Relationship Between Total Body Protein and Cross-Sectional Skeletal Muscle Area in Liver Cirrhosis Is Influenced by Overhydration
Abstract
Sarcopenia as defined by reduced skeletal muscle area (SMA) on cross-sectional abdominal imaging has been proposed as an objective measure of malnutrition, and it is associated with both wait-list mortality and posttransplant complications in patients with cirrhosis. SMA, however, has never been validated against the gold standard measurement of total body protein (TBP) by in vivo neutron activation analysis (IVNAA). Furthermore, overhydration is common in cirrhosis, and its effect on muscle area measurement remains unknown. We aimed to examine the relationship between SMA and TBP in patients with cirrhosis and to assess the impact of overhydration on this relationship. Patients with cirrhosis who had undergone IVNAA and cross-sectional imaging within 30 days were retrospectively identified. Patients with significant clinical events between measurements were excluded. Psoas muscle area (PMA) and SMA at the level of the third lumbar vertebrae were determined. Total body water was estimated from a multicompartment model and expressed as a fraction of fat-free mass (FFM), as determined by dual-energy X-ray absorptiometry, to provide an index of hydration status. In total, 107 patients underwent 109 cross-sectional imaging studies (87 computed tomography; 22 magnetic resonance imaging) within 30 days of IVNAA. Median time between measurements was 1 day (IQR, –1 to 3 days). Between 43% and 69% of the cohort was identified as sarcopenic, depending on muscle area cutoff values used. TBP was strongly correlated with SMA (r = 0.78; P < 0.001) and weakly correlated with PMA (r = 0.49; P < 0.001). Multiple linear regression showed SMA was significantly and positively associated with FFM hydration (P < 0.001) independently of TBP. In conclusion, SMA is more closely related to TBP than is PMA, and it should be preferred as a measure of sarcopenia. Overhydration significantly affects the measurement of cross-sectional muscle area.
Abbreviations
-
- ALD
-
- alcoholic liver disease
-
- ANCOVA
-
- analysis of covariance
-
- CT
-
- computed tomography
-
- DXA
-
- dual-energy X-ray absorptiometry
-
- FFM
-
- fat-free mass
-
- GI
-
- gastrointestinal
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HI
-
- hydration index
-
- HU
-
- Hounsfield units
-
- IQR
-
- interquartile range
-
- IVNAA
-
- in vivo neutron activation analysis
-
- L3
-
- third lumbar vertebrae
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MELD-Na
-
- Model for End-Stage Liver Disease–sodium
-
- MRI
-
- magnetic resonance imaging
-
- PACS
-
- Picture Archiving and Communication System
-
- PMA
-
- psoas muscle area
-
- PMI
-
- psoas muscle index
-
- SD
-
- standard deviation
-
- SMA
-
- skeletal muscle area
-
- SMI
-
- skeletal muscle index
-
- TBP
-
- total body protein
-
- TBW
-
- total body water
Sarcopenia is characterized by reduced skeletal muscle mass and/or strength, and it is a key marker of frailty associated with aging, malignancy, and chronic disease.1, 2 Sarcopenia and protein-energy malnutrition are common in patients with chronic liver disease,3 affecting up to 70% of patients on the waiting list for liver transplantation.2, 4, 5 Several studies have demonstrated strong associations between sarcopenia and wait-list mortality6, 7 as well as short-term and longterm outcomes following transplantation.8-10 Similar trends have also been described for patients with abdominal malignancies and those undergoing major surgery.11, 12 Given its strong association with clinical outcomes, some authors have proposed using sarcopenia as an objective prognostic indicator in the assessment of patients for transplantation. However, no consensus exists on the most appropriate definitions, cutoffs, or techniques for clinical use.2, 9, 13, 14
Clinician judgment alone is unreliable for the assessment of malnutrition. Therefore, objective measures of sarcopenia are necessary for accurate risk stratification and guidance for clinical decision making.15 Because of their clinical availability, computed tomography (CT) and magnetic resonance imaging (MRI) are the most commonly used modalities for the identification of reduced muscle mass.2 Cross-sectional skeletal muscle area (SMA) at the level of the third lumbar vertebrae (L3) has previously been correlated with total body skeletal muscle volume as measured by MRI in healthy volunteers,16 as well as with fat-free mass (FFM) and appendicular skeletal muscle mass measured by dual-energy X-ray absorptiometry (DXA) in cancer patients and patients with cirrhosis.4, 17 However, the relationship between SMA and total body protein (TBP) remains unknown.18 Gold standard measurements of TBP by in vivo neutron activation analysis (IVNAA) represent whole-body protein stores, including skeletal muscle and other solid organs, as well as plasma and structural proteins and, thus, can provide an assessment of malnutrition.3, 19
Overhydration and ascites are common in patients with chronic liver disease, often signifying advanced disease associated with considerable morbidity and mortality.20-22 Tissue edema and anasarca secondary to hepatic failure may expand muscle volumes and confound the assessment of sarcopenia in patients with cirrhosis.3, 23, 24 The impact of overhydration on measurement of cross-sectional muscle area has not previously been investigated.23
- To examine the relationship between cross-sectional measurements of skeletal muscle (SMA and psoas muscle area [PMA]) and IVNAA measurements of TBP in patients with cirrhosis.
- To assess the impact of overhydration on this relationship.
Patients and Methods
Adult patients with chronic liver disease enrolled in previous body composition studies in the Department of Surgery, University of Auckland, New Zealand, from 2004 to 2010 were retrospectively identified and assessed for inclusion.3, 25-28 Diagnosis of cirrhosis was based on clinical, laboratory, and radiological evidence or liver histology. The severity of cirrhosis was scored using Model for End-Stage Liver Disease (MELD) and Model for End-Stage Liver Disease–sodium (MELD-Na) scores.21, 29 All participants had previously undergone IVNAA measurements of TBP in a dedicated laboratory within the Department of Surgery. Clinical notes were obtained from the Clinical Records Department and MRI and CT imaging from the Picture Archiving and Communication System (PACS) at Auckland City Hospital. Institutional ethical approval was obtained prior to data retrieval and analysis (UAHPEC 015477).
For inclusion, participants were required to have had cross-sectional abdominal imaging (CT or MRI) within a 30-day interval of IVNAA. All radiological scans were obtained for routine clinical purposes, most commonly as part of an assessment for liver transplantation. Patients with acute hospital admissions, surgical procedures (including abdominal paracentesis), or other significant clinical events (major bleeding, encephalopathy, sepsis, or changes to diuretic dosing) during the interval between IVNAA and abdominal imaging were excluded. Patients with documented congestive heart failure, end-stage renal disease, or other causes of overhydration were excluded. Patients with cross-sectional imaging not including L3 were also excluded, as were data from IVNAA or radiological studies performed following liver transplantation. For patients with more than 1 cross-sectional imaging study within 30 days of IVNAA, the scan closest to IVNAA measurement was selected for evaluation.
Anthropometry
Body weight and height were prospectively assessed at the time of IVNAA. Measurements were obtained to the nearest 0.1 kg using a beam balance and adjusted for the estimated weight of clothing. Height was measured using a stadiometer and used to calculate body mass index (BMI).
Total Body Protein
Total body nitrogen was measured by prompt gamma IVNAA as previously described and validated (precision of 2.7% and accuracy of within 4%).30-33 TBP was calculated as 6.25 times total body nitrogen. Significant protein depletion was defined as TBP below the fifth percentile for measurements in 386 healthy volunteers (fifth percentile was 9.74 kg for men and 6.64 kg for women).34
Hydration Status
Total body water (TBW) was derived from IVNAA and DXA (Model DPX+, software version 3.6y, extended research analysis mode; GE-Lunar, Madison, WI) measurements as previously described in detail.35 In brief, TBW was calculated as the difference between body weight and the sum of TBP, total body fat by DXA, bone mineral content by DXA, nonbone minerals (estimated), and glycogen (estimated). Error propagation calculations suggest this technique has a precision of close to 1% and an accuracy better than 3% for the estimation of TBW.3, 35 FFM was calculated as body weight minus DXA total body fat. A hydration index (HI) was calculated for each patient, defined as the ratio of TBW to FFM. Significant overhydration was defined as HI ≥ 0.76, corresponding to 2 standard deviations (SDs) above the mean (0.73) of this variable in 229 healthy volunteers undergoing the same measurements.3
Cross-Sectional Image Analysis
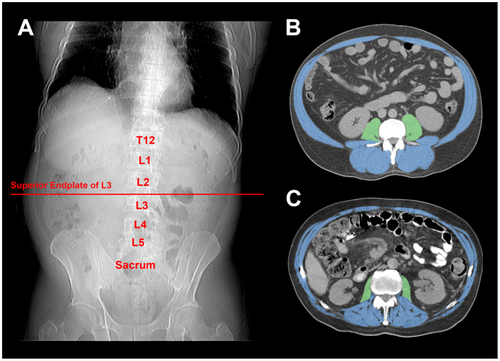
Cross-sectional slices at the level of the superior endplate of the L3 were manually identified in the digital MRI and CT images and analyzed using National Institutes of Health ImageJ, version 1.48.36 This anatomical plane has previously been identified as most appropriate for the estimation of total body muscle volume16 and has been conventionally used in studies investigating abdominal cross-sectional muscle area as a measure of sarcopenia.2, 11, 12 PMA and SMA were calculated and recorded in centimeters squared using previously described methods (Fig. 1)37, 38 by a study investigator (C.I.W.) who was blinded to TBP and TBW measurements. PMA was calculated by manually tracing around each of the psoas muscles, followed by thresholding to Hounsfield units (HU) specific to skeletal muscle (–29 to +150 HU). SMA was determined by manually deselecting the intraabdominal contents, abdominal wall skin, and subcutaneous tissues, followed by thresholding to skeletal muscle HU as above. The skeletal muscle index (SMI) and psoas muscle index (PMI) were calculated by dividing SMA and PMA, respectively, by height squared and expressed as cm2/m2. These values were used to determine sarcopenia according to several published cutoffs that have been derived in cirrhotic and noncirrhotic cohorts.7, 9, 39, 40
Statistical Analysis
Statistical analysis was performed using SAS, release 9.4 (SAS Institute, Cary, NC) and SPSS for Macintosh, version 23 (IBM Corp, Armonk, NY). P < 0.05 was chosen for statistical significance. Data are presented as mean ± SD or, for nonnormally distributed data, median (interquartile range [IQR]) unless stated otherwise. For between-group comparisons, Student t test or Mann-Whitney U test was used as appropriate. Bivariate associations were examined by using Pearson correlation coefficients, and Fisher’s exact test was used for categorical data. Sensitivity and specificity for previously published cross-sectional muscle area cutoffs were calculated for their association with significant TBP depletion. Analysis of covariance (ANCOVA) was used to adjust the means for TBP for comparison of SMA between normally hydrated and overhydrated groups. To further assess the impact of overhydration on SMA, a multiple linear regression model was constructed using TBP and HI as independent variables and SMA as the dependent variable. Bland-Altman plots41 were constructed for patients with both CT and MRI measurements of cross-sectional muscle area within a 30-day interval to assess agreement between these 2 modalities.
Results
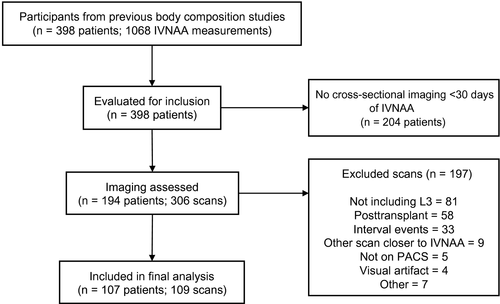
In total, 398 patients with cirrhosis (undergoing 1068 IVNAA measurements) were identified and assessed for inclusion (Fig. 2). After screening, 107 patients were included who underwent a total of 109 cross-sectional imaging studies (87 CT; 22 MRI) within 30 days of IVNAA measurement. Demographic and clinical data of the included participants are provided in Table 1.
| Patients (n = 107) | |
|---|---|
| Age, years | 54 (22-68) |
| Sex, male | 76 (71.0) |
| BMI, kg/m2 | 27.0 (19.0-43.9) |
| Ethnicity | |
| European | 76 (71.0) |
| Maori/Pacific | 21 (19.6) |
| Asian | 10 (9.3) |
| Child-Pugh grade | |
| A | 36 (33.6) |
| B | 28 (26.2) |
| C | 43 (40.2) |
| MELD score | 12 (5-28) |
| MELD-Na score | 14 (5-31) |
| Ascites | |
| None | 58 (54.2) |
| Diuretic-controlled | 32 (29.9) |
| Diuretic-resistant | 17 (15.9) |
| Etiology* | |
| HBV | 28 (26.2) |
| HCV | 49 (45.8) |
| ALD | 17 (15.9) |
| Other | 23 (21.5) |
| Concurrent HCC | 50 (46.7) |
| On diuretics | 53 (49.5) |
NOTE:
- Data are presented as median (range) or n (%).
- * Percentages do not add up to 100% due to patients with multiple etiologies.
The majority of included cross-sectional imaging studies were performed for liver transplant assessment or hepatocellular carcinoma (HCC) screening or surveillance (105 of 109; 96%). The remainder were performed for suspected biliary (n = 1) or urinary (n = 1) calculi, diagnosis or surveillance of other tumors (n = 1), or indications were not documented (n = 1). The median time between IVNAA measurement and cross-sectional imaging was 1 day (IQR, –1 to 3 days).
Mean SMA and PMA for all 109 images were 140.7 ± 31.2 cm2 and 13.4 ± 5.4 cm2, respectively. There were 27 (25%) patients who were protein depleted with TBP below the fifth percentile for healthy volunteers. Table 2 provides clinical and muscle area data comparing patients with and without protein depletion. SMA, SMI, and PMA were significantly reduced in protein-depleted patients, whereas PMI was not significantly different between patients with and without protein depletion. The percentage of patients identified as sarcopenic by cross-sectional muscle area ranged from 43.1% to 68.8%, depending on the cutoff threshold used (Table 3).
| All (n = 109) | Not Depleted (n = 82) | Depleted* (n = 27) | P Value | |
|---|---|---|---|---|
| Age, years | 54 (22-68) | 55 (25-68) | 54 (22-68) | 0.49 |
| Sex, male | 76 (69.7) | 55 (67.1) | 21 (77.8) | 0.29 |
| BMI, kg/m2 | 26.8 (19.0-44.0) | 26.8 (19.0-44.0) | 26.8 (20.4-39.4) | <0.001 |
| Ethnicity | 0.04 | |||
| European | 76 (69.7) | 56 (68.3) | 20 (74.1) | |
| Maori/Pacific | 23 (21.1) | 21 (25.6) | 2 (7.4) | |
| Asian | 10 (9.2) | 5 (6.1) | 5 (18.5) | |
| Child-Pugh grade | 0.11 | |||
| A | 38 (34.9) | 33 (40.2) | 5 (18.5) | |
| B | 28 (25.7) | 20 (24.4) | 8 (29.6) | |
| C | 43 (39.4) | 29 (35.4) | 14 (51.9) | |
| MELD score | 12 (5-28) | 10 (5-26) | 14 (6-28) | 0.01 |
| MELD-Na score | 14 (5-31) | 12 (5-29) | 16 (7-31) | <0.001 |
| Ascites | 0.001 | |||
| None | 60 (55.0) | 53 (64.6) | 7 (25.9) | |
| Diuretic-controlled | 32 (29.4) | 21 (25.6) | 11 (40.7) | |
| Diuretic-resistant | 17 (15.6) | 8 (9.8) | 9 (33.3) | |
| Etiology† | ||||
| HBV | 30 (27.5) | 23 (28.0) | 7 (25.9) | 0.83 |
| HCV | 49 (45.0) | 42 (51.2) | 7 (25.9) | 0.02 |
| ALD | 17 (15.6) | 11 (13.4) | 6 (22.2) | 0.27 |
| Other | 23 (21.1) | 14 (17.1) | 9 (33.3) | 0.07 |
| Concurrent HCC | 52 (47.7) | 44 (53.7) | 8 (29.6) | 0.03 |
| On diuretics | 55 (50.5) | 38 (46.3) | 16 (59.3) | 0.24 |
| SMA, cm2 | 140.7 ± 31.2 | 147.0 ± 30.7 | 121.8 ± 24.6 | <0.001 |
| SMI, cm2/m2 | 48.0 ± 9.1 | 49.8 ± 9.0 | 42.6 ± 7.2 | <0.001 |
| PMA, cm2 | 13.4 ± 5.4 | 13.9 ± 5.8 | 11.8 ± 3.7 | 0.03 |
| PMI, cm2/m2 | 4.6 ± 1.7 | 4.7 ± 1.9 | 4.2 ± 1.3 | 0.10 |
- NOTE: Data are presented as n (%), median (range), or mean ± SD.
- * Protein depletion defined as TBP less than the fifth percentile for the distribution in healthy volunteers.
- † Percentages do not add up to 100% due to patients with multiple etiologies.
| Reference* | Index† | Type | All (n = 109) | Not Depleted (n = 82) | Depleted‡ (n = 27) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Prado et al.39 (2008) | |||||||
| Lung/GI cancer, association with longterm mortality | SMI | Nonsarcopenic | 51 (46.8) | 47 (57.3) | 4 (14.8) | 85.2 | 57.3 |
| Sarcopenic | 58 (53.2) | 35 (42.7) | 23 (85.2) | ||||
| Martin et al.40 (2013) | |||||||
| Lung/GI cancer, association with longterm mortality | SMI | Nonsarcopenic | 62 (56.9) | 53 (64.6) | 9 (33.3) | 66.7 | 64.6 |
| Sarcopenic | 47 (43.1) | 29 (35.4) | 18 (66.7) | ||||
| Carey et al.7 (2017) | |||||||
| Cirrhosis, association with wait-list mortality | SMI | Nonsarcopenic | 61 (56.0) | 53 (64.6) | 8 (29.6) | 70.4 | 64.6 |
| Sarcopenic | 48 (44.0) | 29 (35.4) | 19 (70.4) | ||||
| Golse et al.9 (2017) | |||||||
| Cirrhosis, association with 1-year survival | PMA | Nonsarcopenic | 34 (31.2) | 30 (36.6) | 4 (14.8) | 85.2 | 36.6 |
| Sarcopenic | 75 (68.8) | 52 (63.4) | 23 (85.2) |
- NOTE: Data are given as n (%) unless otherwise noted.
- * For each reference, patient group and outcome used for determination of cutoff threshold for sarcopenia are provided.
- † SMI and PMA determined at 3rd lumbar vertebrae.
- ‡ Protein depletion defined as TBP less than the fifth percentile for the distribution in healthy volunteers.
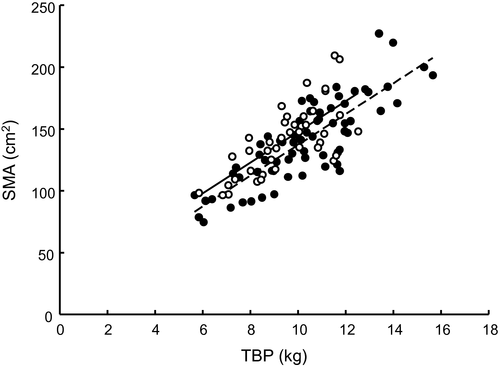
SMA was strongly positively correlated with TBP (r = 0.78; P < 0.001). A weaker positive correlation was observed between PMA and TBP (r = 0.49; P < 0.001). Serum albumin at the time of measurement was not correlated with SMA (r = 0.05), PMA (r = 0.17), or TBP (r = 0.06).
Overhydration as defined by HI was present in 37 of 106 (34.9%) patients with hydration data available (Table 4). Overhydration was seen in 15 of 27 (55.6%) patients with protein depletion and 22 of 79 (27.8%) nondepleted patients (P = 0.01). Univariate analysis revealed no significant difference in mean SMA or PMA between normally hydrated and overhydrated patients (Table 4). For the protein-depleted patients, SMA was significantly higher in overhydrated compared with normally hydrated patients (134.8 ± 5.1 versus 105.4 ± 5.9 cm2; P < 0.001), whereas PMA did not differ between these groups (12.3 ± 1.0 and 11.2 ± 1.0 cm2, respectively; P = 0.47). The relationships between SMA and TBP are shown in Fig. 3 for the normally hydrated and overhydrated patients. The correlation between SMA and TBP improved slightly when removing overhydrated patients from the model (r = 0.83 versus 0.78). Elevation of the regression lines for the normally hydrated and overhydrated groups differed significantly (ANCOVA, adjusted means 137.2 ± 2.3 and 150.0 ± 3.1 cm2, respectively; P = 0.001). Using HI as a continuous variable, multiple linear regression showed that this index was significantly and positively associated with SMA when the effect of TBP was controlled for (P < 0.001). We used the following regression equation for predicting SMA:
| All (n = 106) | Not overhydrated (n = 69) | Overhydrated* (n = 37) | P value | |
|---|---|---|---|---|
| Age, years | 54 (22-68) | 55 (25-68) | 54 (22-68) | 0.90 |
| Sex, male | 76 (71.7) | 45 (65.2) | 31 (83.8) | 0.04 |
| BMI, kg/m2 | 26.8 (19.0-44.0) | 27.6 (19.0-44.0) | 26.5 (20.4-39.4) | 0.78 |
| Ethnicity | 0.04 | |||
| European | 76 (71.7) | 44 (63.8) | 32 (86.5) | |
| Maori/Pacific | 20 (18.9) | 16 (23.2) | 4 (10.8) | |
| Asian | 10 (9.4) | 9 (13.0) | 1 (2.7) | |
| Child-Pugh grade | <0.001 | |||
| A | 35 (33.0) | 30 (43.5) | 5 (13.5) | |
| B | 28 (26.4) | 20 (29.0) | 8 (21.6) | |
| C | 43 (40.6) | 19 (27.5) | 24 (64.9) | |
| MELD score | 12 (5-28) | 11 (5-26) | 14 (6-28) | 0.004 |
| MELD-Na score | 14 (5-31) | 12 (5-29) | 16 (7-31) | 0.003 |
| Ascites | <0.001 | |||
| None | 57 (53.8) | 47 (68.1) | 10 (27.0) | |
| Diuretic-controlled | 32 (30.2) | 20 (29.0) | 12 (32.4) | |
| Diuretic-resistant | 17 (16.0) | 2 (2.9) | 15 (40.5) | |
| Etiology† | ||||
| HBV | 27 (25.5) | 22 (31.9) | 5 (13.5) | 0.04 |
| HCV | 49 (46.2) | 27 (39.1) | 22 (59.5) | 0.045 |
| ALD | 17 (16.0) | 8 (11.6) | 9 (24.3) | 0.09 |
| Other | 23 (21.7) | 17 (24.6) | 6 (16.2) | 0.32 |
| Concurrent HCC | 49 (46.2) | 34 (49.3) | 15 (40.5) | 0.39 |
| On diuretics | 53 (50.0) | 27 (39.1) | 26 (70.3) | 0.002 |
| Protein depletion | 27 (25.5) | 12 (17.4) | 15 (40.5) | 0.01 |
| SMA, cm2 | 141.7 ± 31.1 | 140.2 ± 33.1 | 144.3 ± 27.3 | 0.52 |
| SMI, cm2/m2 | 48.3 ± 9.1 | 48.7 ± 9.2 | 47.5 ± 9.1 | 0.54 |
| PMA, cm2 | 13.5 ± 5.5 | 13.6 ± 5.9 | 13.3 ± 4.6 | 0.79 |
| PMI, cm2/m2 | 4.6 ± 1.8 | 4.7 ± 1.8 | 4.4 ± 1.6 | 0.38 |
- NOTE: Data are presented as n (%), median (range), or mean ± SD.
- * Overhydration was defined as HI ≥ 0.76, ie, 2 SDs above the mean for this variable in healthy volunteers.
- † Percentages do not add up to 100% because of patients with multiple etiologies.
SMA (cm2) = 12.7 (± 0.9) × TBP (kg) + 245.1 (± 68.7) × HI – 170.5 (± 54.7)
(standard error of estimate = 18.6 cm2, r2 = 0.65, n = 106).
Comparison of SMA in patients with moderate-to-large ascites to those with no or mild ascites, adjusting for TBP, showed no significant difference (adjusted means, 143.5 ± 4.4 and 141.2 ± 2.1 cm2, respectively; P = 0.65). In contrast, comparison of SMA in patients with no ascites but significant overhydration to all other patients yielded a significant difference in the adjusted means (153.2 ± 4.2 versus 139.0 ± 2.0 cm2, respectively; P = 0.003).
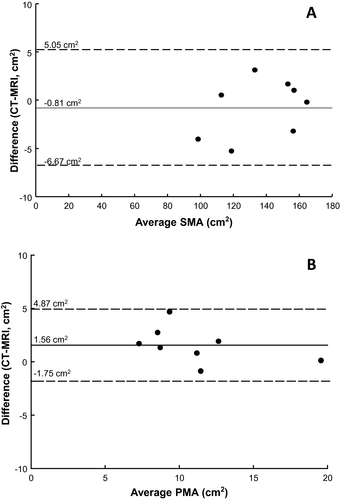
A total of 8 patients had both CT and MRI measurements of SMA and PMA available within 30 days of each other. No clinically significant events occurred between imaging studies, which occurred a median of 1 day apart (IQR, –8 to 7 days). Measurements of SMA and PMA using the 2 modalities were strongly correlated (r = 0.99 and r = 0.93, respectively). Limits of agreement analysis demonstrated a mean SMA difference of 0.8 cm2 (95% CI, –6.7 to 5.1 cm2) and a mean PMA difference of 1.6 cm2 (95% CI, –1.8 to 4.9 cm2; Fig. 4).
Discussion
Hepatic cirrhosis is associated with a chronic inflammatory state, leading to muscle catabolism, protein-energy malnutrition, and sarcopenia.3, 42 This is the first published study correlating cross-sectional SMA with the gold standard measurement of TBP by IVNAA and the first to compare the use of PMA and total SMA from cross-sectional imaging. In this cohort of patients with cirrhosis, TBP as measured by IVNAA was more strongly correlated with SMA at the L3 level than PMA. Furthermore, independently of TBP, increased hydration was associated with significantly increased SMA.
Several studies have investigated the relationship between cross-sectional SMA and other measures of body composition with varying results. A seminal article by Shen et al.16 investigated 328 healthy volunteers undergoing whole-body MRI. SMA measured at a level 5 cm above the L4/5 intervertebral disc was most strongly correlated with total skeletal muscle volume as measured by MRI (r = 0.93). This study provided the foundation for future work in various patient groups that have assessed cross-sectional muscle area at the L3 level and its prognostic utility. Mourtzakis et al.17 demonstrated a strong relationship between SMA measured by CT and FFM measured by DXA in 31 patients with advanced lung or colorectal cancer (r = 0.94). Most recently, Giusto et al.4 studied patients with cirrhosis being evaluated for liver transplantation and showed SMI measured by CT at L3 was weakly correlated with DXA-measured FFM indexed for height (r = 0.44 for 46 males; r = 0.54 for 13 females). DXA measurements of FFM are confounded by fluid retention commonly seen in cirrhosis, and this may explain, at least partially, the divergent findings of Mourtzakis et al.17 and Giusto et al.4 Furthermore, patterns of muscle loss may not be identical in cancer and cirrhosis, affecting the ability of lumbar SMA to predict total body muscle mass.
The present study assessed TBP, independent of hydration status, and showed a moderately strong association between SMA and TBP (r = 0.78) and a weaker association between PMA and TBP (r = 0.49). SMA measurements are obtained from multiple muscle groups and, therefore, are likely more reflective of overall skeletal muscle mass than PMA alone. IVNAA measurements of TBP are also affected by nonskeletal muscle protein and, therefore, may be confounded by organomegaly or large tumor volumes. However, protein is preferentially lost from muscle in malnutrition, and therefore, TBP is likely to be sensitive to changes in skeletal muscle mass.43
Overhydration may confound the assessment of sarcopenia by cross-sectional imaging in patients with cirrhosis. The present study has identified that hydration status is positively associated with cross-sectional SMA. Thus, overhydration may expand muscle volumes and affect the assessment of sarcopenia in patients with cirrhosis. This effect of overhydration is supported by the significantly raised SMA that we observed in overhydrated patients without ascites when compared with all other patients, when TBP is controlled for. Increased fluid content in biopsied human muscle tissue has been shown in critically ill surgical patients,44 multitrauma and burn injury patients,45 and malnourished cancer patients.46 Hydration status and hyponatremia are also independent predictors of mortality in cirrhosis, and therefore, these variables should be considered alongside sarcopenia in future prognostic studies.21, 22
Although only 8 patients in this cohort had both CT and MRI scans available for comparative analysis, we could not identify any significant differences in measurement of cross-sectional muscle area values between the 2 modalities, in keeping with an analysis of scans from 61 patients recently published by Tandon et al.47 The strong agreement between these 2 modalities supports the use of either CT or MRI imaging in future studies investigating cross-sectional muscle area and sarcopenia.
Between 43% and 69% of patients in this cohort were deemed sarcopenic based on previously published cross-sectional muscle area cutoff values derived from cohorts of patients with cancer or cirrhosis.7, 9, 39, 40 These definitions have been derived from associations with mortality and may not accurately reflect changes in body composition. The relatively small cohort of patients in the present study precluded the calculation of cutoff values for prediction of protein depletion as measured by IVNAA. On the basis of the Carey et al. cutoffs in patients with cirrhosis,7 60% of patients in the present study who met the criteria for sarcopenia (SMI < 50 cm2/m2 for men and < 39 cm2/m2 for women) did not have significant protein depletion measured by IVNAA (defined as TBP values below the fifth percentile for healthy volunteers). However, reduced muscle mass, which does not meet the criterion for significant protein depletion, may nevertheless have an impact on clinical outcomes and mortality, potentially explaining this observation.
There is considerable inconsistency in the techniques, cutoffs, and muscle groups used to assess sarcopenia, which has limited the ability to synthesize evidence in several recent systematic reviews.2, 11, 12 A consensus definition of sarcopenia based on cross-sectional imaging should be developed with consistent cutoffs and techniques agreed on for use in future studies.
There are several limitations of the present analysis, most importantly its retrospective design. This prevented scheduling of IVNAA and cross-sectional imaging on the same day, resulting in the potential for changes in body composition between the 2 measurements. However, this was at least partially overcome by only including patients with measurements within 30 days, and the exclusion of those with documented significant clinical events occurring between the 2 measurements. In this study, only 1 cross-sectional slice was analyzed and correlated with TBP measurements. Sarcopenia is not a uniform condition. Therefore, multislice or volumetric analysis may provide more accurate estimates of body composition. This remains an area for further investigation.4, 48 We did not account for the effects of splenomegaly or tumor burden, and these remain as potential confounders of TBP measurements. It should be noted that IVNAA is a specialized body composition research tool and is not routinely available for clinical use in the evaluation of patients with end-stage liver disease. Our study was essentially a proof-of-concept that aimed to explore associations between SMA and TBP and overhydration. We have not investigated the clinical relevance of our finding of a relationship between SMA and hydration status; a much larger study would be required to adequately address this issue. Finally, although functional tests of skeletal muscle strength are likely to be an important part of global frailty assessment, these were beyond the scope of the present study.15
In summary, TBP measured by IVNAA was strongly correlated with cross-sectional SMA at the level of the L3 in patients with cirrhosis. Psoas area was weakly correlated with TBP and may be of limited use in the assessment of body composition. Overhydration had a significant effect on the measurement of cross-sectional muscle area from CT or MRI imaging, and this should be accounted for in future studies of sarcopenia and its impact. Future efforts should aim to establish a consensus definition of sarcopenia, based on imaging modalities, for use in studies assessing its utility in patients with cirrhosis and other chronic diseases.
Potential conflict of interest
Nothing to report.