Recurrence of autoimmune liver disease and inflammatory bowel disease after pediatric liver transplantation
Potential conflict of interest: Nothing to report.
Rodrigo Liberal, Diego Vergani, and Giorgina Mieli-Vergani are supported by grants from the Rosetree Foundation, Edgware, UK, and PSC Partners Seeking a Cure, Greenwood Village, CO.
Abstract
Approximately 10% of children with autoimmune hepatitis (AIH) and 30% of those with sclerosing cholangitis (SC) require liver transplantation (LT). LT is indicated in patients who present with fulminant hepatic failure (ie, with encephalopathy) and in those who develop end-stage liver disease despite treatment. After LT, recurrent AIH is reported in approximately 30% of patients and recurrent SC in up to 50%. Diagnosis of recurrence is based on biochemical abnormalities, seropositivity for autoantibodies, interface hepatitis on histology, steroid dependence, and, for SC, presence of cholangiopathy. Recurrence of SC after LT is often associated with poorly controlled inflammatory bowel disease (IBD). Recurrence may even appear years after LT; therefore, steroid-based immunosuppression should be maintained at a higher dose than that used for patients transplanted for nonautoimmune liver diseases. Although the impact of recurrent disease on graft function is controversial, it seems that in pediatric LT recipients recurrence of AIH or SC is associated with compromised graft survival. Exacerbation of preexistent IBD may be observed after LT for SC or AIH, and IBD appears to have a more aggressive course than before LT. In addition, IBD can develop de novo following LT. Liver Transplantation 22 1275–1283 2016 AASLD
Abbreviations
-
- AIH
-
- autoimmune hepatitis
-
- AIH-1
-
- autoimmune hepatitis type 1
-
- AIH-2
-
- autoimmune hepatitis type 2
-
- AILD
-
- autoimmune liver disease
-
- ANA
-
- anti-nuclear antibody
-
- anti-LKM-1
-
- anti-liver kidney microsomal type 1
-
- ASC
-
- autoimmune sclerosing cholangitis
-
- CMV
-
- cytomegalovirus
-
- d-AIH
-
- de novo autoimmune hepatitis
-
- DR3
-
- D related antigen 3
-
- DR4
-
- D related antigen 4
-
- H & E
-
- hematoxylin-eosin
-
- HLA
-
- human leukocyte antigen
-
- IBD
-
- inflammatory bowel disease
-
- IgG
-
- immunoglobulin G
-
- LT
-
- liver transplantation
-
- MMF
-
- mycophenolate mofetil
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- NR
-
- not reported
-
- PSC
-
- primary sclerosing cholangitis
-
- SC
-
- sclerosing cholangitis
-
- SMA
-
- smooth muscle antibody
-
- UC
-
- ulcerative colitis
Liver transplantation (LT) is an effective therapeutic approach for adult and pediatric patients with end-stage acute and chronic liver diseases. It is a treatment option for selected pediatric patients with end-stage liver disease caused by autoimmune liver diseases (AILDs), either autoimmune hepatitis (AIH) or sclerosing cholangitis (SC).1, 2 LT is indicated for AIH and SC patients with end-stage chronic liver disease, hepatic malignancy, or intractable symptoms, as well as for AIH patients presenting with severe acute liver failure unresponsive to steroid treatment.1 In these patients, the current 5-year post-LT survival rate is 80%-90%.3 Transplant recipients, however, can experience various complications, including chronic rejection, recurrence of primary disease, chronic hepatitis, cancer, and hepatic and/or extrahepatic autoimmunity.4 It is well established that there is an increased risk of disease recurrence in the graft of pediatric patients transplanted for AILD.5 In addition, AIH and autoimmunity can arise de novo after LT for non-AILD conditions.6 Similarly, pediatric patients transplanted for AILD have an increased risk of reactivation or de novo development of inflammatory bowel disease (IBD) after LT.7
Here we review the indications and outcomes of LT in pediatric patients with AILD, and we discuss the latest developments in the understanding of the diagnosis, clinical course, and treatment of recurrent and de novo AILD after LT.
Pediatric Autoimmune Liver Diseases
AIH and SC are the major forms of immune-mediated liver disease occurring in pediatrics.2 The target of the autoimmune attack in AIH are the hepatocytes, whereas in SC, the biliary epithelial cells lining the bile ducts are also targeted.8
AIH is a progressive inflammatory liver disorder characterized serologically by high levels of transaminases and immunoglobulin G (IgG), and presence of autoantibodies, and histologically by interface hepatitis, in the absence of a known etiology.2 In children and adolescents, AIH often presents acutely and has a more aggressive course than in middle-age and elderly patients.9 Usually, it responds satisfactorily to immunosuppressive treatment, which should be started as soon as the diagnosis is made because if it is left untreated, AIH progresses rapidly to cirrhosis and liver failure.10 On the basis of the type of antibodies detected at the time of diagnosis, 2 types of AIH are recognized: autoimmune hepatitis type 1 (AIH-1) defined by the presence of anti-nuclear antibody (ANA) and/or anti–smooth muscle antibody (SMA), and autoimmune hepatitis type 2 (AIH-2), which is characterized by positivity for anti-liver kidney microsomal type 1 (anti-LKM-1) and/or anti-liver cytosol type 1 antibodies.11 Although AIH-1 affects both children and adults, AIH-2 is predominantly a pediatric condition accounting for one-third of the juvenile patients.9 Although severity of disease is similar in the 2 types, children with AIH-2 have higher levels of bilirubin and transaminases at presentation than those with AIH-1.9 In addition, AIH-2 is more frequently associated with presentation with acute liver failure compared to AIH-1. The severity of interface hepatitis at diagnosis is similar in both types, but cirrhosis on initial biopsy is more frequent in AIH-1 than in AIH-2, suggesting a more chronic course of disease in the former.9
SC is a chronic cholestatic disorder characterized by progressive inflammation and fibrosis of intrahepatic and/or extrahepatic bile ducts that leads to bile duct obliteration with formation of multifocal bile duct strictures.12 The term primary sclerosing cholangitis (PSC) used in adult hepatology is not accurate to define pediatric SC,13 because in children, SC is often associated with florid autoimmune features such as elevated titers of ANA and/or SMA, elevated IgG, and interface hepatitis on histology.14 This condition, often associated with IBD, is referred to as autoimmune sclerosing cholangitis (ASC) and is as prevalent as AIH-1 in childhood, but in contrast to AIH it affects equally boys and girls.14 ASC responds satisfactorily to immunosuppression in regard to the parenchymal inflammation, but the bile duct disease progresses in 50% of patients.2 In pediatrics, only those rarer SC cases without the autoimmune features mentioned above should be defined as PSC.13
LT for Pediatric Autoimmune Liver Disease
AIH accounts for 2%-5% of pediatric LTs performed in Europe and the United States.15, 16 Because AIH is a relatively rare condition, there are few studies reporting its outcome.
In 1997, Gregorio et al.9 reported the 20-year experience of King's College Hospital in the management of children with AIH. Of 52 patients followed up for a mean period of 5 years, 32 of whom with AIH-1 and 20 with AIH-2, 10 (19%) were listed for LT. Of these 10 children, 5 had fulminant hepatic failure: 1 died awaiting LT and 4 underwent transplantation. The remaining 5 patients underwent transplantation due to development of chronic liver failure 8-14 years after the initial diagnosis.9 Another series from the United Kingdom reported that out of 85 children with AIH, 13 (15%) underwent transplantation.17 The rate of LT was higher and occurred earlier for AIH-2 (28%, mean interval from diagnosis to LT of 1 month) compared to AIH-1 (12%, mean interval from diagnosis to LT of 26 months).17 A much higher percentage of children with AIH requiring LT was reported by Bahar et al.18: out of 51 children followed up for a median of 8 years, 55% (n = 28) required LT. This discrepancy may have several explanations: late referral and treatment, missed diagnosis of ASC, or different ethnic background.18 In contrast to these 3 series from tertiary referral centers, a recent population-based study conducted in Utah showed a LT rate of just 9% for children with AIH.19
SC accounts for some 3% of LTs performed in pediatric-aged patients.20 LT is indicated in SC patients who progress to end-stage liver disease,13 and it remains the only proven life-extending therapy available.12 Similar to AIH, several series have reported the outcomes of children with SC. Overall, LT rate ranges between 15% and 45%, and the interval between SC diagnosis and LT ranges from 6 to 12 years.1 In one of the earliest series, Wilschanski et al.21 reported that out of 32 children with a mean follow-up time of approximately 4 years, 10 had been listed for LT. The King's College Hospital prospective study showed that 4 out of 27 patients with ASC underwent LT during the study period,14 although it is likely that the rate of LT will increase when the longterm outcome and transition into adulthood data will be analyzed. In a retrospective study, Feldstein et al.22 reported that 20% of 52 pediatric SC patients required LT over a mean of approximately 7 years of follow-up, with the median (50%) survival free of LT being approximately 13 years.22 Another retrospective series reported that over a median follow-up time of 6 years, 9 out of 47 (19%) children with SC required LT.23 Lastly, the Utah pediatric population-based study mentioned earlier reported that the probability of developing complicated liver disease eventually needing LT was 37% for PSC and 25% for ASC.19
Recurrence of AIH After Pediatric LT
Although children transplanted for AIH have generally a good outcome, AIH may recur in the allograft despite immunosuppression.5 The reported recurrence rate of AIH after LT is variable and depends on the criteria used for its diagnosis, the immunosuppressive regimen, length of follow-up, and performance of “per protocol” biopsies.5 Mean time from LT to recurrence is 5 years,15 and recurrence rate increases with the postsurgery interval, but it may occur as early as 35 days after LT.24
Following the initial description in adult patients,25 Birnbaum et al.26 reported in 1997 that AIH recurred in 5 pediatric patients, who had been transplanted for AIH, at a mean time of 11 months after LT. Later, other series reported that the recurrence rate in children transplanted for AIH is nearly 40%17, 18 (Table 1).
The diagnosis of recurrent AIH is based on the reappearance of clinical symptoms and signs, elevation of transaminases and IgG levels, autoantibodies, and interface hepatitis, along with response to prednisolone and azathioprine15, 27 (Table 2). These criteria are basically those included in the International Autoimmune Hepatitis Group scoring systems,28-30 which are used to diagnose AIH in the native liver. Although they have not been tested systematically for the diagnosis of recurrent AIH, they may provide a useful diagnostic tool in view of the similarity between AIH in the native liver and recurrent disease in the allograft.31
| Liver transplant for AIH |
| Elevation of transaminases |
| Interface hepatitis |
| Elevation of IgG |
| Presence of autoantibodies (ANA, SMA, and/or anti-LKM-1) |
| Corticosteroid dependency |
| Exclusion of other causes of graft dysfunction |
Although there are no consistent risk factors for the development of recurrent AIH after LT, some features associated with recurrence have been reported. First, possession of either human leukocyte antigen (HLA)–D related antigen 3 (DR3) or -D related antigen 4 (DR4) by the recipient has been shown to confer risk of recurrence.32, 33 Second, recurrent AIH has been associated with discontinuation of corticosteroids, and therefore caution should be exercised in weaning patients off immunosuppression.34-36 Third, the severity of necroinflammatory activity in the native liver at the time of LT correlates with the risk of AIH recurrence.24, 37 Lastly, recurrent AIH develops less frequently in patients transplanted for acute liver failure compared to those with a chronic presentation.38 Although early studies pointed to an association between tacrolimus-based immunosuppression and the risk of AIH recurrence,24, 39 a systematic review reported that primary immunosuppression with either cyclosporine or tacrolimus did not influence the risk of recurrence.40 Although no data pertaining to pediatric patients are available, a recent study has shown that there are no differences in terms of recurrence rate among adult AIH patients receiving first-degree living related, living unrelated, or deceased donor LT.41
Most transplant recipients with recurrent AIH respond to reintroduction or an increase in the dose of corticosteroids and azathioprine, which should be implemented as soon as the diagnosis is made.3, 33, 34 In the case of treatment failure, alternatives that have been tried with success include the following: addition of mycophenolate mofetil (MMF) in lieu of azathioprine to the standard therapeutic regimen,15 replacement of tacrolimus with cyclosporine,42 and replacement of calcineurin inhibitors with sirolimus.43
The consequences of recurrent disease on graft function are controversial. In adults, recurrent AIH has been associated with an aggressive course after LT, with a higher rate of progression to cirrhosis, graft failure, and need for retransplantation, although it has also been reported that recurrent AIH does not have an impact on patient and graft survival.44, 45 Interestingly, 1 study reported that the risk for graft loss after disease recurrence was higher among adult patients with AIH compared to those with primary biliary cirrhosis. The authors have, however, observed a lower rate of recurrence and absence of graft loss caused by recurrent AIH among patients transplanted between 2000 and 2004, compared with those who underwent transplantation before 2000, speculating that increased awareness of the existence of recurrent AIH, along with the maintenance of corticosteroid therapy in patients transplanted for AIH, improved patient outcomes.46 In the first pediatric report, out of the 5 patients who developed recurrent AIH, 3 progressed to end-stage liver disease requiring retransplantation.26 In the Birmingham cohort, 2 patients with disease recurrence progressed to graft failure requiring a second LT; interestingly, the 2 patients had been originally transplanted for AIH-2, whereas none of those with AIH-1 who developed recurrence progressed to graft failure.17 Further support to the negative impact of disease recurrence in allograft survival comes from a United Network for Organ Sharing database; out of 174 children with AIH transplanted between 2002 and 2012, 19% lost the graft to recurrent disease.47 Successful management of recurrent AIH relies greatly on its early diagnosis and prompt treatment. Because histologic evidence can precede clinical evidence of recurrence, it might be useful to include a follow-up liver biopsy in the protocol for the management of patients transplanted for AIH.48
Recurrence of SC After Pediatric LT
Recurrence of SC after pediatric LT has also been reported. Its prevalence ranges from 10% to 50% of LT recipients20, 22, 49, 50; this wide range depends on the length of the follow-up time because the risk for the development of recurrent SC increases over time (Table 3).
| Reference | Number of LTs for SC | Median Follow-up Time, Years | Recurrent SC, n (%) | Median Time From LT to Recurrence, Months |
| Feldstein et al.22 (2003) | 11 | 7 | 3 (27) | 40 |
| Scalori et al.49 (2007) | 6 | 13 | 3 (50) | 32 |
| Miloh et al.23 (2009) | 9 | 6 | 1 (11) | NR |
| Chai et al.17 (2010) | 5 | 13 | 2 (40) | 33 |
| Miloh et al.20 (2011) | 61 | 3 | 6 (10) | 19 |
| Venkat et al.50 (2014) | 12 | NR | 4 (33) | 52 |
The diagnosis of recurrent SC is suggested by histological and/or cholangiographic findings (Fig. 1). Histological findings that may be observed include the presence of fibrous cholangitis, fibro-obliterative lesions with or without ductopenia, fibrosis or cirrhosis, and/or interface hepatitis, whereas the colangiography generally shows diffuse biliary stricturing.51 However, other causes for the development of nonanastomotic biliary strictures in the graft should be considered and carefully excluded. These include the following: ischemic biliary insults (especially hepatic artery thrombosis), ABO incompatibility between donor and recipient, bacterial or fungal cholangitis, and chronic ductopenic rejection.52 Importantly, because most patients who underwent LT for SC have a Roux-en-Y loop rather than a duct-to-duct anastomosis, endoscopic diagnosis is difficult,53 and therefore, bile duct strictures may be better demonstrated by magnetic resonance cholangiography or percutaneous transhepatic cholangiography.31
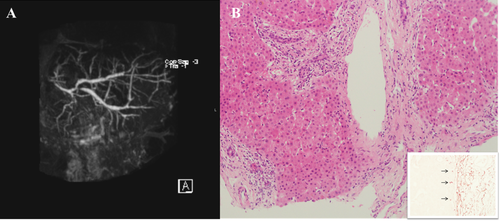
(A) Cholangiographic and histological appearance of recurrent ASC. MRCP showing widespread cholangiopathy with mild bile duct dilatation in a girl transplanted 2 years earlier for end-stage ASC. Courtesy of Maria Sellars, Department of Radiology, King's College Hospital. (B) Liver biopsy, taken at the same time of the MRCP, shows portal cholangiolitic changes (H & E, 200 × magnification). The orcein staining shows periportal deposits of copper-binding protein (inset arrows, 400 × magnification). The histological changes indicate an early stage cholangiopathy. Courtesy of Alberto Quaglia, Institute of Liver Studies, King's College Hospital.
Because pediatric series on recurrent SC are limited to very few patients, there are no consistent risk factors associated with its development. However, some pediatric studies point to an association between active IBD after LT and the development of recurrent SC.20, 49 Similarly, a study in adult patients transplanted for PSC showed that persistent ulcerative colitis (UC) requiring maintenance steroids was associated with an increased risk of developing recurrent disease in the graft, whereas in those patients with concomitant UC, colectomy before or during LT conferred protection against the development of recurrent disease.54 In adult PSC, 2 small studies from Japan suggested that there is an increased rate of PSC recurrence in first-degree relative living related LT compared to deceased donor LT.55, 56 However, this was not confirmed in a larger Canadian study which included 138 PSC patients. No data are available regarding pediatric patients.41
There is no established treatment for recurrent SC after pediatric LT. The use of ursodeoxycholic acid has been advocated in the setting of transplanted adult PSC patients because it seems to improve biochemical indices of liver disease, but it remains unknown whether ursodeoxycholic acid has an impact on outcomes.57 If dominant strictures are present, they should be dilated by interventional cholangiographic means whenever possible.57
Although in adults the impact of recurrence on graft survival remains controversial, in the pediatric setting it is clear that recurrent SC is associated with seriously compromised graft survival.49 In the King's College Hospital prospective study, two-thirds of patients who experienced recurrent SC eventually required retransplantation.49
De Novo Autoimmune Hepatitis After Pediatric LT
De novo autoimmune hepatitis (d-AIH) after LT affects patients transplanted for disorders other than AILD. In the first report, 4% of children transplanted in a single center for various nonautoimmune conditions developed a form of graft dysfunction with features identical to those of classical AIH, namely, hypergammaglobulinemia, positivity for autoantibodies, and histological features of chronic hepatitis with portal and periportal inflammation (Fig. 2).6 The index case did not respond to antirejection treatment, but only to the classical treatment of AIH. None of the children underwent transplantation for AILD, none were hepatitis C virus positive, and all had serum concentration of calcineurin inhibitor within therapeutic antirejection levels at the time of d-AIH diagnosis.6 Since that report, several other groups have reported the occurrence of d-AIH after pediatric LT. Its prevalence in children ranges from 2% to 6%.58-61 The indications for LT reported so far include the following: extrahepatic biliary atresia, Alagille syndrome, acute liver failure, alpha-1-antitrypsin deficiency, primary familial intrahepatic cholestasis, and Budd-Chiari syndrome.6, 58-61
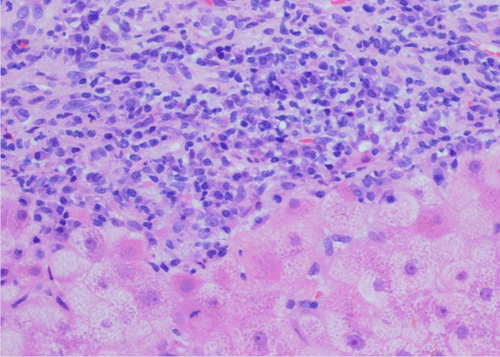
d-AIH after LT. The portal tract is densely infiltrated by mononuclear cells—including several plasma cells—which invade the parenchyma, disrupting the limiting plate. Provided by Yoh Zen, Institute of Liver Studies, King's College Hospital.
Miyagawa-Hayashino et al.62 described the appearance of d-AIH as a complication in living donor LT recipients. In this study, all but 1 patient were below the age of 18 years. Thirteen patients developed a form of graft dysfunction with serological and histological features compatible with AIH at a median interval after living donor LT of 3.1 years.62 Lastly, in the largest and only case-control study published to date in children, 41 (5.2%) patients out of 788 LTs performed at a single center developed d-AIH; all had histological features typical of classical AIH and two-thirds had positive ANA and anti–double-stranded DNA at the time of diagnosis.61
Awareness that treatment with prednisolone alone or in combination with azathioprine or MMF is successful in d-AIH has led to excellent graft and patient survival.5 Children should be given a starting dose of 1-2 mg/kg predniso(lo)ne, without exceeding a daily dose of 60 mg, in combination with azathioprine (1-2 mg/kg); the steroids should then be tapered over 4-8 weeks, to reach a maintenance dose of 5-10 mg/day. In the absence of response, azathioprine should be replaced by MMF.5 The importance of maintenance therapy with steroids in d-AIH was shown in a study comparing treatment with and without steroids: whereas all steroid-untreated patients developed cirrhosis and either died or required retransplantation, none of the steroid-treated patients had progressive disease.63
IBD After LT
IBD, particularly UC, occurs in up to 80% of patients with PSC/ASC as well as in up to 20% of patients with AIH.9, 64 Exacerbation of preexistent UC has been described following LT for PSC or AIH, and it appears to have a more aggressive course than before LT.65-68 Approximately 9% of patients require colectomy after LT due to refractory IBD.7 IBD can also develop de novo following LT.66
The course of IBD following LT has been described mainly in the adult population, though IBD may also recur or arise de novo in pediatric LT recipients. In 1 study, the risk for UC was reported to increase on tacrolimus-based immunosuppression, whereas the risk was decreased in patients receiving a regimen containing cyclosporine; however, a confounding factor is that patients receiving cyclosporine were also treated with azathioprine, whereas those on tacrolimus were not.69 Another study reported that cytomegalovirus (CMV) donor-recipient mismatch confers an increased risk for IBD after LT; however, CMV infection itself can cause a colitis that closely resembles UC, and may thus also constitute a confounding factor.70
Management of IBD, and in particular of UC, after LT is not well established and the risk/benefit of treatment with biological agents in this setting remains to be determined.12 The rate of proctocolectomy for refractory IBD is higher after transplantation than before LT.65 Because the cumulative risk for development of recurrent or de novo UC after LT increases during the first 10 years after LT, and because there is a high risk for the development of colonic neoplasia, careful observation and annual surveillance with colonoscopy are recommended for transplant recipients with concomitant UC.66, 71-73
Conclusion
The outcome of LT in pediatric AILD is excellent, although the disease recurs in the graft in a significant proportion of patients. Diagnosis of recurrent AIH is based on biochemical, serological, and histological criteria and, for recurrent SC, also on cholangiography. Recurrence of AIH is associated with possession of either HLA-DR3 or -DR4, early discontinuation of steroids, and severity of necroinflammatory activity at time of LT, whereas the recurrence of SC is often associated with poorly controlled IBD. Recurrence may even appear years after LT, therefore steroid-based immunosuppression should be maintained at a higher dose than that used for patients transplanted for non-AILD. Early diagnosis of recurrent disease is essential for appropriate life- and graft-saving treatment. AIH can arise de novo following LT for non-AILD conditions. d-AIH should be suspected and investigated in all patients with graft dysfunction not attributable to rejection or surgical complications and, once diagnosed, should be treated promptly. IBD may occur after LT for whatever indication but affects in particular patients transplanted for PSC and AIH, in whom it may represent a flare-up of a disease process present before LT.