Reconstruction of Anomalous Portal Venous Branching in Right Lobe Living Donor Liver Transplantation: Malatya Approach
Potential conflict of interest: Nothing to report.
Sezai Yilmaz: Planning and conducting the study, interpreting data, and drafting the manuscript. Cuneyt Kayaalp: Conducting the study and drafting the manuscript. Burak Isik: Conducting the study and interpreting data. Veysel Ersan: Planning and conducting the study and drafting the manuscript. Emrah Otan and Sami Akbulut: Drafting the manuscript. Ramazan Kutlu, Aysegul Sagir Kahraman: Collecting and interpreting data. Abuzer Dirican, Cengiz Ara, Mehmet Yilmaz, Bulent Unal, Cemalettin Aydin, Turgut Piskin, Dincer Ozgor, Mustafa Ates, Fatih Ozdemir, Volkan Ince, Cemalettin Koc, Adil Baskiran, Sait Murat Dogan, Bora Barut, Fatih Sumer, Serdar Karakas, and Koray Kutluturk: Collecting data. Saim Yologlu and Harika Gozukara: Statistical analysis.
Abstract
Reconstruction of anomalous portal vein branching (APVB) during right lobe living donor liver transplantation (LDLT) can be challenging. The goal of this article is to describe our surgical technique, named the Malatya Approach, in case of APVB during right lobe LDLT. The technique unifies the APVB and obtains a funnel-shaped common extension with a circumferential fence by a saphenous vein conduit. In total, 126 (10.6%) of 1192 right lobe grafts had APVB that were divided into 2 groups according to the adopted surgical techniques: the Malatya Approach group (n = 91) and the previously defined other techniques group (n = 35). Both groups were compared regarding portal vein thrombosis (PVT), postoperative 90-day mortality and survival. PVT developed in 3 patients (3.3%) in the Malatya Approach group and developed in 10 (28.6%) patients for the other group (P < 0.001). There were 8 (8.8%) 90-day mortalities in the Malatya Approach group (1 PVT related) and 15 patients (9 PVT related) died in the other techniques group (P < 0.001). Mean follow-up time for both groups was similar (999.1 days for the Malatya Approach group versus 1024.7 days for the other group; P = 0.47), but longterm survival in the Malatya Approach group was better than in the other group (84.6% versus 40%; P < 0.001). Multivariate analysis revealed that the Malatya Approach group showed less PVT development and longer survival (P < 0.001). This technique is promising to avoid PVT and mortalities in cases of APVB during right lobe LDLT. Liver Transplantation 23 751–761 2017 AASLD.
Abbreviations
-
- APVB
-
- anomalous portal vein branching
-
- CI
-
- confidence interval
-
- CT
-
- computed tomography
-
- CVIG
-
- cryopreserved iliac vein graft
-
- GRWR
-
- graft-to-recipient weight ratio
-
- HAT
-
- hepatic artery thrombosis
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- LDLT
-
- living donor liver transplantation
-
- LPV
-
- left portal vein
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MPV
-
- main portal vein
-
- PVT
-
- portal vein thrombosis
-
- RAPV
-
- right anterior portal vein
-
- RPV
-
- right portal vein
-
- RPPV
-
- right posterior portal vein
-
- SD
-
- standard deviation
-
- VCI
-
- vena cava inferior
Living donor liver transplantation (LDLT) is an effective treatment for end-stage liver disease, especially in areas where deceased grafts are in short supply. Although the left-sided grafts are commonly used for children and for small-sized adults, the right lobe graft is the main source of LDLTs for most adults. However, anomalous portal vein branching (APVB) that results in 2 portal venous openings in a right lobe graft is one of the common anatomic variations with a reported incidence of 6%-22%.1-4 Reconstruction of these vessels during transplantation can be challenging, and even donors with such APVB have often been disqualified as right lobe living donors.2, 5 Several reconstruction methods have been attempted to enable the use of right lobe liver grafts from donors with APVBs.6-10 All these surgical techniques have several pitfalls. Here, we described our experience with portal vein reconstruction in 126 patients with APVB in right lobe LDLT. Moreover, this study was intended to describe in detail a surgical technique, named the Malatya Approach, and its clinical outcomes.
Patients and Methods
- Normal: The donor right portal vein (RPV) branch with 1 common opening in 1066 patients of type 1 were anastomosed to the recipient's main portal trunk.
- Unification: Two portal vein orifices were joined as a common orifice on the back table. The joined common orifice was anastomosed to the recipient main portal vein (MPV; Fig. 2A).
- Y-graft interposition: With a cryopreserved iliac vein graft (CVIG), both donor portal veins were anastomosed to the recipient's MPV (Fig. 2B).
- Double anastomosis: Two portal veins of the graft were anastomosed to the RPV and left portal vein (LPV) branches of the recipient (Fig. 2C).
- Complicated venoplasties: There were some unclassified, but previously published venoplasty techniques (Fig. 2D).6, 7, 12, 13
- Malatya Approach: This technique, with its various modifications, is described in detail below.
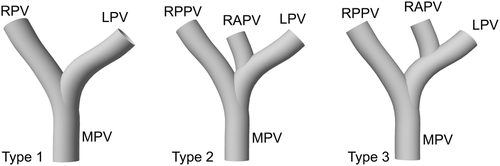
Varotti-Emre classification of portal vein anatomy.
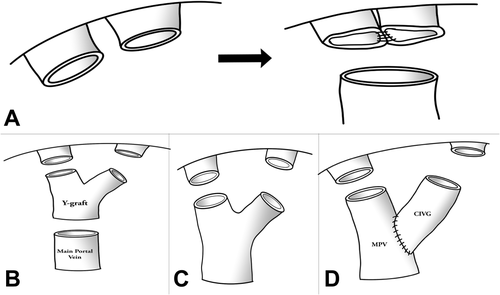
(A) Joined common orifice was anastomosed to the MPV. (B) Y-graft interposition technique in type 2 APBV. (C) Double anastomoses in type 3 APBV. (D) Complicated venoplasty in type 3 APBV.
| Type 1 | Type 2 | Type 3 | Total | |
|---|---|---|---|---|
| Normal | 1066 | — | — | 1066 |
| Unification | — | 9 | 1 | 10 |
| Y plasty | — | 5 | 9 | 14 |
| Double anastomosis | — | — | 4 | 4 |
| Complicated venoplasties | — | — | 7 | 7 |
| The Malatya Approach | — | 53 | 38 | 91 |
| Total | 1066 | 67 | 59 | 1192 |
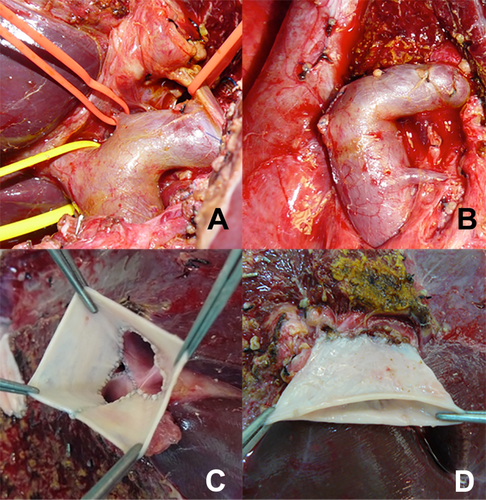
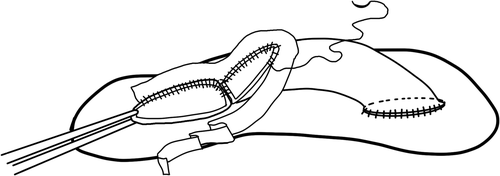
- a. Classical Malatya Approach: The right hepatic lobectomy in living donors with APVB was performed, as described.11 After the parenchymal transection, including right hepatic duct and right hepatic artery, the RPV branches were divided separately with 2 isolated openings 2-3 mm apart from the confluence. This left the MPV of the donor and the confluence intact. The graft was flushed with a lactated Ringer and then histidine tryptophan ketoglutarate solution through each of the portal vein branches on the back table. Patients with type 2 APVB may display snout-shaped RPV or a narrow bridge of tissue connecting the 2 RPV branches. However, in order to prevent narrowing in the remnant portal vein of the donor, the right posterior portal vein (RPPV) and right anterior portal vein (RAPV) branches of the portal vein should be divided separately and possibly far from the trunk of the common LPV of the donor. The semiseparate divided branches of the RPV must be rendered completely separate at the bench. Otherwise, unifying the orifices of the portal vein will not be ergonomic. We successfully applied this surgical technique to the cases where the distance between the 2 portal veins of the right lobe graft were 3 cm (Fig. 3A). Two portal venous openings are held together like an equilateral triangle, and the closest edges are joined with 7/0 Prolene sutures (Fig. 3B,C). The objective here is to apply a joining suture without narrowing or stretching both lumens. If the unifying includes half of the orifices of the 2 portal veins, that is half of the circles, it can be seen that both of the lumens narrow. Then an autologous saphenous vein is cut longitudinally and sutured around the joined vessel like a circumferential fence (Fig. 3D). After completion of the circumferential fence with continuous 7/0 Prolene sutures, the saphenous vein is cut with a slope 2 mm apart from the point where continuous Prolene sutures approximated. The objective is to obtain a width of saphenous vein conduit that is as long as the unified branches of the portal vein branches at the side of the liver graft but a wider length at the side where the anastomosis with recipient portal vein will be performed. This may also be called a funnel-shaped extension. The cut saphenous vein ends were sutured in a 2-layer fashion, and a circumferential fence procedure was completed. Finally, unified portal vein orifices were extended at least 2 cm with an autologous vein and a single lumen, resulting in a wider orifice than the original one (Fig. 3E). The anastomosis between the formed conduit and recipient MPV trunk can then be performed easily. The illustrations of the procedure are shown in Fig. 4A-F. In the anastomosis between the conduit and the MPV, the most important point while performing the corner sutures in portal veins of the graft and recipient is to obtain the orientation which will supply equal and well-balanced flow of the portal vein of the recipient to 2 portal vein orifices of the graft. Thus, there will not be any expansions or narrowing in favor, or against, in the orifices of the portal vein. Otherwise, ischemia will develop in almost half of the grafts after revascularization of the right lobe graft portal vein. We had 3 portal venous branches in 5 right lobe grafts. Even if there are 3 portal venous branches in the right lobe, it is possible to obtain a good conduit to perform easy portal vein anastomosis with this technique. Two of the branches were dominant, but all 3 portal vein orifices were unified and a single lumen was obtained after performing a circumferential fence with a saphenous vein (Fig. 5A-D).
- b. Malatya Approach with quilt venoplasty: In some cases, the approximation of 2 portal venous openings was not possible. In these cases, we had to place a quilt venoplasty between portal vein orifices. We had 5 grafts like this, having 3 portal venous branches. After that, a circumferential fence with the saphenous vein was performed (Fig. 6A-C).
- c. Malatya Approach with vessel extension: Portal vein of the anterior sector was extended with CVIG and joined with the portal vein of the posterior sector in 2 patients because the distance between the portal vein orifices was too long. Later a single lumen was obtained after performing a circumferential fence with the saphenous vein (Fig. 7).
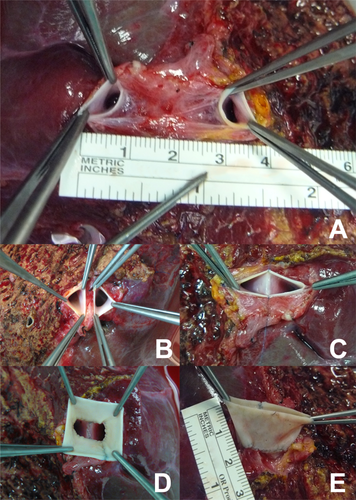
(A) Separate portal vein branches in right lobe graft. (B) Portal vein branches are held like equilateral triangles. (C) The closest edge of the triangles are joined. (D) Autologous saphenous vein is sutured around the joined vessel like a circumferential fence. (E) An extended conduit with single lumen for easy anastomosis is obtained.
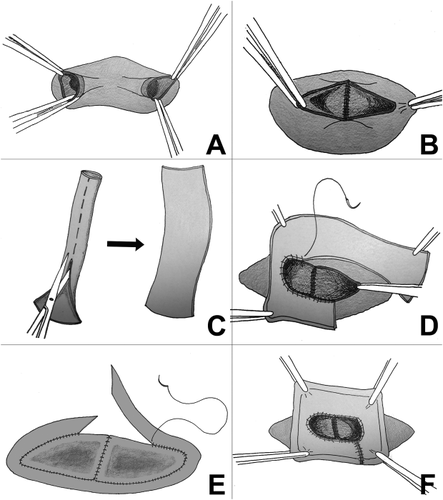
Illustrations of the Malatya Approach.
(A,B) Three portal veins in the right lobe graft and (C,D) their reconstruction with the Malatya Approach.
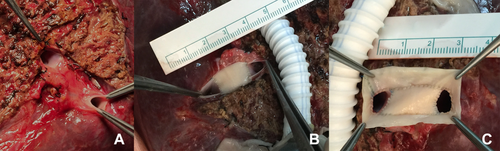
Malatya Approach with quilt venoplasty.
Malatya Approach with vessel extension.
After the completion of graft vascular reconstruction, hepatic inflow and outflow were routinely examined with intraoperative color Doppler ultrasonography. No specific anticoagulant therapy was administered. Posttransplant follow-up of portal vein patency was checked with color Doppler ultrasonography daily for the first week and a dynamic liver CT scan between postoperative days 7 and 10. A dynamic CT scan was delayed until the renal functions improved in patients with renal dysfunction. In patients with persistent renal dysfunction, we used color Doppler ultrasonography instead of a CT scan.
All patients received the same immunosuppresive protocol, consisting of prednisolone, mycophenolate mofetil, and tacrolimus. In patients with deteriorating renal function or hepatocellular carcinoma (HCC), everolimus was added.
Patients with APVB were divided into 2 groups according to the adopted surgical techniques in this study: group 1, Malatya Approach (91 patients) and group 2, other procedures for APVB (35 patients). Both groups were compared via portal vein thrombosis (PVT), relaparotomy, and survival. The groups were also compared in terms of hepatic artery thrombosis (HAT) and bile peritonitis. The study was carried out with the written approval of the ethics committee of Inonu University.
STATISTICAL ANALYSIS
SPSS version 21.0 (SPSS, Inc, Chicago, IL) was used for the statistical analysis. Data are presented as mean ± standard deviation (SD) for quantitative variables and as numerical value and percentage for qualitative variables. The first 3 months and overall survival rates were calculated using the Kaplan-Meier estimation method and examined using the log-rank test. Multivariate Cox regression analysis was used to determine the covariate-adjusted effect of operation type on survival. For determination of the covariate-adjusted effect of operation type on PVT development, multivariate logistic regression analysis was used. The effect of operation type on PVT, HAT, relaparotomies, and bile peritonitis were examined by Pearson chi-square, Fisher's exact test. P values < 0.05 were considered statistically significant.
Results
Clinicopathological profiles of the donors and the recipients are summarized in Table 2. There was no difference between the 2 groups regarding the recipient and the donor features. Graft-to-recipient weight ratios (GRWRs) of the grafts were also comparable. Although type 3 APVB was less in the Malatya Approach group (41.8% versus 60.0%), it was not statistically significant (P = 0.08).
| Total | Malatya Approach | Others | P Values | |
|---|---|---|---|---|
| Recipients | 126 | 91 | 35 | |
| Sex, male/female, n | 96/30 | 68/23 | 28/7 | |
| Age, years, mean ± SD (range) | 46.6 ± 1.3 (3-68) | 47.2 ± 1.6 (3-68) | 44.9 ± 2.3 (21-66) | 0.77 |
| Primary diagnosis, n | ||||
| HBV | 57 | 39 | 18 | |
| Cryptogenic | 28 | 23 | 5 | |
| Fulminant | 10 | 7 | 3 | |
| HCV | 8 | 6 | 2 | |
| Budd-Chiari | 6 | 4 | 2 | |
| Wilson's disease | 4 | 3 | 1 | |
| HCC | 21 (18 concurrent patients) | 15 (13 concurrent patients) | 6 (5 concurrent patients) | |
| Others | 10 | 7 | 3 | |
| MELD | 17.7 ± 0.7 | 17.3 ± 0.9 | 18.9 ± 1.4 | 0.33 |
| Donors | ||||
| Sex, male/female, n | 72/54 | 54/37 | 18/17 | |
| Age, years, mean ± SD (range) | 30.1 ± 0.8 (18-55) | 29.6 ± 0.9 (18-55) | 31.3 ± 1.6 (18-54) | 0.31 |
| Grafts | ||||
| GRWR, %, mean ± SD (range | 1.15 ± 0.25 (0.60-1.82) | 1.10 ± 0.30 (0.70-1.82) | 1.08 ± 0.19 (0.60-1.48) | 0.78 |
| GRWR ≥ 0.8%, n | 117 | 84 | 33 | 0.7 |
| GRWR < 0.8%, n | 9 | 7 | 2 | |
| Portal vein, n (%) | ||||
| Type 1 | 1066 (89.5) | 0 | 0 | |
| Type 2 | 67 (5.6) | 53 (58.2) | 14 (40) | 0.08 |
| Type 3 | 59 (4.9) | 38 (41.8) | 21 (60) |
We only measured the velocity values of the MPV and its branches after the transplantation in the Malatya Approach group and the other group in 12 and 7 patients, respectively. Portal vein velocity measurements of the patients in the Malatya Approach group were all higher than the other group (main trunk before the anastomosis, 59 ± 5 cm/second versus 47 ± 3 cm/second, P < 0.001; lateral branch, 66 ± 5 cm/second versus 55 ± 4 cm/second, P < 0.001; medial branch, 60 ± 4 cm/second versus 47 ± 4 cm/second, P < 0.001).
Mean follow-up time of both groups were similar (999.1 days for the Malatya Approach group versus 1024.7 days for the other group, P = 0.47). There was a total of 13 PVTs (10.3%): 3 in the Malatya Approach group (3.3%) and 10 in the other group (28.5%) throughout the entire follow-up (P < 0.001). PVT was the most important determinant for the 90-day hospital mortality. In the postoperative 90-day period, PVT was diagnosed in only 1 patient in the Malatya Approach group (coexisting with HAT) and in 9 patients in the other group. In these patients, color Doppler ultrasonography did not reveal flow decrease in portal vein branches before PVT development in the early postoperative period. However, the following day color Doppler ultrasonography revealed PVT. Although soft thrombus tissue was extracted with thromboendovenectomy during relaparotomy, prognosis of all these patients resulted in mortality due to graft failure. Among the 9 patients of the other group, 5 patients were type 3 and 4 patients were type 2 APVB. PVT was diagnosed in 2 of the unification patients, 3 of the Y-plasty patients, 2 of the double anastomosis patients, and 2 of the complicated venoplasty patients. Eight patients in the Malatya Approach group and 15 patients in the other group died in the first 3 months (P < 0.001). In terms of overall mortality, 35 (27.8%) patients died in the long term. Of the patients who died, 14 (15.4%) were in the Malatya Approach group and 21 (60%) were in the other (P < 0.001). Mean survival time was longer in the Malatya Approach group (1997 ± 89 days versus 1361 ± 272; P < 0.001; Table 3; Fig. 8).
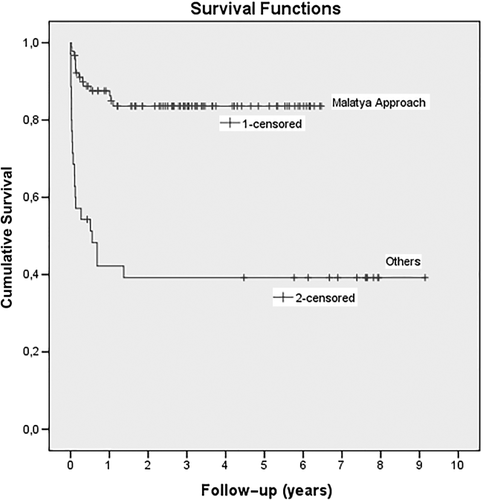
Survival rates according to the groups. (Censored observations represent still alive patients when they are lost to follow-up or when the study ends).
| Malatya Approach (n) | Others (n) | P Values | |
|---|---|---|---|
| Patients | 91 | 35 | |
| Alive (overall) | 77 | 14 | |
| Dead (overall) | 14 | 21 | |
| Overall survival, mean ± SD | 1997 ± 89 | 1361 ± 272 | <0.001 |
| 90-day mortality | 8 | 15 | <0.001 |
| PVT | 1a | 9 | |
| Sepsis | 5 | 5 | |
| Neurological complications | 2 | 1 | |
| 1-year mortality | 14 | 21 | |
| PVT | 1a | 9 | |
| Sepsis | 7 | 7 | |
| Neurological complications | 3 | 1 | |
| Biliary complications | 2 | 3 | |
| Myocardial infarction | — | 1 | |
| Hepatic artery aneurysm rupture | 1 | — | |
| HAT | 1 | 2 | |
| PVT | 3 | 10 | <0.001 |
| Bile peritonitis | 4 | 3 | |
| Relaparotomies | 12 | 16 | <0.001 |
| Intraabdominal hemorrhage | 5 | 5 | |
| VCI stenosis | 1 | 0 | |
| Bile peritonitis | 4 | 3 | |
| HAT revascularization | 1 | 1 | |
| PVT | 1 | 7 |
- a The patient also had HAT.
The relaparotomies were less in the Malatya Approach group (P < 0.001). There were no significant differences between the 2 groups regarding causes of the relaparotomy, except for PVT (Table 3). Right hepatic artery anomalies were encountered in only 2 right lobe grafts with portal vein anomaly. In these grafts, right hepatic artery bifurcated early and the placement of 1 of the sectorial arteries was in front of the common hepatic duct and the other was behind. A double artery anastomosis was performed in 1 of these patients. The ductus was transected from the distal portion in the other case because it was wider than 1 cm and the diameters of the sectorial arteries were nearly 1 mm. This provided a single anastomosis to the right hepatic artery trunk, which was nearly 2 mm wide. Then a duct-to-duct anastomosis with a T-tube was performed in the living donor. The postoperative course was uneventful both in the donor and the recipient. In these series, there was 1 bile duct in 45 donors, 2 bile ducts in 71 donors, 3 bile ducts in 9 donors, and 4 bile ducts in 1 donor.
To eliminate the effects of the covariates on survival when comparing with operation types, we used the multivariate Cox regression method. Operation type was considered as an independent variable, and the covariates included in multivariate analysis were relaparatomy, HAT, PVT, recipient age, donor age, GRWR, and bile peritonitis. When the Malatya Approach was considered as a reference category, the covariate-adjusted hazard ratio of operation type was found to be 4.53 (95% confidence interval [CI], 2.01-10.20; P < 0.001). For comparing PVT development between the operation types to exclude the covariate effects, multivariate logistic regression analysis was used. The covariate-adjusted odds ratio of operation type was found to be 7.58 (95% CI, 1.72-33.44; P < 0.001) when the Malatya Approach was considered as reference category and the outcome variable was PVT development. Hosmer-Lemeshow goodness-of-fit test for multivariate logistic regression was chi-square = 2.336 (P = 0.89).
A narrowing after closing of the orifices of the portal vein branches was seen in 2 living donors. The narrowing was resolved with patch plasty in both donors. No living donor with APVB showed any abnormal portal blood flow in the remnant left lobe on routine follow-up imaging studies performed before being discharged. No major donor complications requiring reoperation or endoscopic or radiologic intervention occurred in any of the 126 living donors with APVB.
Discussion
Even though the procedures performed to reconstruct APVBs in right lobe grafts have changed over time at our institute, the Malatya Approach has been accepted as the standard procedure since 2010 in nearly all patients. The cases performed before 2010, when the Malatya Approach was accepted as a standard procedure for APVB, constitute half of all patients in this study. We also performed other procedures besides the Malatya Approach after 2010 in a couple of patients but obtained better results with the Malatya Approach. For example, redundancy or shortness cannot be predicted before perfusion in Y-plasty performed with autologous or cryopreserved vascular allografts. Limited and short vascular graft utilization can solve this problem. The Malatya Approach requires a funnel-shaped circumferential fence instead of tubular vascular allograft which is prone to kinking, shrinkage, etc. However, it is possible to speak of a positive era effect on the good results obtained with the Malatya Approach.
Unification venoplasty, which is the first step of our technique, resulting in a single orifice can be performed even in 2 portal vein branches located far apart, approximately 3 cm. However, performing an anastomosis after unification is difficult because the opposite edges of the portal vein openings after back-wall plasty retract toward the parenchyma. Suturing an autologous saphenous vein around the joined vessel like a circumferential fence will enable a portal vein anastomosis to be performed easily by extending and widening the vein. Furthermore, suturing of the saphenous vein to joined portal venous branches, even if the wall of the portal vein is thin due to extensive dissection, is problem-free on the bench by taking safe bites. In the Malatya Approach after the unification of 2 portal branches, the circumferential fence with the saphenous vein is not a tubular interposition but a funnel-shaped interposition. This structure is larger than any luminal vascular allograft. The saphenous vein is not a cryopreserved allograft and thus more sound than a Y-allograft. The autologous portal vein is like a sieve; therefore, after perfusion, bleeding is frequent.
We compared PVT and the mortality rates of 2 groups in the first postoperative 3 months. In both groups, early PVT development is thought to be due to the reconstruction techniques. In the late period, independent of the reconstruction techniques in both groups, the development of PVT could be attributed to various possible causes. It is known that in the right lobe LDLT with APVB grafts, there is a high rate of biliary anomalies. Severe biliary strictures and biliary sepsis lead to stiffness in the liver grafts along with cholestasis. The portal vein and hepatic artery flow in these graft livers has always been less compared with a normal liver. Presumably, such a liver and decreased portal vein flow may invite PVT after liver transplantation. In this early period, the mortality rates for the Malatya Approach group and the other group were 8.8% and 42.9%, respectively. PVT occurred only in 1 (1.1%) patient for Malatya Approach, which was diagnosed intraoperatively and corrected with reorientation of the anastomosis. There were 9 (25.7%) PVT patients in group 2; all of which were lost. Overall, the mortality rates were favorable for the Malatya Approach patients. Additionally, the overall PVT rate was also lower among patients who underwent the Malatya Approach. In the other group, there were 10 PVT patients.
There was no difference between the groups regarding portal vein anatomy. However, the Malatya Approach group had less type 3 APVB compared with the second group (41.8% versus 60%, respectively). Although more difficult and separated branched portal veins seem to appear in the other group, we used the Malatya Approach in type 2 and 3 for 91 patients, resulting in successful prognosis. There was no significant difference regarding mortality and PVT development between type 2 and 3 APVB with the Malatya Approach.
The 2 groups were also compared in terms of relaparotomy rates and indications. Relaparotomy rates were higher in the second group (13.2% to 45.7%). Intra-abdominal bleeding and PVT were more frequent indications for relaparotomy in the second group.
In LDLT, donor safety is of the utmost importance. Discoid patch excision of the donor portal vein was done with 2 portal venous branches in the harvesting operation to create a common orifice in the right lobe grafts.6, 14 The defects in the remnant lobe portal vein were repaired by segmental resection and end-to-end anastomosis or autologous vein patchplasty. However, these patients showed some evidence of PVT or stenosis requiring re-exploration or insertion of the stent in the donor.6 We should avoid procedures that could risk donor portal vein blood flow. Sometimes efforts to create a common orifice may result in an incomplete endothelial bridge between the orifices instead of a single orifice in the right lobe. This bridge may prevent unification and proper portal vein anastomosis.15
Some surgeons have presented the feasibility of double portal vein anastomoses. Double portal vein anastomoses can be performed with RPV and LPV in the recipient, but double portal vein anastomoses may also result in PVT because of an angulation of the MPV axis. Moreover, in patients where GRWR < 1%, double portal vein anastomoses are prone to PVT due to rapid regeneration causing progressive malalignment.6 Among the 4 patients of double portal vein anastomosis in our study, 2 had PVT. The fixed portal vein orifices with the Malatya Approach are not affected from liver regeneration.
Unification venoplasty, resulting in a single orifice, can be performed in patients with APVB even though right lobe graft has type 3. Xu et al. and Guler et al. also reported PVT patients among APVB patients following the unification and single anastomosis as in our study.8, 12
Y-plasty with Y-shaped vein allograft, such as a common iliac vein, and other complicated venoplasties are difficult procedures regarding orientation, twisting, length, and placement of the vessel grafts.6, 12, 13 Several groups suggested autologous portal vein Y-graft interposition as an option for procedure.10, 15-18 The portal vein of the explant could be excised and used as a Y-graft. From the viewpoint of hemodynamics and surgical technique, a double anastomosis at the bench and subsequent single anastomosis to the main PV in the recipient may provide a more natural alignment of vein openings, but buckling deformities would be inevitable due to reperfusion and postoperative regeneration. On the other hand, fixation of portal vein orifices in the level of parenchyma and anastomosis in the Malatya Approach performed with a large conduit are more durable to reperfusion and regeneration. Lee et al. defined and applied a Y interposition technique with autologus portal vein in 76 patients and required stent application in 5 patients (6.5%) due to portal vein stenosis or buckling deformities.16 Shehata et al. reported similar problems.19 The presence of chronic PVT or other changes on the wall of the recipient portal vein can exclude the use of autologous PV grafts. Besides, the recipient portal vein is also extremely thin. During removal of cirrhotic liver, dissection close to the hylus and of cavernomatous venous structures results in sieve-like autologous portal vein.
Xu et al. suggested U-graft anastomosis with CVIG for APVB reconstruction.8 Both ends of the redundant iliac vein are anastomosed to 2 portal veins, and the middle of the iliac vein is opened and anastomosed to the recipient portal vein trunk. Redundant iliac vein graft can cause stasis and thrombosis. Another form of this technique is suggested by Yaprak et al.13 The technique's complexity and difficulty could likely cause PVT after reperfusion. Chan et al. suggested that autologous MPV trunk should be interposed between the joining portal vein openings and the MPV trunk.9 However, in this technique, recipient portal vein trunk can cause shrinkage and the diameter of the joined portal vein openings can be larger than the diameter of the autologous MPV trunk.
Right lobe liver grafts with a type 2 and 3 portal vein often have a concurrent variant bile duct anatomy with respect to the right livers with a type 1 portal vein, making the use of these grafts technically more challenging.2, 4, 10, 12 In our study, there were 81 (64.3%) donors with APVB accompanied with biliary variations. There was not a statistically significant biliary peritonitis leading to relaparotomy, and rates were relatively high in the other group in comparison to the Malatya Approach group. These results can be interpreted to show that the Malatya Approach did not make any obstacles for complex biliary anatomy reconstruction.
In conclusion, we believe that our study is the most comprehensive among literature regarding APVB in right lobe liver grafts. The results reflect realistic outcomes in terms of PVT and mortality confirming that the use of this technique and its variations can be the standard procedure for reconstruction of RL grafts with double portal vein orifices. Our experience suggests that this novel technique, without increasing management difficulty, can be safe and feasible for the reconstruction of APVB with short and far apart PV branching. As reported by Professor Sung Gyu Lee, the circumferential fence was being performed on right hepatic vein orifices of inferior vena cava. Additionally, an article published by the same institution defined that an autologous saphenous vein could be attached to snout-shaped RPV circumferentially.10 It is apparent that the idea of the circumferential fence of the saphenous vein was inspired by Professor Lee. However, this technique was first applied as an operative procedure for right lobe APVB patients in our clinic. This procedure at the bench enables us to reconstruct only 1 portal vein anastomosis, leading to a decrease in the warm ischemia time and an augmentation of technical feasibility in comparison with other techniques. The major limitation of this study is the lack of a portal vein flow measurement study and the possibility of era effect on good results. We believe that the Malatya Approach with its modifications is applicable to all patients with APVB in right lobe LDLT, except for donors with right anterior portal vein originating from the umbilical portion of the LPV.
Acknowledgments
We thank Cheryl Collins and Meredith A. Collins for English language editing.