≥F3 was 0.61, 0.66, and 0.66, respectively, which was not significantly different from that of other liver fibrosis markers or indexes.
| ≥F1 | P Value | ≥F2 | P Value | ≥F3 | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| AUROC | 95% CI | AUROC | 95% CI | AUROC | 95% CI | ||||
| M2BPGi | 0.61 | 0.53-0.69 | 0.66 | 0.56-0.75 | 0.66 | 0.47-0.81 | |||
| Platelet count | 0.57 | 0.48-0.65 | 0.39 | 0.72 | 0.63-0.79 | 0.34 | 0.80 | 0.64-0.99 | 0.23 |
| Hyaluronic acid | 0.55 | 0.46-0.64 | 0.16 | 0.64 | 0.55-0.73 | 0.64 | 0.64 | 0.47-0.79 | 0.79 |
| Type 4 collagen | 0.60 | 0.51-0.69 | 0.86 | 0.74 | 0.63-0.80 | 0.27 | 0.72 | 0.55-0.84 | 0.59 |
| APRI | 0.63 | 0.54-0.71 | 0.75 | 0.70 | 0.62-0.77 | 0.36 | 0.76 | 0.64-0.85 | 0.29 |
| FIB-4 index | 0.54 | 0.45-0.62 | 0.07 | 0.65 | 0.56-0.73 | 0.77 | 0.74 | 0.58-0.85 | 0.24 |
| ARFI | 0.68 | 0.56-0.78 | 0.31 | 0.67 | 0.54-0.77 | 0.95 | 0.75 | 0.48-0.91 | 0.18 |
NOTE:
- P values were calculated by DeLong test (versus M2BPGi value).
The AUROC to predict the liver necroinflammatory index is shown in Table 3. The AUROC of M2BPGi to predict ≥A1 and ≥A2 was 0.75 and 0.88, respectively. The AUROC of M2BPGi to predict ≥A2 was significantly higher than that of some liver fibrosis markers (ie, platelet count and hyaluronic acid). However, it was not significantly different from other liver inflammation markers (ie, lactate dehydrogenase [LDH], AST, and ALT). It is noteworthy that the AUROC of M2BPGi to predict ≥A1 was significantly higher than that of any other liver fibrosis marker or index (ie, platelet count, hyaluronic acid, type 4 collagen, APRI, FIB-4 index, and ARFI) and serum liver inflammation marker (ie, LDH, AST, and ALT). The cutoff value, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR) for each fibrosis and necroinflammatory stage are shown in Table 4.
| ≥A1 | P Value | ≥A2 | P Value | |||
|---|---|---|---|---|---|---|
| AUROC | 95% CI | AUROC | 95% CI | |||
| M2BPGi | 0.75 | 0.69-0.81 | 0.88 | 0.75-0.95 | ||
| Platelet count | 0.55 | 0.47-0.62 | <0.001 | 0.51 | 0.28-0.74 | <0.001 |
| Hyaluronic acid | 0.66 | 0.59-0.73 | 0.005 | 0.68 | 0.43-0.86 | 0.006 |
| Type 4 collagen | 0.58 | 0.50-0.66 | <0.001 | 0.73 | 0.53-0.87 | 0.08 |
| ARPI | 0.62 | 0.54-0.69 | <0.001 | 0.92 | 0.85-0.96 | 0.40 |
| FIB-4 index | 0.64 | 0.56-0.70 | <0.001 | 0.83 | 0.67-0.92 | 0.28 |
| ARFI | 0.61 | 0.51-0.71 | 0.009 | 0.50 | 0.13-0.87 | 0.13 |
| LDH | 0.68 | 0.60-0.74 | 0.04 | 0.89 | 0.77-0.95 | 0.97 |
| AST | 0.68 | 0.61-0.75 | 0.005 | 0.94 | 0.83-0.98 | 0.39 |
| ALT | 0.65 | 0.58-0.72 | 0.02 | 0.90 | 0.77-0.96 | 0.81 |
| T-Bil | 0.54 | 0.47-0.61 | <0.001 | 0.46 | 0.29-0.64 | <0.001 |
NOTE:
- P values were calculated by DeLong test (versus M2BPGi value).
| Cutoff Value | Sensitivity | Specificity | PPV | NPV | PLR | NLR | |
|---|---|---|---|---|---|---|---|
| Fibrosis stage | |||||||
| ≥F1 | 1.13 | 0.391 | 0.878 | 0.923 | 0.277 | 3.196 | 0.694 |
| ≥F2 | 1.15 | 0.566 | 0.744 | 0.395 | 0.854 | 2.215 | 0.583 |
| ≥F3 | 1.16 | 0.643 | 0.699 | 0.120 | 0.968 | 2.133 | 0.511 |
| Necroinflammatory index | |||||||
| ≥A1 | 0.70 | 0.743 | 0.667 | 0.677 | 0.734 | 2.230 | 0.385 |
| ≥A2 | 1.11 | 1.000 | 0.677 | 0.088 | 1.000 | 3.096 | 0 |
Next, we examined the utility of M2BPGi to diagnose allograft rejection. We assessed rejection by calculating the RAI. The RAI was 0 in 139 (59.7%), 1 in 50 (21.5%), 2 in 25 (10.7%), 3 in 16 (6.9%), and 5 in 3 patients (1.3%).
First, we analyzed the correlation between the necroinflammatory index and RAI (Fig. 2A). Spearman correlation test revealed a significant positive correlation between the RAI and necroinflammatory index (r = 0.32, P < 0.001).
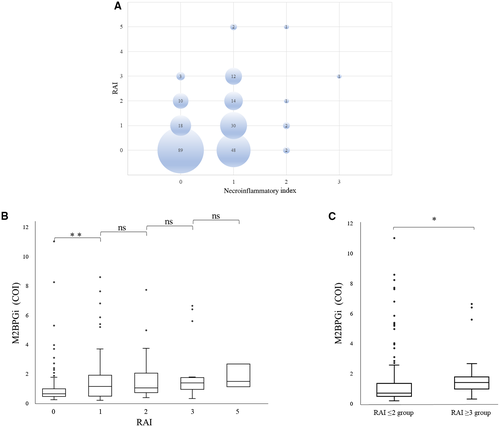
Second, on the basis of this result, we found a relationship between the RAI and the M2BPGi value. The median and quartile values of M2BPGi in RAI 0, 1, 2, 3, and 5 were 0.61 (0.44, 0.97), 1.12 (0.45, 1.895), 1.02 (0.705, 2.03), 1.365 (0.925, 1.7175), and 1.46 (1.11, 2.66), respectively. The M2BPGi value in RAI 1 was significantly higher than that in RAI 0 (P = 0.03). However, there was no statistically significant difference in M2BPGi between RAI 1 and 2, between RAI 2 and 3, and between RAI 3 and 5 (Fig. 2B).
The number of patients in RAI 3 and 5 was very low. Therefore, we regrouped the patients into groups based on RAI ≤2 and RAI ≥3. The median and quartile values of M2BPGi in RAI ≤2 and ≥3 groups were 0.695 (0.4675, 1.3325) and 1.39 (0.97, 1.77), respectively. The M2BPGi value in the RAI ≥3 group was significantly higher than that in the RAI ≤2 group (P = 0.001; Fig. 2C). The AUROC of M2BPGi to predict RAI ≥ 3 was 0.72. The cutoff value, sensitivity, specificity, PPV, NPV, PLR, and NLR in the RAI ≥ 3 group were 0.91, 0.842, 0.594, 0.155, 0.977, 2.071, and 0.266, respectively.
Relationship Between M2BPGi Value and HCV Infection
We analyzed the effect of HCV infection on the M2BPGi value. There were 29 patients whose primary disease was HCV, and all of them had recurrence of HCV after LT. At the time of the liver biopsy, 20 patients had viremia, whereas 9 patients had achieved a sustained virological response (SVR).
We compared the M2BPGi value between non-HCV patients, hepatitis C virus patients with viremia (HCV-viremia), and hepatitis C virus patients who had achieved sustained virological response (HCV-SVR). The median and quartile values of M2BPGi in non-HCV, HCV-viremia, and HCV-SVR groups were 0.69 (0.465, 1.17), 2.43 (2.18, 5.315), and 0.715 (0.35, 0.9475), respectively. The M2BPGi value in the HCV-viremia group was significantly higher than that in the non-HCV (P < 0.001) and HCV-SVR (P < 0.001) groups (Fig. 3A).
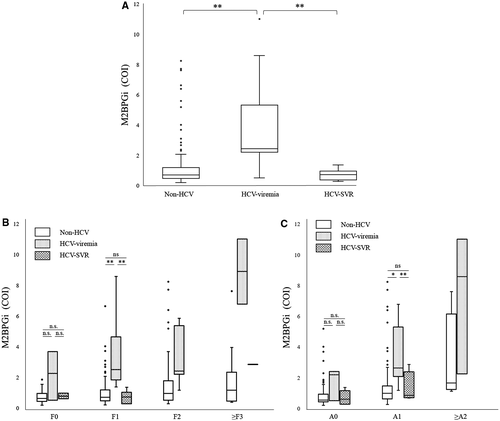
For further analysis, we stratified all patients according to the liver fibrosis score or liver necroinflammation score (Fig. 3B,C). The number of patients in non-HCV, HCV-viremia, and HCV-SVR groups for each fibrosis stage or necroinflammatory index are shown in Table 5. According to the liver fibrosis score, in F1 patients, the M2BPGi value in the HCV-viremia group was significantly higher than that in the non-HCV (P = 0.002) and HCV-SVR (P < 0.001) groups. However, there was no significant difference between these 3 groups of F0 patients. We could not perform statistical analysis of F2 and ≥F3 patients because of the small number of patients in each group.
| Group | Fibrosis Stage | Necroinflammatory Index | |||||
|---|---|---|---|---|---|---|---|
| F0 | F1 | F2 | ≥F3 | A0 | A1 | ≥A2 | |
| Non-HCV | 44 | 117 | 32 | 11 | 112 | 88 | 4 |
| HCV-viremia | 3 | 8 | 7 | 2 | 3 | 14 | 3 |
| HCV-SVR | 2 | 6 | 0 | 1 | 5 | 4 | 0 |
Similarly, according to the liver necroinflammation score, in A1 patients, the M2BPGi value in the HCV-viremia group was significantly higher than that in the non-HCV (P = 0.04) and HCV-SVR (P < 0.001) groups. However, there were no significant differences between these 3 groups of A0 patients. We could not perform statistical analysis of ≥A2 patients because of the small number of patients in each group.
Expression of M2BPGi in Liver Biopsy Specimens
We performed immunostaining of liver specimens from LT patients. Both WFA- and M2BP-positive cells were found in the liver specimens (Fig. 4A). These cells were also positive for CD68, a specific maker of Kupffer cells in the liver, indicating that Kupffer cells were expressing M2BPGi in the liver (Fig. 4B).
Discussion
Recently, M2BPGi was reported to be a novel marker of liver fibrosis in glycoproteomics studies.(26-28, 35) M2BP is extensively glycosylated and related to extracellular proteins, such as collagens. As a glycol alteration of M2BP, WFA was found to be useful to diagnose liver fibrosis. This glycoprotein (WFA-M2BP) is named M2BPGi. Serum M2BPGi is detected as an alteration of glycoproteins caused by liver fibrosis by using a sandwich immunoassay with WFA and anti-M2BP, which can be measured within 20 minutes using the HISCL M2BPGi Assay Kit (Sysmex). In this study, both WFA- and M2BP-positive cells were identified in liver specimens of LT patients, and these cells were thought to express M2BPGi. Simultaneously, these cells were positive for CD68, indicating that the M2BPGi-positive cells were Kupffer cells.
In addition to its utility as a liver fibrosis marker, another clinical utility of M2BPGi is prediction of liver decompensation(36) and development of HCC.(20) Furthermore, Okuda et al. showed that M2BPGi was useful to predict posthepatectomy liver failure in patients with HCC.(25) However, recent studies have demonstrated that the M2BPGi value can be affected by not only the extent of liver fibrosis, but also other factors. The M2BPGi value is significantly higher in HCV patients than in patients with other etiologies. This suggests that its value can be influenced by the underlying etiology, although it may merely reflect the fact that the intensity of the liver inflammation is stronger in HCV patients than in patients with other etiologies.(37) After treatment for HCV, the M2BPGi value decreases rapidly with the decrease in viral load preceding the improvement of liver fibrosis.(26, 38, 39) Morio et al. reported that the M2BPGi value increased during acute liver injury and decreased after recovery.(36) These studies suggest that the M2BPGi value is influenced by the presence of inflammation, as is seen in other noninvasive liver fibrosis tests, such as liver stiffness measurement,(40) and serum biomarkers.(41) The processes of liver damage, inflammation, and activation of the fibrogenic process are closely linked.(42) These facts prompted us to investigate the utility of M2BPGi in transplant recipients because it is interesting to determine how M2BPGi levels change in post-LT recipients, who are susceptible to various histological modifications such as acute rejection and chronic rejection, which are not observed in nontransplant patients with chronic liver disease. In this study, the M2BPGi-expressing cells in the liver were Kupffer cells, which are a type of macrophage, and this observation may strongly support our finding that the M2BPGi value reflects the activity of the fibrogenic process rather than the amount of accumulated extracellular matrix.
Only 1 study has evaluated the utility of M2BPGi in LT recipients.(43) It was suggested that M2BPGi was a novel fibrosis marker for LT patients, which increased in patients with acute cellular rejection. The study was conducted in pediatric patients whose primary disease was relatively homogeneous, primarily biliary atresia. Our study validated the utility of M2BPGi in a posttransplant setting in more patients with a wide range of underlying diseases.
Our results indicated that M2BPGi was not as useful in posttransplant recipients as nontransplant patients for diagnosis of liver fibrosis. The AUROC of M2BPGi to predict ≥F1, ≥F2, and ≥F3 was 0.61, 0.66, and 0.66, respectively, which was not better than that of other liver fibrosis markers or indexes. These values appeared to be lower than the reported AUROC values of M2BPGi to predict advanced fibrosis in nontransplant patients with HBV or HCV (0.76-0.81).(44, 45) These results suggest limited utility of serum markers to predict fibrosis with values that can be influenced by various factors other than fibrosis.
However, M2BPGi levels increased more sharply with an increasing necroinflammatory index than with an increasing fibrosis index. Indeed, Spearman correlation test and the bootstrap method revealed that the M2BPGi value had a stronger correlation to the necroinflammatory index than the fibrosis stage.
In particular, the AUROC to predict ≥A1 was 0.75, which was significantly higher than the AUROC of any other liver inflammation marker (ie, LDH, AST, and ALT). This result suggested that M2BPGi is useful to diagnose mild liver inflammation that cannot be detected by other conventional laboratory tests. Neuberger et al. reported that among 364 biopsies without abnormal laboratory findings, only 33 biopsies had normal histology.(5) This is one reason that a protocol biopsy is recommended for liver recipients without abnormal blood test results. In this regard, M2BPGi may be helpful to identify patients with abnormal histology but without abnormal findings in conventional laboratory tests. Although it is controversial whether therapeutic intervention should be considered for patients who are A1 with normal transaminase levels or whether therapeutic intervention improves the prognosis, a study by Sanada et al. demonstrated that A1 patients likely progress to F2 in several years.(46) Regular M2BPGi measurements may allow efficient identification of such patients with rapid fibrosis progression.
We suggest M2BPGi measurement, for example, once every 3-6 months for LT patients. We believe this can identify some patients with otherwise normal blood tests who require careful follow-up. However, we may be able to omit protocol liver biopsies for longterm stable liver recipients whose M2BPGi value is <0.70, predicting ≥A1 with a sensitivity and specificity of 0.743 and 0.667, respectively.
HCV recurs almost universally after LT.(47, 48) M2BPGi is a serum marker that was originally identified in a patient population consisting mainly of HCV patients.(26) A study has shown that the value increases more sharply in HCV patients.(21) The present study demonstrated that this was also true for patients after LT. We found that the M2BPGi value was significantly higher in patients with HCV viremia than in patients with other primary diseases. Moreover, the M2BPGi value in the HCV-SVR group was lower than that in the HCV-viremia group, which was also consistent with reports in a nontransplant setting. Although this study does not provide consecutive measurements in the same patients, this finding reinforces our finding that the M2BPGi value reflects necroinflammatory changes rather than the amount of fibrous tissue, because a rapid decrease in the amount of fibrous tissue is unlikely even in patients with SVR. Regular M2BPGi measurement would have been useful to determine appropriate treatment timing for posttransplant HCV patients in the interferon era by sensitively identifying a histological abnormality, although nowadays almost all HCV patients achieve SVR with direct-acting antiviral therapy. Therefore, M2BPGi measurement has little effect on clinical decisions regarding whether or when to administer antiviral therapy for patients with HCV after LT.
This study has several limitations. First, this is a single institutional study involving a small number of patients. A multicenter prospective study with a larger cohort is needed to further assess the efficacy of M2BPGi in liver recipients. Second, the number of patients with severe fibrosis or inflammation was relatively small. The numbers of patients with ≥F3 and ≥A2 were 14 and only 7, respectively, which did not sufficiently demonstrate the utility of M2BPGi for these patients. However, recruiting many posttransplant patients with severe fibrosis or inflammation is unrealistic under circumstances where liver recipients have been carefully observed and treated to prevent graft fibrosis or inflammation. Detection of patients with early stage liver fibrosis or inflammation is far more significant for recipients. In this regard, M2BPGi, which can detect early stage inflammation, may have some utility for transplant patients.
In conclusion, the present study revealed M2BPGi, a so-called fibrosis marker, as a disease activity marker in transplant recipients as demonstrated by its closer correlation to the necroinflammatory index than the fibrosis index. This suggests that M2BPGi is a more clinically useful biomarker because it is able to detect necroinflammatory changes that cannot be detected by routine blood tests early as a precursor to fibrosis.