Liver transplantation for adenomatosis: European experience
Grants and financial support: Nothing to report.
Abstract
The aim of this study was to collect data from patients who underwent liver transplantation (LT) for adenomatosis; to analyze the symptoms, the characteristics of the disease, and the recipient outcomes; and to better define the role of LT in this rare indication. This retrospective multicenter study, based on data from the European Liver Transplant Registry, encompassed patients who underwent LT for adenomatosis between January 1, 1986, and July 15, 2013, in Europe. Patients with glycogen storage disease (GSD) type IA were not excluded. This study included 49 patients. Sixteen patients had GSD, and 7 had liver vascular abnormalities. The main indications for transplantation were either a suspicion of hepatocellular carcinoma (HCC; 15 patients) or a histologically proven HCC (16 patients), but only 17 had actual malignant transformation (MT) of adenomas. GSD status was similar for the 2 groups, except for age and the presence of HCC on explants (P = 0.030). Three patients with HCC on explant developed recurrence after transplantation. We obtained and studied the pathomolecular characteristics for 23 patients. In conclusion, LT should remain an extremely rare treatment for adenomatosis. Indications for transplantation primarily concern the MT of adenomas. The decision should rely on morphological data and histological evidence of MT. Additional indications should be discussed on a case-by-case basis. In this report, we propose a simplified approach to this decision-making process.
Abbreviations
-
- β-CAT
-
- β-catenin
-
- ELTR
-
- European Liver Transplant Registry
-
- GSD
-
- glycogen storage disease
-
- HCA
-
- hepatocellular adenoma
-
- HCC
-
- hepatocellular carcinoma
-
- HNF
-
- hepatocyte nuclear factor
-
- IHCA
-
- inflammatory hepatocellular adenoma
-
- LA
-
- liver adenomatosis
-
- LT
-
- liver transplantation
-
- MA
-
- multiple adenoma
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MODY
-
- maturity onset diabetes of the young
-
- MT
-
- malignant transformation
-
- VASC
-
- liver vascular abnormality
Multiple adenomas (MAs) occur either in normal livers or in cases of underlying liver pathology, such as glycogen storage disease (GSD) or congenital or acquired vascular anomalies. Initially, Flejou et al.1 described a special entity, liver adenomatosis (LA), in cases with more than 10 adenomas in a normal liver in the absence of GSD or vascular disease. Great progress has been made in the diagnosis, imaging, and pathogenicity of adenomas. In particular, the quality of nontumoral parenchyma is important in the management of MAs. So, considering the recent advances in this field, the term of adenomatosis has been extended and refers today to a high number of these tumors in the liver.2 LA is a heterogeneous disease, and imaging often underestimates the number of lesions present. Chiche et al.3 described 2 forms of LA: a massive form with an enlarged liver and typically large and necrotic tumors, and a multifocal form with a normal-sized liver in which 1 or 2 adenomas may predominate over the smallest lesions.
The risks of hemorrhage and malignant transformation (MT) justify tumor resection in many cases of solitary hepatocellular adenoma (HCA).4-10 However, this surgical approach can be difficult or impossible in cases of LA due to the large number of adenomas. Therefore, liver transplantations (LTs) have been reported in the literature.11-17 Nevertheless, the management of LA remains controversial despite some progress in the prediction of the potential risk of complications (which is likely related to the size and biomolecular subtypes of the adenomas), and LA management ranges from simple follow-up to partial or total liver resection.18-21
This report describes a large retrospective European cohort of LT patients treated for adenomatosis and examines the main factors leading to transplantation and the transplantation results, with the goal of identifying the optimal role for LT in LA. This study also provides recommendations for LT decision-making in LA.
Patients and Methods
Prospectively collected data from the European Liver Transplant Registry (ELTR)22 were reviewed after obtaining approval from the board of the European Liver and Intestine Transplant Association. Patients undergoing LT for LA between January 1, 1986, and July 15, 2013, were selected. Patients with GSD type IA who underwent LT for LA were included.
The ELTR has collected prospective data on LT in 145 centers all over Europe since 1968. We selected cases with the diagnostic code “adenomatosis” in the ELTR database. A standard computerized file was provided to the contributing centers with detailed instructions for the collection of accurate and uniform information. However, participation in the ELTR is voluntary and may contain coding errors. Therefore, we directly contacted the centers and, notably, all of the French and Belgium centers to check the validity of the ELTR list. Patients were included only if the primary indication was LA (exclusion of hepatocellular insufficiency).
Data Collection
Each transplant center received the project summary and was contacted to obtain detailed medical files after patient identification. All of the centers were asked to send the medical files (main preoperative consultation; radiological, surgical, histopathological reports; and date of last news or death). The files were processed at the coordinating center at Bordeaux, France. Processing included record review to confirm the diagnosis and exclude possible errors.
Histological and primarily pathomolecular data were very laborious to obtain. Twelve centers sent us pathologic tissue blocs for secondary examination by our pathological expert in Bordeaux (Paulette Bioulac-Sage).
Disease
The clinical manifestations that led to the adenomatosis diagnosis were reported as follows: “abdominal pain and hepatomegaly”; “hemorrhage,” which included patients who had severe bleeding episodes; or “others,” which included rare signs, such as pruritus. When diagnosis of LA was done fortuitously by imaging or in the follow-up of different pathologies, the patient was classified in “no symptoms.”
We identified 2 forms of adenomatosis as defined by Chiche et al.,3 ie, massive or multifocal forms.
After careful review of all the clinical reports, indications for LT were reclassified into 5 main groups: “hepatocellular carcinoma (HCC) confirmed,” which included patients who had the diagnosis of MT by a biopsy or on a surgical specimen; “suspicion of HCC” as noted in the files as indications for LT; “evolution,” which included patients who had LA with progression in size or number of adenomas; “hemorrhage,” for patients who had iterative or life-threatening hemorrhagic manifestations; and “symptoms” if patients presented with severe pain or disability associated with liver enlargement.
The populations with and without GSD were studied separately to identify whether this underlying disease should be considered a separate entity, and the risk factors of MT were investigated.
Statistical Analysis
Patient and follow-up data were obtained from the electronic medical record we created. Proportions were compared using the chi-square test or Fisher's exact test as indicated. P values < 0.05 were considered significant in all analyses. Statistical analyses were performed using the R program, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria) and the statistical software STATA (Statacorp, College Station, TX).
There were missing data regarding, for instance, oral contraception or the number of HCAs. We included missing data in the statistical analyses when these data represented more than 10% of the value.
Results
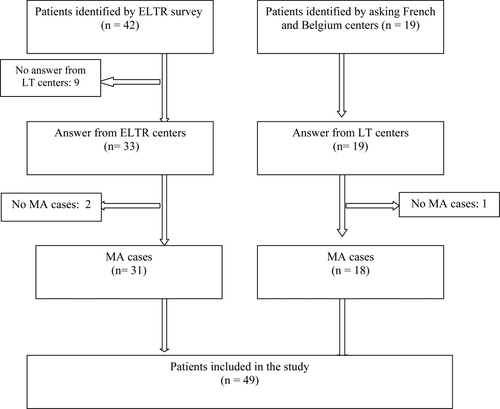
According to the ELTR, 64 patients were identified as having a LT for LA in 22 centers in 5 European countries during the study period. Several centers did not provide data, others reported more or less cases than the registered number in the ELTR database, and several coding errors were identified.
Additional cases were collected after direct calls to the French centers. Thirteen French centers reported 24 patients, but only 5 of these patients were listed in the ELTR database. Two Belgium centers reported 6 patients; 4 Italian centers reported 7 patients; 2 Spanish centers reported 3 patients; and 1 German center reported 9 patients.
Eventually, a total of 49 patients underwent a LT for histologically confirmed adenomatosis throughout this long period, quite consistently, because 30 underwent transplantation before 2006 and 19 underwent transplantation after (Fig. 1). Table 1 shows the patient characteristics. Thirty-one of the 49 (63%) patients were women, and 18 (37%) patients were men. Information on estrogen intake was not available in 12/31 women, even after advanced research. In the other 19 female patients, only 6 had a history by oral contraceptive intake. The following initial clinical manifestations of the disease were noted: 22 follow-up cases (14 for glycogenosis); 6 cases of abdominal pain with hemorrhagic manifestations; 6 cases of accidental discovery, including trauma in most cases; and 4 cases of hepatomegaly. Twenty-seven of the 49 (55%) patients were younger than 30 years. We obtained information for maturity onset diabetes of the young (MODY) 3 diabetes23-25 for 2 patients, and 2 patients had a family history of adenomatosis, including 2 sisters in this study. Sixteen (33%) patients had GSD type IA in this study.
| Value (n = 49) | |
|---|---|
| Patients characteristics | |
| Sex | |
| Female | 31 (63) |
| Male | 18 (37) |
| Age | |
| ≤30 years | 27 (55) |
| >30 years | 22 (45) |
| GSD | 16 (33) |
| MODY 3 | 2 (4) |
| Clinical manifestation | |
| No | 33 (67) |
| Pain and hepatomegaly | 10 (20) |
| Hemorrhage | 6 (12) |
| Liver and adenomatosis characteristics | |
| Underlying liver pathology | |
| GSD | 15 (31) |
| Vascular abnormalities | 6 (12) |
| GSD and vascular abnormalities | 1 (2) |
| Form | |
| Massive | 28 (57) |
| Multifocal | 21 (43) |
| Number of HCAs | |
| Missing data | 15 (31) |
| ≤10 | 4 (8) |
| >10 | 30 (61) |
| Biggest size (cm) | |
| ≤4 | 9 (18) |
| >4 | 40 (82) |
| Bleeding history | 11 (22) |
| Transplant history | |
| Liver surgery before LT | 21 (43) |
| Embolization before LT | 2 (4) |
| Transplantation type | |
| ≥ 2 LT | 5 (10) |
| Kidney and LT | 2 (4) |
| Living donor | 3 (6) |
| Indication of LT | |
| HCC confirmed | 16 (33) |
| HCC suspected | 15 (31) |
| Disease progression | 8 (16) |
| Hemorrhage | 5 (10) |
| Symptoms | 5 (10) |
- NOTE: Data are given as n (%).
Disease
Twenty-eight (57%) patients had the massive form of adenomatosis, and 21 (43%) patients had the multifocal form. Table 1 shows the characteristics of the cases. Thirty (61%) patients had more than 10 HCA on the final hepatectomy, and 4 (8%) patients had exactly 10 or fewer. Fourty (82) patients had LA >4 cm, as compared to 9 (18%) patients who exhibited LA ≤4 cm (patients with GSD or with a history of previous hepatectomies for larger adenomas).
We identified 7 (14%) patients with underlying vascular disease, which is a well-known factor26-29 in the pathogenesis of benign liver tumors and pseudotumors. The histology was confirmed in those cases by 2 pathologists. One patient had a history of Tetralogy of Fallot that was treated with surgery and a splenorenal shunt. Four patients had portocaval shunts in childhood (including one for agenesis of portal vein) one had a portocaval congenital shunt and one Osler Weber Rendu disease. One patient had GSD with vascular abnormalities.
Indication for LT and Transplantation Management
Table 1 shows the following indications for LT: 15 (31%) patients received LT for suspicion of MT; 16 (33%) patients underwent transplantation for HCC that was histologically proven before LT (by biopsy [n = 4] or on a surgical specimen of a previous hepatectomy [n = 12]). Patients who had biopsy underwent transplantation for HCC; and in patients who had HCC on a surgical specimen, LT was decided either as a preventive procedure, considering the risk of malignancy in the remaining adenomas or as a curative after recurrence. Eight (16%) patients underwent LT due to disease progression (size and number of adenomas); 5 (10%) patients underwent LT for hemorrhagic manifestations—1 life-threatening cataclysmic hemorrhagic justified the indication of LT in an emergency, and 4 patients received emergency surgery with liver resection for intraperitoneal bleeding; and 5 (10%) patients received LT for symptomatic adenomas without the possibility of simple hepatic resection. Twenty-one of the 49 (43%) patients had a history of 1 or several hepatic resections, and 2 (4%) patients had received embolization for hemorrhage before LT. Five (10%) patients received more than 1 LT; 2 (4%) patients underwent simultaneous kidney transplantation (patients with GSD); and 3 (6%) patients received a LT from a living donor.
Pathological Characteristics
We obtained 23 pathomolecular characteristics. The results (hepatocyte nuclear factor [HNF] 1α, inflammatory (IHCA), β-catenin [β-CAT], or mixed subtypes) are reported in Table 2. In patients without GSD, HNF1α mutation was observed in 10/16 patients; in patients with GSD and available data, the inflammatory subtype was predominant with or without β-CAT mutation. This study identified 17 (35%) patients with MT (Table 3). Sixteen (33%) patients had HCC that was confirmed before LT and diagnosed by biopsy or hepatic surgery. Only 1 patient was transplanted for the suspicion of HCC and showed MT on the liver explant. Table 3 shows the clinical and molecular characteristics of patients with HCC: 8 of the 17 patients had multifocal MT and each pathomolecular type has been observed. Only 1 of the patients transplanted for suspicion of MT (including the 10 GSD patients) showed actual HCC.
| Number | Liver | HCC | Form | Biomolecular Group | Number of HCA | Size |
|---|---|---|---|---|---|---|
| 1 | GSD | Multiple | IHCA | 20 | 6 | |
| 2 | GSD | + | Massive | β-CAT + β-IHCA | >10 | 15 |
| 3 | GSD | + | Multiple | IHCA | 22 | 4 |
| 4 | GSD | Multiple | β-CAT+ β-IHCA + IHCA | >10 | 2 | |
| 5 | GSD | Multiple | β-CAT+ β-IHCA + IHCA | >10 | 3 | |
| 6 | GSD | Multiple | IHCA | >10 | 5 | |
| 7 | GSD | + | Massive | IHCA | >10 | 11 |
| 8 | VASC | + | Massive | HNF1α | >10 | 4 |
| 9 | VASC | + | Multiple | HNF1α | NR | 8 |
| 10 | VASC | + | Massive | β-CAT | 50 | 13 |
| 11 | VASC | Massive | HNF1α | 2 | 17 | |
| 12 | VASC | Multiple | Unclassified | >10 | 4 | |
| 13 | Massive | HNF1α | >10 | 10 | ||
| 14 | Massive | HNF1α | >10 | 8 | ||
| 15 | Multiple | IHCA | 8 | 8 | ||
| 16 | Multiple | HNF1α | >10 | 5 | ||
| 17 | + | Massive | IHCA | 39 | 8 | |
| 18 | + | Massive | β-CAT + β-IHCA | 50 | 9 | |
| 19 | Massive | HNF1α | >10 | 8 | ||
| 20 | Massive | HNF1α | >10 | 10 | ||
| 21 | Multiple | HNF1α | >10 | 6 | ||
| 22 | Massive | HNF1α | 22 | 18 | ||
| 23 | Massive | IHCA | >10 | 8 |
| Number | Age, years | Sex | Liver | Form | HCC Before TH | Explant HCC | Number of HCC on Liver Explant | Number of HCC Total | Biomolecular Group |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | Female | Multiple | Yes | Yes | Multifocal | Multiple | ||
| 2 | 34 | Female | Massive | Yes | Yes | Multifocal | Multiple | IHCA | |
| 3 | 43 | Male | Multiple | Yes | Yes | Multifocal | Multiple | ||
| 4 | 47 | Female | Massive | Yes | Yes | Multifocal | Multiple | β-CAT/CAT/IHCA and IHCA | |
| 5 | 38 | Female | Multiple | Yes | ND | Single | |||
| 6 | 41 | Female | Multiple | Yes | Yes | Unifocal | Single | ||
| 7 | 51 | Female | Multiple | Yes | Yes | Unifocal | Multiple | ||
| 8 | 47 | Female | Massive | Yes | Yes | Unifocal | Single | ||
| 9 | 43 | Female | Multiple | Yes | No | No | Single | ||
| 10 | 31 | Male | VASC | Massive | Yes | Yes | Multifocal | Multiple | β-CAT |
| 11 | 26 | Male | VASC | Massive | Yes | Yes | Multifocal | Multiple | HNF1α |
| 12 | 27 | Male | VASC | Multiple | Yes | Yes | Unifocal | Single | HNF1α |
| 13 | 39 | Female | GSD+VASC | Massive | Yes | Yes | Multifocal | Multiple | β-CAT/IHCA and IHCA |
| 14 | 32 | Male | GSD | Multiple | Yes | No | No | Single | IHCA |
| 15 | 21 | Female | GSD | Massive | No | Yes | Unifocal | Single | IHCA |
| 16 | 25 | Female | GSD | Massive | Yes (B) | No | No | Single | |
| 17 | 23 | Female | GSD | Multiple | Yes (B) (Borderline) | No | No | Single |
GSD Status
Table 4 compares the patients with GSD type IA and without. Patients in the GSD group were significantly younger (P = 0.034) and had larger livers than the group without GSD (P = 0.002) at the time of LT. The patients without GSD more often (10/17) had the diagnosis of HCC confirmed before LT (P = 0.030). There was no significant difference in the global risk of HCC (confirmed before or after LT) between GSD and non-GSD patients.
| No GSD (n = 33) | GSD (n = 16) | P Value | |
|---|---|---|---|
| Sex | 0.35 | ||
| Female | 23 (70) | 8 (50) | |
| Male | 10 (30) | 8 (50) | |
| Age | 0.034 | ||
| ≤30 years | 14 (42) | 13 (81) | |
| >30 years | 19 (58) | 3 (19) | |
| Patient weight (kg) | 0.015 | ||
| ≤60 | 11 (33) | 11 (69) | |
| >60 | 22 (67) | 5 (31) | |
| LA form | 0.45 | ||
| Massive | 19 (58) | 8 (50) | |
| Multifocal | 14 (42) | 8 (50) | |
| Number of HAs | 0.49 | ||
| Missing data | 8 (24) | 7 (44) | |
| ≤10 | 3 (9) | 1 (6) | |
| >10 | 22 (67) | 8 (50) | |
| Biggest size (cm) | 0.07 | ||
| ≤4 | 3 (9) | 6 (38) | |
| >4 | 30 (91) | 10 (63) | |
| Liver weight (kg) | 0.002 | ||
| Missing data | 5 (15) | 1 (6) | |
| ≤2.5 | 19 (58) | 3 (19) | |
| >2.5 | 9 (27) | 12 (75) | |
| Vascular liver abnormalities | 6 (18) | 1 (6) | 0.96 |
| Hemorrhagic intratumoral | 10 (30) | 1 (6) | 0.11 |
| Indication of LT | |||
| HCC confirmed | 12 (36) | 4 (25) | |
| Suspicion of HCC | 5 (15) | 10 (63) | |
| Disease progression | 6 (18) | 2 (13) | |
| Hemorrhagic | 5 (15) | 0 (0) | |
| Symptomatic without possibility of liver resection | 5 (15) | 0 (0) | |
| HCC confirmed on the liver explant | 10 (59) | 2 (13) | 0.030 |
| HCC confirmed before or after LT | 12 (36) | 5 (31) | 0.62 |
- NOTE: Data are given as n (%).
Longterm Outcomes
No patient was lost during follow-up. The median duration of survival was 108 months (24-168 months), with a range of 0-316 months. Three patients died within 90 days after LT because of severe pulmonary edema, sepsis (biliary duct necrosis), and hemorrhagic shock after his second transplantation. These 2 last operative deaths occurred before 2000 and concerned patients with vascular abnormalities or previous liver surgery. Two patients died after 3 years of follow-up; of these, 1 patient died of lymphoma, and the other patient died in unknown circumstances. Three of the 17 patients with HCC in liver explants experienced recurrence after LT and died of the disease. So, in 2014, 41/49 patients were alive and well.
The median duration of GSD was 74 months (13-141 months), with a range of 0 to 316 months. The indications for retransplantation: hemorrhagic shock in 1 patient and severe biliary complications (ischemic cholangiopathy in 2 patients and chronic rejection in 2 patients).
Analyses of the factors associated with MT revealed that only age > 30 years and history of partial hepatectomies were statistically significant (Table 5). The characteristics of the LA and the underlying disease were not significantly associated with MT.
| No HCC (n = 32) | HCC (n = 17) | P Value | |
|---|---|---|---|
| Sex | 0.54 | ||
| Female | 19 (59) | 12 (71) | |
| Male | 13 (41) | 5 (29) | |
| Age | 0.016 | ||
| ≤30 years | 22 (69) | 5 (29) | |
| >30 years | 10 (31) | 12 (71) | |
| GSD | |||
| Yes | 11 (34) | 5 (29) | 0.75 |
| No | 21 (66) | 12 (71) | |
| Vascular abnormalities | 0.23 | ||
| Yes | 3 (9) | 4 (24) | |
| No | 29 (91) | 13 (76) | |
| LA form | 0.38 | ||
| Massive | 20 (62) | 8 (47) | |
| Multifocal | 12 (38) | 9 (53) | |
| Surgical history | 0.013 | ||
| Yes | 10 (31) | 11 (65) | |
| No | 22 (69) | 6 (35) | |
| Hemorrhagic form | 0.46 | ||
| Yes | 9 (28) | 2 (12) | |
| No | 23 (72) | 15 (88) |
- NOTE: Data are given as n (%).
Discussion
LA is a benign disease, although 2 potential lethal risks may justify aggressive management: cases of the massive form with complications, such as iterative and/or severe bleeding, and cases showing MT.
This ELTR study is the largest cohort of LT in LA reported in the literature. Although we only identified 21 patients who received LT for adenomatosis without GSD in the English language literature, our series of 33 patients without GSD is the largest reported to date (Table 6).
| Reference | n | HCC explant transformation |
|---|---|---|
| Patients without GSD | ||
| Leese et al.30 (1988) | 1 | HCC |
| Marino et al.31 (1992) | 4 | No |
| Yoshidome et al.32 (1999) | 6 | ND |
| Chiche et al.3 (2000) | 2 | HCC |
| Yunta et al.33 (2001) | 1 | HCC |
| Fujita et al.27 (2006) | 1 | No |
| Wellen et al.17 (2010) | 1 | No |
| Dokmak et al.18 (2009) | 1 | ND |
| Di Sandro et al.34 (2009) | 1 | HCC |
| Vennarecci et al.21 (2013) | 1 | HCC |
| Gordon-Burroughs et al.35 (2014) | 1 | HCC |
| Fernández-Vega et al.36 (2014) | 1 | No |
| Patients with GSD IA | ||
| Matern et al.14 (1999) | 14 | No |
| Labrune15 (2002) | 1 | HCC |
| Lerut et al.13 (2003) | 1 | HCC |
| Franco et al.37 (2005) | 1 | HCC |
| Arikan et al.38 (2006) | 13 | No |
| Davis and Weinstein39 (2008) | 4 | No |
| Reddy et al.16 (2009) | 1 | HCC |
| Manzia et al.40 (2011) | 2 | HCC |
This study first described a population of transplanted patients to analyze the primary factors that lead to transplantation in current practice. Several interesting findings from the data emerged from our analyses. First, we observed a predominance of women (63%) but a lower incidence compared to the usual HCA population. The median age was 28 years (23-41), and more than half of the patients were younger than 30 years old. Notably, we expected to find a large number of massive and highly symptomatic forms, but we found that 75% of the transplanted patients were asymptomatic. Specifically, 43% of the patients presented multifocal disease, and the maximal size of the adenomas in 9 (18%) patients was ≤4 cm in liver explants, but this concerned patients who had undergone previous hepatectomies or patients with liver disease hampering the possibility of partial hepatectomies.
Concerning the indication of LT, because hemorrhage appeared to be the main indication in this disease in the case reports of the literature we expected to collect a higher percentage of patients transplanted for hemorrhage in this study. However, only 5 patients underwent transplantation for this complication: 1 patient had dramatic hemorrhage with a history of several previous partial resections, and 4 patients presented with underlying liver disease that contraindicated resection. Iterative severe or life-threatening hemorrhages are rare in LA. Moreover, we have an expanding armamentarium and experience in controlling bleeding angiographically, so this indication of LT should remain exceptional. It should be restricted to hemorrhages in case of failure or impossibility of embolization and/or partial hepatectomy, because of the location of the bleeding tumor, the number of hemorrhages, or the underlying liver disease.
The concern of malignancy happens to be, in this series, the major indication. Two situations were observed before transplantation: (1) a proven diagnosis of MT of 1 adenoma or (2) the fear of malignancy. In the first case, the transformation of 1 or more HCAs among lots of other nodules was a major concern, which explains the decision to perform LT. In 1 patient, this decision was likely too late because the disease was obviously progressive and HCC in the liver explant was very advanced.
The second situation, the fear of malignancy, is more debatable. Notably, we observed that 14 (75%) patients with GSD underwent transplantation for HCC suspicion or disease progression, although only 4 (25%) patients with GSD had HCC that was histologically confirmed before LT. Twelve (36%) patients without GSD underwent transplantation with histological proof of HCC, and 11 (33%) patients underwent transplantation for HCC suspicion or disease progression without histological proof. The risk of malignancy was clearly overestimated in the group with GSD because the literature considers this underlying disease as a risk factor for the MT of adenomas.8 These data are supported by a high frequency of β-CAT–mutated adenomas5, 7 in this pathology. We identified nearly 37 patients transplanted for MAs and GSD in the literature, but only 6 patients showed evidence of HCC in liver explants. Therefore, 85% of the patients who underwent transplantation for adenoma exhibited no HCC on liver explants, both in our study and in the literature. However, patients with GSD underwent transplantation earlier than other patients (median age was statistically lower), perhaps before MT.
MT was a frequent feature in patients with vascular disease associated with adenomas, as this presentation occurred in 4 of the 7 patients. However, it was not statistically relevant in our analyses of risk factors, likely because of the low number of these cases.
In the 9 patients with normal livers, (1) the median age was higher; (2) the form of LA, either massive or multifocal, was usually evolutive; and (3) the MT was multifocal in liver explants in 5 of the 9 patients.
These data demonstrate that follow-up must be recommended in LA to diagnose this lethal complication. However, implementation of this follow-up approach is not clearly established. Magnetic resonance imaging may be the best method to study increases in adenoma size or intratumoral modifications with apparitions of a portal washout, and biopsy or surgical exploration should also be discussed. The means and timing of follow-up in LA should likely be adaptable in this heterogeneous disease. Follow-up must be frequent and accurate in evolutive forms of adenomas and in patients with vascular disease or a β-CAT mutation, whereas follow-up can be less frequent in nonevolutive forms of the disease and in patients with small adenomas, particularly young patients, using ultrasonography for basic routine examinations. In addition, patient age >30 years was statistically associated with malignancy.
Analyses of biomolecular pathways revealed another interesting finding of this study. Biomolecular studies are not performed in all centers, which is why we did not obtain complete data for our 49 patients. Nevertheless, even when incomplete, these data are informative. The HNF1α subgroup was the most frequent feature in LA without liver disease or GSD, as reported in the literature; however, we also observed that different biomolecular subgroups of adenomas could be present in the same patient41 and that MT was possible across biomolecular subtypes, even in the HNF1α group (2 HCCs with underlying vascular disease of the liver). Therefore, more data are mandatory to better understand the link between HCC and biomolecular subtype, and it is clear that the risk of malignancy, in the case of adenomatosis, should not be stated only on the biopsy of 1 sole adenoma and does not exclusively concern β-CAT–mutated adenomas.
The clinical results of LT in this series were relatively good (41 patients alive and well), although 43% of the patients had undergone previous liver surgery and some had vascular abnormalities. Nevertheless, we must take into account the 3 operative deaths and 5 retransplantations, which is not negligible. Transplantation teams have to assess the potential technical difficulties of LT related to prior vascular or hepatic surgery. This past history may be a factor of operative bleeding and/or morbimortality. So, the performance of preventive LT is debatable, although the opposite risk is delayed transplantation after the MT of several tumors. The patients with MT who recurred after LT died of the disease. These 3 patients had massive LA with multifocal HCC identified on liver explants. One of these 3 patients had vascular abnormalities; 1 patient had GSD and vascular abnormalities; and 1 patient had no underlying liver disease (no GSD, no vascular abnormalities). However, all 3 patients had advanced HCC in liver explants with vascular involvement (obvious for 2 patients on preoperative imaging), which is a well-known very poor prognostic factor. Like in transplantation for HCC, diagnosis of vascular invasion on imaging should indeed be an absolute contraindication to transplantation. In conclusion, MT seems to be a good indication if it is not too advanced. This implies that in the case of suspicion of MT, diagnosis has to be rapidly confirmed in order to discuss LT without delay.
Even with this study, it is difficult or impossible to provide evidence-based recommendations for the indication of LT in LA. It should be discussed with caution because of the benign nature of this disease, the mortality of LT, and liver graft shortage. Some authors have stated that there is no indication for transplantation in LA,18 and this series demonstrates the opposite. Nevertheless, 1 major problem is the access to LT for these patients without cirrhosis and therefore with low Model for End-Stage Liver Disease (MELD) score. Several years ago (approximately before 2006-2007 in Europe), before MELD score was adopted for organ allocation, patients with LA had access to transplant quite easily. Today, with the European allocation's rules based on MELD score, those patients can be transplanted only as MELD exceptions or with living donors (3 in this series). That is why it is important to define the good candidates for transplantation. In most patients, it seems that the indication for LT will not depend on a single factor.
- Major criteria: HCC (biopsy or surgical specimen).
- Minor criteria:
- More than 2 serious (life-threatening) hemorrhages.
- More than 2 previous hepatectomies.
- Beta-mutated or inflammatory adenomas.
- Underlying liver disease (major steatosis or vascular abnormalities).
- Age > 30 years.
| Criterion | Number of Criteria Required | |
|---|---|---|
| Major criteria | Histological proof of MT in 1 (or more) adenoma | 1 |
| Minor criteria | More than 2 life-threatening hemorrhages More than 2 previous hepatectomies Beta-mutated or inflammatory adenomas Underlying liver disease Age > 30 years | 3 or more |
Our results highlight the crucial necessity of precise histological study of several adenomas (pathomolecular characteristics and mandatory proof of MT) and nontumoral livers before any discussion of LT. This study also supports that the restrictive definition of LA that excludes GSD or vascular disease is not justified even if these underlying pathologies influence the management of these patients.
In conclusion, this study demonstrates that LA is an extremely rare indication for LT, representing approximately 0.03% of LTs in Europe, which should be validated in cases of exceptionally aggressive types of this disease. Considering the characteristics of this population (previous liver surgery, liver underlying disease, vascular abnormalities), LT is associated with a significant rate of morbimortality. On the other hand, many complications of this benign disease can be handled by modern radiological or surgical approaches. If not, mostly in case of MT, and after assessment of the risk-benefit ratio, this European experience shows that LT can be justified. However, the access to LT depends on an expert decision because the system of graft allocation is based on the MELD score, and LA has no impact on liver function. Therefore, the identification of several criteria is mandatory to aid the decision-making process.
The strength of this multicenter series was the collection of information on extremely rare diseases. These results should serve as a catalyst to initiate prospective multicenter studies to address the many remaining issues of this rare disease, such as the identification of high-risk patients, prediction of malignancy, and the timing of LT. A prospective worldwide registry of patients transplanted for LA, with extensive study of history, pretransplant modern imaging, and precise pathological examination of the explanted liver with biomolecular characterization would be the best way to progress in that heterogeneous and complex rare disease.