An early look at the Organ Procurement and Transplantation Network explant pathology form data
The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplantation Network (OPTN) contractor. The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the US government.
Abstract
In April 2012, the Organ Procurement and Transplantation Network (OPTN) implemented an online explant pathology form for recipients of liver transplantation who received additional wait-list priority for their diagnosis of hepatocellular carcinoma (HCC). The purpose of the form was to standardize the data being reported to the OPTN, which had been required since 2002 but were submitted to the OPTN in a variety of formats via facsimile. From April 2012 to December 2014, over 4500 explant forms were submitted, allowing for detailed analysis of the characteristics of the explanted livers. Data from the explant pathology forms were used to assess agreement with pretransplant imaging. Explant data were also used to assess the risk of recurrence. Of those with T2 priority, 55.7% were found to be stage T2 on explant. Extrahepatic spread (odds ratio [OR] = 6.8; P < 0.01), poor tumor differentiation (OR = 2.8; P < 0.01), microvascular invasion (OR = 2.6; P < 0.01), macrovascular invasion (OR = 3.2; P < 0.01), and whether the Milan stage based on the number and size of tumors on the explant form was T4 (OR = 2.4; P < 0.01) were the strongest predictors of recurrence. In conclusion, this analysis confirms earlier findings that showed an incomplete agreement between pretransplant imaging and posttransplant pathology in terms of HCC staging, though the number of patients with both no pretransplant treatment and no tumor in the explant was reduced from 20% to <1%. In addition, several factors were identified (eg, tumor burden, age, sex, region, ablative therapy, alpha-fetoprotein, Milan stage, vascular invasion, satellite lesions, etc.) that were predictive of HCC recurrence, allowing for more targeted surveillance of high-risk recipients. Continued evaluation of these data will help shape future guidelines or policy recommendations. Liver Transplantation 22 757–764 2016 AASLD.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- CI
-
- confidence interval
-
- COD
-
- cause of death
-
- COGF
-
- cause of graft failure
-
- HCC
-
- hepatocellular carcinoma
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NMC
-
- hepatocellular carcinoma not meeting policy criteria
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- OR
-
- odds ratio
-
- PTM
-
- posttransplant malignancy form
-
- RRB
-
- regional review board
Hepatocellular carcinoma (HCC) is one of the most common indications for patients awaiting liver transplantation (LT), with 20% of all candidates added to the waiting list in 2014 having HCC as a primary or secondary diagnosis at the time of listing.1 Candidates with HCC exceptions accounted for approximately one-quarter of all liver transplants in 2014.2, 3 Tumor staging for HCC is based on the Milan criteria, which is determined predominantly by the number and size of the initial presenting tumors.4 Those with stage T2 HCC (ie, 1 nodule of 2.0-5.0 cm or 2 or 3 nodules, all ≤3.0 cm, also known as the Milan criteria5) have a good prognosis for tumor-free survival following LT.6 On the basis of these findings, the liver allocation policy from the Organ Procurement and Transplantation Network (OPTN) has provided additional priority on the waiting list for candidates with stage T2 HCC who are not candidates for resection, with the intent to direct livers to patients before their HCC progresses to the point where transplantation may no longer be feasible due to poorer posttransplant outcomes.
Though US allocation policy is based on the Model for End-Stage Liver Disease (MELD) score, which orders patients with chronic liver disease awaiting LT based on their risk of 3-month wait-list mortality,7 many candidates with HCC cannot be ranked based on the MELD score because the risk of tumor progression (rather than progression of their underlying liver disease) is the key concern for those with HCC. Therefore, per OPTN policy, centers may submit a MELD exception application for candidates with HCC. HCC exception applications that meet policy criteria are automatically approved; otherwise the exception application is submitted to the applicable regional review board (RRB).8 There are 11 geographic regions used for OPTN administrative purposes, each with a review board that is responsible for reviewing and approving or declining requests for exceptions. The OPTN policy for HCC exceptions allows the RRBs to approve exceptions that are outside the policy criteria based on their consensus medical judgment; for example, RRBs may agree to approve a subset of stage T3 cases, or T3 cases that are down-staged to within Milan, or they may approve tumors that do not meet radiographic criteria, potentially because they were completely treated before the application was submitted.
Since 2002, OPTN policy has required submission of the recipient's posttransplant pathology reports to confirm the diagnosis of HCC, though these forms were initially article copies of the original report submitted by facsimile in a nonstandardized format which were used primarily to confirm that HCC was identified in the explant. This method of collection significantly limited data analysis. However, on the basis of the recommendations from a national HCC consensus conference that was held in 2008 and included hepatologists, surgeons, radiologists, pathologists, and other specialists, a standardized explant pathology form, standardized imaging report form, and more rigorous imaging criteria for pretransplant assessment of patients with HCC were developed and adopted.9 The online explant pathology form was approved by the OPTN board, and implemented in April 2012. A subsequent policy implemented in October 2013 refined the radiologic criteria by which the HCC diagnosis is made, with the intent to better identify those patients who should receive exceptions for HCC. A standardized template for reporting imaging findings was also made available.
This report is the first to analyze a large standardized national database of explant pathology for patients undergoing LT for HCC. The aim of this analysis is to determine whether the explant pathology is congruent with the pretransplant imaging diagnosis of HCC as well as to determine early estimates of posttransplant tumor recurrence stratified by tumor characteristics provided on the explant pathology form. The correlation of pretransplant imaging and posttransplant pathology may be valuable in further refining liver allocation policy in order to optimize outcomes.
Patients and Methods
General
This study used data from the OPTN. The OPTN data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN.
All analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC) and were based on OPTN data as of December 4, 2015. All P values were 2-sided. Tests for differences in the proportions of patient demographics between the HCC exception types were based on the chi-square distribution. Tests for differences in continuous variables were based on the t distribution. Logistic regression using backward elimination was used to determine factors that are significantly related to HCC recurrence.10 A time-to-event analysis was not possible because the date of recurrence was not available for all patients. In approximately 75% of cases, the recurrence was reported on the posttransplant malignancy form (PTM), whereas in the remaining 25% the recurrence was noted as the cause of death (COD) or graft loss on the posttransplant follow-up form, which does not include the date of recurrence.
Analysis of Explant Forms
OPTN explant pathology forms submitted electronically for liver transplants occurring between April 12, 2012 and December 31, 2014, were included in the analyses (n = 4550). Each transplant recipient was categorized based both on the type of HCC exception and the HCC stage (Milan) calculated from the number and size of tumors provided on the explant pathology form. There are 3 types of MELD HCC exceptions included in the analysis: stage T2 meeting policy criteria (T2), hepatocellular carcinoma not meeting policy criteria (NMC; primarily due to the tumor being beyond criteria, or being completely ablated prior to requesting MELD exception), and those listed with a non-HCC exception (“other”) where the diagnosis and narrative clearly indicated that the exception priority was being asked for HCC. Recent data presented to the Liver and Intestinal Organ Transplantation Committee suggest that approximately 70% of the “other, specify” cases are submitted following a missed extension deadline, which may be the only reason that the case did not meet criteria.11
- Was evidence of HCC (viable or nonviable) found in the explant?
- Pretransplant treatment for HCC?
- Number and size of tumors (on HCC application and found in the explant).
- Level of tumor differentiation.
- Vascular invasion, lymph node involvement, extrahepatic spread, and satellite lesions.
A primary aim of this analysis was to compare the data reported on the standardized explant pathology form to the imaging data.
Posttransplant Outcomes
HCC recurrence was noted if, at any point in time following the transplant, the PTM indicated recurrent cancer, if the COD text field denoted HCC recurrence, or if the cause of graft failure (COGF) was “recurrent disease.” A time-to-recurrence analysis was not performed because the date of recurrence is only reported on the PTM form, representing only 75% of patients identified. Variables investigated in the initial model included the following: initial presenting tumor burden; age, sex, region, length of time to transplant from initial listing and from exception application; pretransplant ablative therapy; pretransplant alpha-fetoprotein (AFP); evidence of HCC on explant; Milan stage on explant; vascular invasion; satellite lesion; lymph node involvement; extrahepatic spread; and level of tumor differentiation. Because many of these variables are required only if evidence of HCC was seen on explant, the cohort for recurrence was restricted to those with evidence of HCC.
Results
Pretransplant Exception Application Data
- The number of tumors cannot be 0 on an initial application (46.3%).
- One tumor was indicated, and its size was <2 cm (19.5%).
- The candidate has stage T2 not meeting imaging criteria (11.8%).
- Original presenting tumor number and/or size were greater than stage T2 HCC (9.2%).
Thus, it is important to reiterate that “not meeting criteria” does not necessarily mean that the HCC is greater than stage T2. Nearly 95% of the applications indicated that the candidate had undergone pretransplant ablative therapy for HCC, which is why there were so many with reported tumor sizes of 0 (complete ablation). The remaining 2.9% of the patients who were not automatically approved in UNet were initially listed with an “other, specify” MELD exception. As with those patients who are designated as HCC exceptions, the OPTN has a mechanism to ensure that explant pathology forms are generated in those cases when an HCC exception ultimately is converted to an “other, specify” exception. There were significant differences in the percentage of cases not meeting policy criteria across regions, ranging from 17.5% to 31% (P < 0.001).
Demographics by final exception type prior to transplant are provided in Table 1. There were no major differences in sex, age, or diagnosis based on the type of HCC exception application at the time of transplant. The overall median age was 59.5 years. Overall, 77.3% were male. Not surprisingly, the most common diagnosis at listing was malignant neoplasms, followed by noncholestatic cirrhosis. Those with an “other, specify” application were more likely to be Asian or Hispanic. Asians were more likely to have a diagnosis of type B surface antigen positive cirrhosis than other ethnic groups, at 14.2%, versus 1% or less for other groups (data not shown).
| HCC Stage T2 | NMC | HCC Coded As “Other, specify” | Total | |
|---|---|---|---|---|
| Age in years, median | 59.4 | 59.9 | 59.4 | 59.5 |
| Calculated laboratory MELD score, median | 12 | 11 | 11 | 11 |
| Sex, male, % | 76.2 | 79.4 | 77.8 | 77.3 |
| Ethnicity, % | ||||
| White | 69.7 | 67.1 | 58.8 | 67.5 |
| Hispanic | 12.6 | 14.3 | 19.5 | 14.0 |
| Black | 9.8 | 9.4 | 10.1 | 9.8 |
| Asian | 6.5 | 8.0 | 10.6 | 7.5 |
| Other | 1.4 | 1.2 | 1.0 | 1.3 |
| Diagnosis at listing, % | ||||
| Malignant neoplasms | 73.5 | 72.8 | 73.5 | 73.3 |
| Noncholestatic cirrhosis | 24.6 | 24.4 | 24.7 | 24.6 |
| Other | 1.9 | 2.8 | 1.8 | 2.1 |
Explant Form Data
Evidence of HCC was seen on explant in 4242 (93.2%) patients. Of the 308 recipients with no evidence of HCC on explant pathology, 87.0% had received pretransplant locoregional treatment for HCC, leaving 40 (0.9% of total) patients with no tumor found in the explant and no pretreatment indicated. Only 8.0% of the cohort had a report of a biopsy being performed; the percent with evidence of HCC on explant was only slightly lower in those with no biopsy versus those with a biopsy (5.4% versus 6.9%; P = 0.23). Of those with evidence of HCC, 23.0% reported well with tumor differentiation; 45.6% were moderate; 7.1% were poor; and treatment resulted in complete tumor necrosis in 17.6% of patients. Most recipients (75.5%) showed no vascular invasion, with 13.1% indicating microvascular invasion and 1.7% with macrovascular invasion. Two percent of pathology reports revealed lymph node involvement, <1% showed extrahepatic spread, and 6.5% reported satellite lesions.
Of the 2642 recipients that were within Milan criteria (stage T2) on imaging (as reported on the last exception application submitted prior to transplant), 601 (22.7%) exceeded criteria based on explant pathology, with 14.7% indicating stage T1, and 6.8% with no evidence of HCC, and the remaining 55.8% meeting T2 criteria (Figs. 1 and 2). Those not meeting policy criteria or with an “other, specify” MELD exception were more likely to be stage T3 or T4 on explant, at 37.5% and 34.9%, respectively. However, after stricter pretransplant imaging criteria were implemented on October 31, 2013, there was a slight decrease in the percentage of stage T4 tumors on explant when compared to exceptions submitted prior to that date (13.4% versus 11.9%; P = 0.21), with a corresponding increase in stage T3 (14.7% versus 18.6%; P = 0.003). The percentage of stage T1 and T2 cases remained approximately the same for those with a T2 or NMC exception. Those cases submitted after October 31, 2013 that did not meet radiologic criteria (but were approved by the RRB) were no more likely to have a biopsy than those who met criteria (P = 0.92).
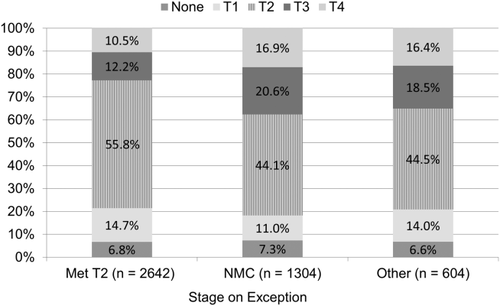
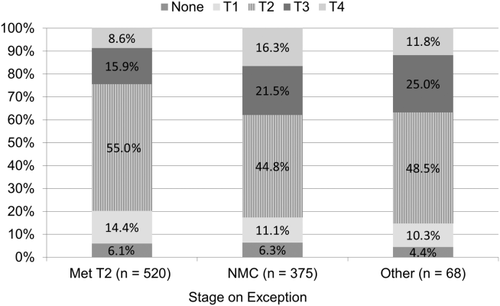
Although most recipients had undergone pretransplant locoregional treatment for HCC, at 92.1% nationally, there were some differences by region. Regions 3, 10, and 11 fell below the average, at 86.5%, 82.4%, and 86.5%, respectively. In general, the percentage increased for those regions with typically longer waiting times to transplant, such as 1 (97.4%) and 9 (97.9%). Although 93.2% of forms indicated evidence of HCC, there were small variations by region. There were significant differences between regions in terms of downstaging (from greater than stage T2 to less than stage T2), ranging from <1% to 12% (P < 0.001).
Recurrence
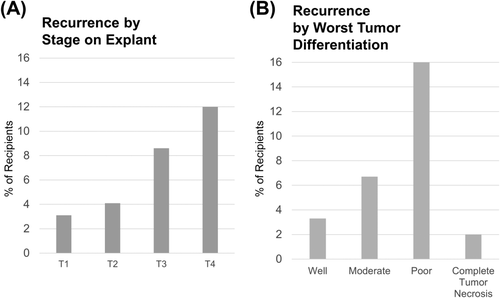
Recurrence was found in 258 of the transplant recipients (5.7%), as shown in Table 2. The majority of patients with recurrence (75.1%) were identified via the PTM. Although the percentage is likely an underestimate due to the short follow-up period (the median time to recurrence for those whose recurrence was reported on the PTM was 345 days), there are some notable findings even with this small sample. As Figs. 3 and 4 show, early recurrence was much higher in stage T3 and T4 HCC on explant and for those with poor tumor differentiation, macrovascular invasion, and extrahepatic spread.
| How Recurrence Was Identified | Value, n (%) |
|---|---|
| PTM + COD or COGF | 91 (2.0) |
| COD only | 49 (1.1) |
| COGF only | 15 (0.3) |
| PTM only | 103 (2.3) |
| None identified | 4292 (94.3) |
| Total | 4550 (100.0) |
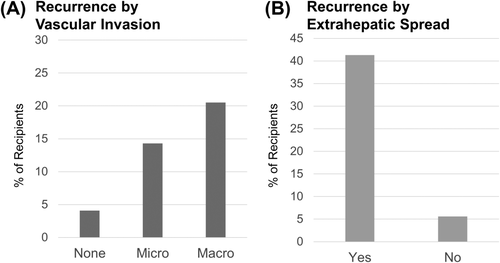
Results of a logistic regression analysis revealed several pretransplant factors as well as factors identified on explant that were predictive of HCC recurrence, as shown in Table 3. AFP (log scale) had a statistically significant impact on recurrence with an odds ratio (OR) of 1.2 per unit increase (P < 0.01). Explant findings that were significantly related to recurrence included extrahepatic spread (OR = 6.8; P < 0.01), poor tumor differentiation (OR = 2.8; P < 0.01), microvascular invasion (OR = 2.6; P < 0.01), macrovascular invasion (OR = 3.2; P < 0.01), whether the Milan stage based on the number and size of tumors on the explant form was T4 (OR = 2.4; P < 0.01) or T3 (OR = 1.9; P < 0.01), lymph node involvement (OR = 1.9; P = 0.03), and whether the HCC had been downstaged from greater than stage T2 to less than stage T2 (OR = 1.8; P = 0.01).
| Variable | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Extrahepatic spread | 6.8 | 2.9 | 15.9 | <0.01 |
| Poor tumor differentiation | 2.8 | 2.0 | 4.0 | <0.01 |
| Microvascular invasion | 2.6 | 1.9 | 3.5 | <0.01 |
| Macrovascular invasion | 3.2 | 1.7 | 5.9 | <0.01 |
| Stage T4 on explant | 2.4 | 1.7 | 3.3 | <0.01 |
| Lymph node involvement | 1.9 | 1.1 | 3.5 | 0.03 |
| Stage T3 on explant | 1.9 | 1.4 | 2.7 | <0.01 |
| Downstaged from > T2 | 1.8 | 1.1 | 2.8 | 0.01 |
| AFP (log) | 1.2 | 1.1 | 1.3 | <0.01 |
- NOTE: The 95% CI was found with the Wald test, and P values were found by chi-square.
The length of time between initial listing or exception application and transplant was not found to be statistically significant for recurrence. Other factors examined that were not found to be significantly associated with recurrence included age, sex, pretransplant ablative therapy, and satellite lesions. The percent with recurrence for those with a biopsy was comparable to those with no biopsy (4.5% versus 4.6%). Statistically, the downstaging effect was not dependent on, or modified by, waiting time (P = 0.69). The c-statistic of the final model was 0.75, indicating good discrimination between recipients with and without recurrence in the original sample.
One other pretransplant factor, being transplanted in region 3, also increased the odds of recurrence (OR = 2.0; P < 0.01). Further analyses did not explain why region 3 would differ from other regions in terms of recurrence rates. Statistically, the effect of waiting time was independent of listing region (region 3 versus all other regions; P = 0.61). Although patients in region 3 have shorter waiting times than other regions, we could not specifically demonstrate short wait time as the etiology for recurrence. These shorter waiting times may mean that patients with poor tumor biology (and poor outcomes) are transplanted before tumor progression beyond Milan criteria becomes evident, as happens in areas with longer waiting times.12 It is also possible that this finding is due to reporting bias or other factors not collected by the OPTN. We will continue to examine this finding for possible explanations. However, because this is not a patient-level factor that would be used for policy-making or posttransplant surveillance, it was removed from the final model.
Discussion
LT for HCC remains an excellent option for patients meeting Milan criteria. The challenge is being able to accurately stage patients with pretransplant imaging. In this report of over 4500 patients transplanted for HCC, final pathology showed 55.9% of patients with T2 imaging before transplant had consistent pathologic findings. Just over one-quarter of recipients had explant pathology of T3 or T4 (T3, 15.5%; T4, 13.3%) similar to a previous report13 regarding imaging accuracy. The lack of significant radiologic agreement with pathologic findings is an important issue as policy and priority in the MELD-based allocation system is predicated on pretransplant staging. The adoption of more consistent and homogeneous imaging modalities and criteria14 were meant to help rectify this problem. Currently, in excess of 90% of patients have pretransplant treatment for HCC, which may limit our ability to interpret the imaging findings in light of the explant findings. In the previous analysis by Freeman et al.,13 only 44% of explant data matched the pretransplant imaging, and nearly 20% of patients did not have any evidence of tumor in the explant with no pretransplant treatment. In our study, <1% had no tumor in the explant and no pretransplant treatment, demonstrating that although the system is still not optimal, notable improvements have been made. Given that candidates with HCC are given considerable priority in the US allocation system,9 it is imperative that we continue to monitor the HCC allocation system in order to make continued improvements.
Recurrence following LT is a critical issue because the intent of LT is to use a limited resource for a longterm cure. The current analysis demonstrated pretransplant and posttransplant factors that were predictive of recurrence. Unfortunately, predictors of recurrence are important, yet may only be partially known prior to transplant. Tumor differentiation, vascular invasion, and extrahepatic spread are all important variables but would not likely be known prior to transplant. In addition, the standardized pathology form captures only the basic elements such as tumor size, location, and presence of metastasis. The form cannot ensure a standardized interpretation of HCC by the pathologist, nor can it differentiate between tumor subtypes such as mixed HCC and cholangiocarcinoma subtypes. With this large cohort of patients, our logistic regression analysis identified a number of factors that are predictors of recurrence. Not surprisingly, extrahepatic spread was associated with the highest odds of recurrence, with an OR of 6.8, consistent with previous reports of smaller cohorts that have also identified them as risk factors as well.15-17
A number of previous reports18-20 have shown that patients with tumors beyond Milan criteria can benefit from transplant with good outcomes. In the current report, there was an increased risk for recurrence for patients who were initially beyond Milan and were downstaged. In addition, this report shows that 22.7% of patients thought to have met T2 criteria before transplant actually were T3 or T4 after transplant. Proponents of expanding exception criteria to beyond Milan stress the excellent outcomes achieved,21 which may well be achieved in selected centers with close follow-up and a waiting period prior to receiving an HCC exception score. However, the inability of pretransplant imaging to more accurately predict the true staging is a significant impediment to expanding HCC exception policy. Patients with HCC currently have increased access to LT, meaning they are transplanted more quickly and have lower pretransplant mortality than non-HCC patients.2 Expanding the number of patients eligible for priority without decreasing the priority scores assigned may have significant negative effects on the ability of non-HCC patients to receive a transplant.
The presence of a large set of pathologic data on these transplant patients is the first of its kind in this field. This early evaluation of the data has helped elucidate and validate earlier findings regarding tumor recurrence. The data have also clarified that although our current pretransplant imaging has improved, there still remains incomplete agreement between pretransplant imaging staging and posttransplant explant pathology staging. Ongoing analysis of the data, including expanding the ascertainment of HCC recurrence to sources beyond the OPTN database, should be used to help in future policy development regarding transplantation and HCC.
Acknowledgments
The authors thank the members of OPTN Liver and Intestinal Organ Transplantation Committee and HCC subcommittee for their contributions to the design and implementation of the explant pathology form and associated policies.