Impaired physical function following pediatric LT
This project was supported by grant number R01 HD045694 of the National Institute of Child Health and Human Development and grant number U01 DK061693 of the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health.
Potential conflict of interest: Nothing to report.
The list of participating centers is included in the supporting material.
Abstract
The purpose of this article is to investigate the spectrum of physical function of pediatric liver transplantation (LT) recipients 12-24 months after LT. Review data were collected through the functional outcomes group, an ancillary study of the Studies of Pediatric Liver Transplantation registry. Patients were eligible if they had survived LT by 12-24 months. Children ≥ 8 years and parents completed the Pediatric Quality of Life Inventory™ 4.0 generic core scales, which includes 8 questions assessing physical function. Scores were compared to a matched healthy child population (n = 1658) and between survivors with optimal versus nonoptimal health. A total of 263 patients were included. Median age at transplant and survey was 4.8 years (interquartile range [IQR], 1.3-11.4 years) and 5.9 years (IQR, 2.6-13.1 years), respectively. The mean physical functioning score on child and parent reports were 81.2 ± 17.3 and 77.1 ± 23.7, respectively. Compared to a matched healthy population, transplant survivors and their parents reported lower physical function scores (P < 0.001); 32.9% of patients and 35.0% of parents reported a physical function score <75, which is > 1 standard deviation below the mean of a healthy population. Physical functioning scores were significantly higher in survivors with optimal health than those with nonoptimal health (P < 0.01). There was a significant relationship between emotional functioning and physical functioning scores for LT recipients (r = 0.69; P < 0.001). In multivariate analysis, primary disease, height z score < –1.64 at longterm follow-up (LTF) visit, > 4 days of hospitalization since LTF visit, and not being listed as status 1 were predictors of poor physical function. In conclusion, pediatric LT recipients 1-2 years after LT and their parents report lower physical function than a healthy population. Findings suggest practitioners need to routinely assess physical function, and the development of rehabilitation programs may be important. Liver Transplantation 22 495-504 2016 AASLD
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- CI
-
- confidence interval
-
- FOG
-
- functional outcomes group
-
- GGT
-
- gamma glutamyltransferase
-
- GI
-
- gastrointestinal
-
- HRQOL
-
- health-related quality of life
-
- ICC
-
- intraclass correlation coefficient
-
- ICU
-
- intensive care unit
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- LTF
-
- longterm follow-up
-
- PedsQL™
-
- Pediatric Quality of Life Inventory
-
- PELD
-
- Pediatric End-Stage Liver Disease
-
- SD
-
- standard deviation
Studies have shown pediatric liver transplantation (LT) recipients have significantly lower health-related quality of life (HRQOL) than the healthy population.1, 2 One domain of HRQOL that may be specifically impaired in pediatric LT recipients is physical function, which includes a child's strength, energy level, perception of pain, and ability to perform daily activities. Pediatric LT recipients are at risk for physical impairment for a variety of reasons. The majority of patients enter transplant with advanced malnutrition, growth arrest, muscle wasting, and deconditioning secondary to chronic liver disease.3 In addition, many patients spend an extended period of time in the intensive care unit (ICU) before and after transplant which may result in ICU-acquired weakness or critical illness polyneuropathy, which is known to contribute to long-lasting functional impairment.4 Limitations in physical function are important not only because they may impair a child's ability to participate in daily life, but also studies in adults have shown that decreased performance on measures of physical capacity are associated with poor adherence to medication, increased morbidity, and increased mortality. In addition, these studies show a correlation between poor physical health and mental well-being.5, 6
Pediatric recipients of heart, lung, and kidney transplants are known to have lasting impairments in physical function. In heart, lung, and heart-lung transplant recipients, physical work capacity is significantly reduced (43%-64%) compared with healthy controls.7 Pediatric kidney transplant recipients have lower cardiorespiratory fitness,8, 9 treadmill test times,8 and VO2 (oxygen consumption) peak9 compared to age-matched healthy children. Less is known about the fitness levels in pediatric LT recipients.2 In a small study of 29 pediatric LT recipients at 51.6 ± 48.3 months after transplant, only 35% of the LT group achieved standards for muscle strength, and none achieved the standards for progressive aerobic cardiovascular endurance run.10
To the best of our knowledge, there are no studies to date specifically focusing on patient and parental perception of physical function after LT. To address this gap in the empirical literature, the purpose of the present study was to conduct a multicenter analysis that examined the degree of physical function at a specific point in time after pediatric LT. We chose the time frame of 12-24 months after transplant because we assumed this is when most children should be completely recovered from their initial transplant operation: their surgical wound has healed; they are eating a full diet; and they are back in school and involved in activities. The specific study goals were (1) to understand the spectrum of physical function of children at 12-24 months after LT; (2) to compare the physical function of children after LT to matched healthy children; and (3) to assess whether transplant survivors with “optimal” health have physical function that differs from those survivors with “nonoptimal” health.
Patients and Methods
The Studies of Pediatric Liver Transplantation registry was founded in 1995 as a prospective data repository for children undergoing LT in the United States and Canada.11 A functional outcomes group (FOG) was conducted as an ancillary study, leveraging the infrastructure and prospective data set of the registry. The data collection methods for this ancillary study have been previously described.12 Briefly, patient and parent/guardian dyads were recruited during a routine follow-up visit at their transplant center between June 2005 and December 2009. Both child self-report and parent proxy report were obtained for pediatric LT patients of ages 8-18 years, whereas parent proxy report alone was obtained for LT patients of ages 2-7 years. The parent/guardian and patient (if age 8 years or older) completed an age-specific version of the Pediatric Quality of Life Inventory (PedsQL) 4.0 generic core scales. The generic core scales includes 8 questions that assess physical functioning and comprise the physical functioning scale: I have problems with walking more than 1 block, running, participating in sports/exercise/play, lifting something heavy, bathing, doing chores, having hurt or aches, and low energy level. A 5-point Likert scale is used with 0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; and 4 = almost always a problem. Items are reverse-scored and linearly transformed to a 0-100 scale so that higher scores indicate better HRQOL (fewer physical problems). Although data on overall HRQOL in pediatric LT recipients have been reported using this database in the past,12, 13 there have been no prior studies specifically analyzing data on patient and parental perceptions of physical function. For this study, we analyzed data from patients in their second year of rehabilitation between 12 and 24 months after transplant. We assume by 1 year after transplant the majority of patients should have a healed wound, stable graft function, and no recent returns to the operating room for vascular or biliary repairs. The analysis included only data from patients for whom longterm follow-up (LTF) forms in the registry were completed. The LTF form contains past as well as present demographic and medical variables. The institutional review boards at participating centers approved the study, and written informed consent was obtained from all parents or guardians before participation. Assent was obtained from children as required by individual institutions.
The primary outcome measure was the physical functioning scale score from the PedsQL 4.0 child self-report survey. Secondary outcomes included the physical functioning scale score from the PedsQL 4.0 parent proxy report and the 8 individual physical health questions from both the PedsQL 4.0 child self-report and the PedsQL 4.0 parent proxy report. These outcomes were compared to a sample of healthy children (n = 1658) matched by age, sex, and race/ethnicity to the LT sample. The cohort of healthy children was derived from the previously conducted PedsQL 4.0 generic core scales initial field test14 and a statewide State Children's Health Insurance Program evaluation.15 To explore the relationship between emotional functioning and physical functioning, we also examined the emotional functioning scale score from the PedsQL 4.0 child self-report survey and parent proxy report in LT recipients.
To set apart the outcomes of patients with routine postoperative courses from those with significant ongoing complications, we compared the physical health of transplant survivors with “optimal” health to those transplant survivors with “nonoptimal” health. We modeled the definition of optimal posttransplant health after studies by Ng et al.13 and Sundaram et al.16 “Optimal” health in our posttransplant population was defined as the absence of posttransplant lymphoproliferative disease, vascular complications, biliary tract complications, reoperations, or diabetes in the past 6 months. Optimal survivors could not have received any supplemental nutrition or seizure medications since the last LTF visit. In addition, they had a total bilirubin < 2.0 mg/dL, alanine aminotransferase (ALT) < 100 IU/L, and gamma glutamyltransferase (GGT) < 200 IU/L.
Statistical analyses
Descriptive statistics were generated for demographic and clinical patient variables and are reported as median and interquartile range (IQR) for continuous variables and n (%) for categorical variables. Mean PedsQL physical functioning scale scores were calculated for all transplant survivors and the matched healthy sample. Independent sample t tests were used to compare mean PedsQL physical functioning scale scores between posttransplant patients and the matched healthy sample. The relationship between the PedsQL physical functioning scale scores and PedsQL emotional functioning scale scores was assessed with Spearman's rank correlation coefficient. To determine the magnitude of the differences, effect sizes were calculated.17 Effect sizes for differences in means are designated as small (0.20), medium (0.50), and large (0.80) in magnitude. Mean PedsQL physical functioning scale scores were also compared between transplant recipients with optimal versus nonoptimal health using independent sample t test. Chi-square goodness of fit statistics were used when comparing categorical responses to the individual questions comprising the physical functioning scale. Agreement between child self-report and parent proxy report was determined through intraclass correlation coefficients (ICCs).18 ICCs are designated as poor to fair agreement (<0.40), moderate agreement (0.40-0.60), good agreement (>0.60-0.80), and excellent agreement (>0.80-1.00).
Varni et al.15 have identified significant cutoff points for those “at risk” for impaired quality of life, determining in a large pediatric population that 1 SD below the mean of the total sample was a clinically meaningful measure of impaired quality of life as those scores were comparable with patients who had a severe chronic health condition. Therefore, we dichotomized a score as “poor” if the total physical function score was more than 1 SD below the mean for our healthy matched population. All other scores were considered “good.”1
Univariate regression analysis was conducted to identify demographic and health status variables associated with impairments in the PedsQL physical functioning scale scores. Variables chosen for univariate analysis were available through the FOG data set, had conceptual and/or empirical support, had <25% missing data, and had at least 10% of patients in each of the 2 categories. All factors were categorical unless otherwise noted. Separate analyses were conducted for the child self-report and parent proxy report, given the possibility that different predictors could emerge. The logistic regression models were generated using predictor variables detailed in Supporting Table 1, which included demographic, pretransplant, and posttransplant variables that were significant in univariate analysis at a level of P ≤ 0.10. Because age at transplant, interval from transplant to FOG visit, and age at FOG visit were all interrelated, we chose to use only the most significant factor in the modeling. The Hosmer-Lemeshow goodness of fit test was used to evaluate the logistic model formulated. Statistical analyses including the logistic regression modeling were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC). The ICCs were generated with SPSS 12.0 (SPSS Inc., Chicago, IL).
| Demographic and Clinical Variables | Value (n = 263) |
|---|---|
| Age at transplant | |
| 0-12 months | 54 (20.5) |
| 1-4 years | 80 (30.4) |
| 5-12 years | 84 (32.0) |
| 13-18 years | 45 (17.1) |
| Sex, female | 139 (52.9) |
| Race/ethnicity | |
| White | 145 (55.1) |
| Black | 36 (13.7) |
| Hispanic | 49 (18.6) |
| Other | 30 (11.4) |
| Unknown | 3 (1.2) |
| Primary disease | |
| Biliary atresia | 84 (32) |
| Other cholestatic | 35 (13.3) |
| Fulminant liver failure | 38 (14.4) |
| Metabolic | 57 (21.7) |
| Other | 49 (18.6) |
| Retransplant, Yes | 27 (10.3) |
| Status at time of transplant | |
| ICU | 65 (24.7) |
| Hospitalized, not in the ICU | 36 (13.7) |
| At home | 162 (61.6) |
| Current/past failure to thrive at time of transplant | |
| Yes | 91 (34.6) |
| No | 158 (60.1) |
| Unknown | 14 (5.3) |
| Supplemental nutrition at the time of transplant | |
| Yes | 72 (27.4) |
| No | 188 (71.5) |
| Unknown | 3 (1.1) |
| Health insurance status | |
| US federal or state-funded services | 79 (30.0) |
| Provincial government | 24 (9.1) |
| HMO or managed care | 47 (17.9) |
| Private insurance | 91 (34.6) |
| Other | 12 (4.6) |
| Unknown | 10 (3.8) |
| Months on waiting list | 2.2 (0.6-6.2) |
| Calculated PELD score at transplant | 8.4 (–0.8 to 21.6) |
| Transplant length of stay, days | 20.0 (14.0-29.0) |
| Weight z score at transplant | −0.6 (–1.9 to 0.2) |
| Height z score at transplant | −1.2 (–2.3 to –0.3) |
- NOTE: Data are given as n (%) or median (IQR).
Results
There were 263 patients who met criteria for the study. There were 98 available child self-report forms and 260 available parent/guardian proxy report forms. Proxy respondents included 84% mothers, 14% fathers, and 2% guardians. Both child self-report and parent/guardian proxy reports were available for 95 patients; 52.9% of patients were female, and 55.1% were white. The median age at transplant was 4.8 years (interquartile range [IQR], 1.3-11.4 years). The median age at time of survey was 5.9 years (IQR, 2.6-13.1 years). All patients underwent transplantation in the period from 2003 to 2007. Approximately one-third of patients (31.9%) underwent transplantation for biliary atresia; 89.7% of the patients were receiving their first LT. Of the 52 patients listed status 1, 19 (36.5%) underwent transplantation for fulminant liver failure; 10 (19.2%) for biliary atresia; 9 (17.3%) for metabolic disease; and 14 (26.9%) for cholestasis, cirrhosis, or other underlying diagnosis. At the time of LT, 25% of patients were in the ICU. Of the 38 patients diagnosed with fulminant liver failure, 24 (63.2%) were in the ICU at the time of transplant; 4 (10.5%) were hospitalized but not in the ICU; and 10 (26.3%) were not hospitalized. The mean calculated Pediatric End-Stage Liver Disease (PELD) score at the time of transplant was 10.5 ± 14.2. Demographic and clinical characteristics of the patient sample are included in Table 1.
The mean physical functioning score on child self-reports was 81.2 ± 17.3; 32.9% of patients reported a physical functioning score < 75, which is 1 SD below the mean of a published healthy sample; 19.6% of patients reported a perfect physical summary score of 100. The mean physical functioning score on parent proxy reports was 77.1 ± 23.7; 35.0% of parent proxies reported a physical function score < 75 and 20.8% of parent proxies reported a perfect physical summary score of 100. There was no significant differences in mean parental reported scores for children < 8 years of age and for those 8 years or older. Physical function scores are reported in Table 2 and displayed in Fig. 1A,B.
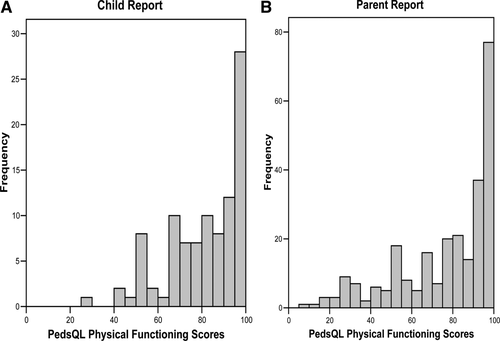
Distribution of scores in the PedsQL physical functioning scale among the LT population. (A) Child report (n = 97); (B) parent report (n = 260).
| Scale | LT (a) | Non-optimal Survivors (b) | Optimal Survivors (c) | Healthy Sample (d) | Mean Differences | Effect Size, a versus d | Effect Size, c versus d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||||
| Child self-report | |||||||||||||||
| Total score | 98 | 77.6 | 16.1 | 35 | 73.8 | 17.5 | 63 | 79.7 | 14.9 | 1010 | 84.7 | 12.6 | a < da c < db | 0.55 | 0.39 |
| Physical functioning | 97 | 81.2 | 17.3 | 35 | 74.7 | 19.9 | 62 | 84.9 | 14.7 | 1010 | 88.6 | 13.2 | a < da c < dc | 0.54 | 0.28 |
| Parent proxy report | |||||||||||||||
| Total score | 261 | 75.8 | 17.9 | 105 | 70.4 | 18.6 | 156 | 79.5 | 16.5 | 1658 | 86.5 | 12.4 | a < da c < da | 0.81 | 0.55 |
| Physical functioning | 260 | 77.1 | 23.7 | 105 | 69.1 | 26.5 | 155 | 82.5 | 19.9 | 1658 | 89.4 | 14.8 | a < da c < da | 0.76 | 0.45 |
- NOTE: Effect sizes are designated as small (0.20), medium (0.50), and large (0.80).
- a P < 0.001.
- b P < 0.01.
- c P < 0.05.
Relationship Between Physical Functioning and Emotional Functioning
The mean emotional functioning score on child self-reports in LT recipients was 76.7.2 ± 20.8. The relationship between the emotional functioning score and the physical functioning score for LT recipients was r = 0.69 (P < 0.001). The mean emotional functioning score on parent proxy reports was 74.7 ± 18.5. The relationship between the emotional functioning score and the physical functioning score for parent proxies of LT recipients was r = 0.43 (P < 0.001).
Comparison to the Healthy Sample
There were 1658 healthy children in the matched sample used as a comparison to the children who received LTs. The mean and SDs of the PedsQL 4.0 generic core scales for patients who underwent pediatric LT and the healthy comparison population are presented in Table 2. Patients who had undergone pediatric LT had significantly lower self-report PedsQL 4.0 total scores and physical functioning scale scores (P < 0.001) with an effect size of 0.55 and 0.54, respectively, representing medium effect sizes. Likewise, parents/guardians of children who underwent pediatric LT reported significantly lower mean PedsQL 4.0 total scores and physical functioning scores than parents/guardians of the healthy population (P < 0.001) with an effect size of 0.81 and 0.76, respectively, representing medium to large effect sizes.
Scores of 3 and 4 (“I usually have this problem” or “I always have this problem”) on individual physical function questions were considered evidence of a significant/pervasive problem that affected the child on a daily basis. There were significant differences in the percentages of poor scores (a score of 3 or 4) on questions about running, playing, lifting, and doing chores in healthy children compared to LT recipients (Fig. 2A). There were significant differences between parent proxy responses for healthy children and parent proxy responses for LT recipients on all 8 individual physical function questions (Fig. 2B).
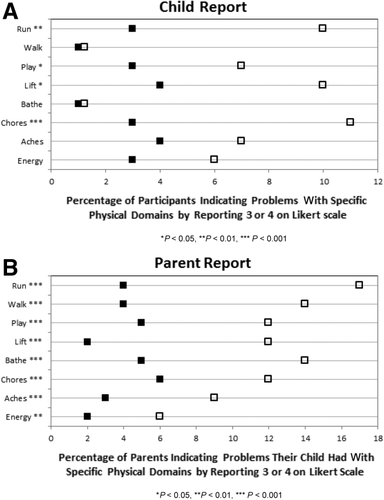
Impairment in individual physical health questions by indicating 3 = “often a problem” or 4 = “almost always a problem” on a 0-4 Likert scale. (A) Child report (n = 97); (B) parent report (n = 260).
Comparison of Survivors With Optimal Health to Survivors With Nonoptimal Health
Optimal health (absence of posttransplant lymphoproliferative disease, reoperations, vascular complications, biliary complications, diabetes, supplemental nutrition, seizure medication, total bilirubin < 2.0 mg/dL, ALT < 100 IU/L, and GGT < 200 IU/L) was seen in 158 (60%) transplant recipients. Of excluded patients, 14 patients had posttransplant lymphoproliferative disease; 16 had undergone a reoperation; 19 had vascular complications; 33 had biliary complications; 9 had diabetes; 15 were on supplemental nutrition; 15 were on seizure medications; 8 had elevated total bilirubin; 23 had elevated ALT; and 17 had elevated GGT. There were 62 (64%) patient reports and 154 (59%) parent proxy reports from survivors with optimal health. There were 35 patient reports and 105 parent proxy reports from survivors with nonoptimal health. The child self-report total physical function scores were significantly higher in posttransplant patients with optimal health (84.9 ± 14.7 versus 74.7 ± 19.8; P = 0.01). Likewise, parent proxy report total physical function scores were significantly higher in those parents with children who had optimal posttransplant health (82.5 ± 19.9 versus 69.1 ± 26.5; P ≤ 0.001).
Comparison of Child Self-Report and Parent Proxy Report
There were 95 child self-report and parent proxy report pairs available. ICCs were determined on this subset to assess agreement in perception of physical function by child self-report and parent proxy report. Overall, the ICC for physical function was 0.68 demonstrating good agreement. When individual physical function questions were assessed and grouped by age (8-12 year olds and 13-18 year olds), there was moderate agreement between child self-report and parent proxy reports (Table 3), with the notable exception of questions about doing chores (ICC = 0.47 in 8-12 year olds and 0.13 in 13-18 year olds).
| ICC, 8-12 year olds (n = 32 pairs) | ICC, 13-18 year olds (n = 63 pairs) | |
|---|---|---|
| Total physical health score | 0.79a | 0.56a |
| Walking | 0.48b | 0.77a |
| Running | 0.69a | 0.75a |
| Participating in sports activity or exercise | 0.69a | 0.72a |
| Lifting something heavy | 0.72a | 0.71a |
| Taking a bath or shower | 0.39b | 0.63a |
| Doing chores like picking up toys | 0.47b | 0.13 |
| Having aches | 0.64a | 0.59a |
| Low energy level | 0.61a | 0.60a |
- NOTE: ICCs are designated as ≤ 0.40 poor to fair agreement, 0.41-0.60 moderate agreement, 0.61-0.80 good agreement, and 0.81-1.00 excellent agreement.
- a P < 0.001.
- b P < 0.01.
Demographic and Medical Predictors of PedsQL Physical Functioning Score
Univariate analysis was performed to understand the relationship between child self-reports of poor physical function (physical summary score < 75) and demographic and medical factors among patients surviving LT. Risk factors found to be significant at the P ≤ 0.10 level were sex and liver dysfunction (as defined by having a total bilirubin ≥ 2.0 mg/dL or an ALT ≥ 100 IU/L or GGT ≥ 200 IU/L). See Supporting Table 1 for a list of all factors used in the univariate analysis. Of the 97 child self-reports with a physical functioning score, 93 had complete data for all of those variables and were included in the generation of the logistic regression model. On multivariate regression analysis of child self-reports, no variables were found to be significant.
Univariate analysis was also performed to understand the relationship between parent proxy reports of poor physical function (physical summary score < 75) and demographic and medical factors. Risk factors found to be significant at the P ≤ 0.10 level were age at transplant, age at FOG visit, primary disease, ever status 1, height z score < –1.64 at LTF visit, liver dysfunction (as defined by having a total bilirubin ≥ 2.0 mg/dL or an ALT ≥ 100 IU/L or GGT ≥ 200 IU/L), and days hospitalized since the last follow-up visit (categorized as 4 or ≥ 4 days). See Supporting Table 1 for a list of all factors used in univariate analysis. Of the 260 parent reports with a physical functioning score, 247 reports had complete data for all of these variables and were included in the generation of the logistic regression model (Table 4). On multivariate regression analysis of parent proxy reports, primary disease other than biliary atresia, history of being listed other than status 1, height z score < –1.64 at last LTF visit, and >4 days hospitalized since the last LTF visit were associated with poor physical functioning (score < 75; Table 4). The computed chi-square was 5.1 with a P value of 0.65, indicating an acceptable goodness of fit.
| Variable | Comparison Group | Reference Group | Final Model Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Primary disease | Other | Biliary atresia | 2.1 (1.1-3.8) | 0.02 |
| Ever status 1 | Yes | No | 0.4 (0.2-0.9) | 0.03 |
| Height z score < –1.64 at LTF visit | Yes | No | 1.9 (1.1-3.5) | 0.03 |
| Days hospitalized since the last LTF visit | >4 days | ≤4 days | 1.8 (1.1-3.2) | 0.03 |
Discussion
The results of this multicenter, prospectively collected, patient-reported outcomes study indicate pediatric LT recipients 1-2 years after transplant and/or their parent proxies report significantly lower physical function scores than a healthy population; 33% of children and 35% of parent proxies report “poor” physical health scores (score < 75) after 1 year after LT. Physical function scores correlated to emotional function scores in LT recipients and their parent proxies. Although these scales measure 2 distinct constructs, the observed correlation implies that physical function is likely impacted by psychological distress such as anxiety, depressed mood, and sleep disorders. Thus, clinicians should couple psychosocial and physical assessments in gauging the level of rehabilitation following transplant. As expected, patients who met our definition of optimal health reported better physical function scores than those with ongoing complications and comorbidities. However, more importantly, physical function remains impaired 1-2 years after LT in a significant proportion of children who otherwise appear healthy. These findings suggest that current approaches to physical rehabilitation following LT in children may be inadequate.
In this study, primary disease other than biliary atresia, height z score < –1.64 at last LTF visit, >4 days of hospitalization since the last LTF visit, and being listed other than status 1 were found to be predictive of poor physical function on parent reports. Although it might be expected that ongoing hospitalizations and poor growth would predict poor physical function, it was interesting to find that an underlying diagnosis of biliary atresia and a history of being listed as status 1 identified a group with a lower risk of poor physical function. Patients with a primary disease other than biliary atresia had 2.1 times greater odds of having a physical function score of <75 than patients with biliary atresia. Although specific data on neurologic status were not collected, one could speculate that children transplanted for metabolic diseases may have underlying developmental delay that would limit physical function. Likewise, children transplanted for malignancy may receive chemotherapy before and after transplantation that would limit physical function. Recent studies have also shown that children with biliary atresia are less likely to have growth impairment following transplant compared to those with metabolic and cholestatic diseases.19, 20 The interrelationship between physical growth and physical function warrants further exploration because such studies may inform posttransplant rehabilitation strategies that leverage improvements in muscle mass or linear growth to maximize the trajectory of improvements in physical outcomes.
Patients who had a history of being listed as status 1 were half as likely to be in the lowest quartile for physical function as compared to those who, presumably, never met status 1 listing criteria. The majority (63%) of patients listed as status 1 had a primary disease classified as chronic (46%) or metabolic (17%) liver disease. Patients with metabolic liver diseases, such as urea cycle defects qualify for status 1 listing with the goal of providing transplantation before they sustain irreversible brain injury. If this is effectively accomplished, they may in fact have better outcomes than patients who suffer the complications of chronic liver disease while awaiting transplantation at a lower status. Children with cirrhosis who develop life-threatening complications of portal hypertension, such as massive gastrointestinal (GI) bleeding also qualify for status 1 listing, which may truncate the progression of muscle wasting and growth failure that is characteristic of end-stage liver failure. It is important to note there were shifts in the criteria for status 1 listing over the time period of this study. Even now, there is great variability in patients who qualify for status 1 listing. Some patients in this group are critically ill in the ICU with acute liver failure while others are at home with stable metabolic disease. Although all patients who have status 1 listing have urgent need for LT, there may be differences in longterm physical function in subgroups of these patients, which this study was not able to capture.
Self-report of physical function by transplant recipients showed only moderate agreement with parent proxy report. This is consistent with previous literature documenting imperfect agreement between self-report and proxy report on HRQOL measurements of children with chronic health conditions.21-23 Parents and children often focus on different aspects of the illness after transplant,24, 25 and there are often dissimilarities in reports of feelings, internalizing symptoms (sadness, pain, fatigue, GI symptoms), and emotional or social functioning.23 It was surprising in our study to find good agreement between patients and their parents on more subjective questions about energy levels and aches, but poor agreement on questions about ability to perform definitive tasks such as bathing or doing chores. Interestingly, our study found better agreement between adolescents and their parents than 8-12 year olds and their parents. Some prior studies have reported wide variation in agreement between adolescent and parent assessments of quality of life,26 whereas others have found that adolescents and their proxies agree on questions about physical function but differ on questions about emotional, social, and school function.27 Perhaps physical function is one domain of HRQOL where parents are able to accurately observe what their adolescent child is experiencing. The discordance between patient and parent reports stresses the importance of questioning both patients and their guardians regarding physical health during posttransplant follow-up visits.
The level of physical function impairment reported in this study is likely an underestimate. Studies have shown both practitioners and patients underreport their physical deficits. In studies of children who have undergone hematopoietic stem cell transplant, objective measurements of physical performance show that 40%-62% of patients have dysfunction; however, clinicians only estimate 10% of survivors have physical health impairment, and 17% of patients report self-impairment.28, 29 Likewise, prior studies have shown pediatric transplant recipients and their parents often underreport problems such as learning disabilities,30 psychosocial functioning,31 and depression.24 A limitation of this study is that we did not collect objective data to measure physical health and performance (treadmill test time, hand grip strength, progressive aerobic cardiovascular endurance run testing, VO2 peak testing, school attendance, participation in organized sports) and correlate it with the self-reports. In future studies, it would be helpful to obtain objective measurements of physical health at LTF visits. Another important future direction for this area of investigation is to determine the longitudinal course of improvement in physical function and to establish the impact that physical function levels in the early posttransplant period have on physical outcomes in LTF and adulthood.
In conclusion, although half of pediatric LT recipients report normal physical function by 1-2 years after transplant, a significant subset of patients continue to report problems with fatigue, aches, and stamina representing an important at-risk group for consideration for additional intervention. Transplant hepatologists and other practitioners taking care of these children need to be cognizant of the potential for physical impairment, especially in patients with primary diagnoses other than biliary atresia, and those who require ongoing hospitalizations. Physical function should be assessed by self-report and parental proxy questionnaires and by objective measures. Practitioners should also assess emotional health in patients with impaired physical function. Development of rehabilitation programs to facilitate optimal physical health and proactive use of these programs in transplant recipients may reduce physical impairments and improve overall HRQOL outcomes for transplant recipients in the future. Future studies are needed to follow these children prospectively to better understand how long these identified deficits persist.
Acknowledgment
The authors thank the centers participating in the Studies of Pediatric Liver Transplantation FOG.