The friendly incidental portal vein thrombus in liver transplantation
Funding sources: Nothing to report.
Potential conflict of interest: Nothing to report.
Abstract
Improved outcomes have been shown in liver transplantation (LT) with portal vein thrombosis (PVT). However, PVT is still discovered incidentally during surgery despite careful preoperative imaging. Data are limited comparing the outcomes of incidental PVT with PVT diagnosed via preoperative imaging before LT. This study aims to compare the overall outcomes of patients with PVT. From 2008 to 2012, 369 patients had LT, and 58 patients with PVT were identified. They were divided into those with non-PVT (group 0; n = 311), preoperatively identified PVT (group 1; n = 28), and incidental PVT (group 2; n = 30). The demographics, characteristics, preoperative assessment, and postoperative outcomes were compared. A survival analysis was also performed. Baseline characteristics and preoperative evaluations of all 3 groups were comparable (P > 0.05) except for Model for End-Stage Liver Disease score, tumor status, platelet levels, and serum bilirubin. A multivariate analysis only showed a high serum bilirubin level to be a predictor of PVT (P = 0.004; odds ratio, 3.395; 95% confidence interval, 1.467-7.861). Postoperative outcomes were also comparable (P > 0.05). Compared to group 2, group 1 had more patients with a Yerdel classification of 3 or 4 with more extensive surgical intervention required (P = 0.02). The survival analysis in all 3 groups was comparable with 5-year survival rate of 87.4%, 84.6%, and 91.8% in group 0, 1, and 2, respectively (P = 0.66). In conclusion, recipients with PVT undergoing LT can have similar outcomes as the non-PVT patients even if PVTs were discovered incidentally. Discovery of incidental PVT only requires thrombectomy with no substantial change of treatment strategy, and the outcome is not adversely affected because most incidental PVTs are of a lower Yerdel grade. Preoperative imaging is useful to identify those with a higher Yerdel grade to allow planning of surgical strategy during transplantation. Liver Transpl 21:944-952, 2015. © 2015 AASLD.
Abbreviations
-
- CT
-
- computed tomography
-
- DDLT
-
- deceased donor liver transplantation
-
- HCC
-
- hepatocellular carcinoma
-
- HV
-
- hepatic vein
-
- INR
-
- international normalized ratio
-
- IVC
-
- inferior vena cava
-
- LDLT
-
- living donor liver transplantation
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- OR
-
- odds ratio
-
- OS
-
- overall survival
-
- PTLD
-
- posttransplant lymphoproliferative disease
-
- PV
-
- portal vein
-
- PVT
-
- portal vein thrombosis
-
- SMV
-
- superior mesenteric vein
-
- U/S
-
- ultrasound
Recent studies have shown that portal vein thrombosis (PVT) is feasible in liver transplantation (LT) compared to the past, where it was once considered a contraindication because of technical difficulty and was associated with a high mortality and morbidity. The early era of PVT management in LT showed significant mortality and morbidity. However, with improved surgical techniques, technical capabilities, and surgical innovations, we have seen a marked improvement in the management of PVT with lower mortality and morbidity as shown in recently published data.1, 2
The incidence of PVT has been reported to be approximately 2%-26% in various studies.3, 4 Therefore, a good preoperative plan and strategy for identifying patients undergoing LT with PVT are vital to ensure good perioperative outcome and long-term consequences. Despite careful preoperative planning with the various imaging techniques that are available, PVT is at times discovered incidentally during surgery and hence, may affect the operative strategy and outcome.5 Since there are limited data to date comparing the outcome of PVT when encountered incidentally during the intraoperative period with PVT diagnosed before surgery by preoperative imaging, this study aims to compare the overall outcomes of patients with PVT in these 2 groups based on data from a center with decades of experience in LT.
PATIENTS AND METHODS
Patient Selection
From January 2008 to December 2012, there were 369 patients who underwent LT at the Department of Surgery, Queen Mary Hospital, The University of Hong Kong. Among these patients, 182 (49.3%) patients had deceased donor liver transplantation (DDLT) and 187 (50.7%) patients had living donor liver transplantation (LDLT).
Analyses were performed in those who were diagnosed with PVT during LT from our prospectively collected data. PVT is usually not a contraindication for LT in our center. Recipients with PVT usually had conventional thrombectomy performed with some having to undergo a thromboendovenectomy or an interposition graft. A flowmeter using the Transonic Flow-QC (Transonic Systems, Inc., Ithaca, NY) is usually used to measure portal vein flow after PVT removal to ensure adequate portal flow with a portal venography performed via the inferior mesenteric vein occasionally.
All patients undergoing LT will have standard radiological imaging before transplantation. The commonly used modality for imaging was a contrast-enhanced computed tomography scan, and a few of the patients had transabdominal ultrasound or magnetic resonance imaging as part of their workup.
All patients who were diagnosed with evidence of PVT during radiological imaging were included in the study and defined as group 1. Patients with no evidence of PVT noted during radiological imaging but who were intraoperatively found to have PVT were included as well; this group of patients was defined as those with “incidental” PVT or group 2. For comparative reasons, the remaining patients with no PVT were known as group 0.
Classifying on the Basis of the Yerdel Classification
The standard classification of PVT commonly used is the Yerdel classification according to the extent of thrombosis and is described as follows: grade 1, minimally or partially thrombosed portal vein in which the thrombosed area is mild or at the most confined to <50% of the vessel lumen with or without minimal extension into the superior mesenteric vein (SMV); grade 2, where >50% occlusion of the portal vein (PV), including total occlusions, with or without minimal extension into the SMV; grade 3, complete thrombosis of both PV and proximal SMV, whereas distal SMV is open; and grade 4, complete thrombosis of the PV and proximal as well as distal SMV.2
In this study where incidental PVT was also identified, Yerdel grades 1 and 2 were classed together for comparative reasons because it was difficult to distinguish between these 2 grades intraoperatively.
Outcome Measurement, Statistical Analysis, and Overall Survival (OS) Analysis
The baseline characteristics and demographics of patients undergoing transplantation between groups 1 and 2 as well as the group with no PVT were compared. The preoperative, intraoperative, and postoperative variables along with the type of management undertaken were analyzed and compared. Also, patient and graft survival analyses were also performed.
Categorical variables were compared using Pearson chi-square or Fisher's exact test when appropriate. Continuous variables were expressed as median with range and were compared using a Kruskal-Wallis test. The likelihood of developing PVT based on patients’ characteristics was also determined by a multivariate analysis by the logistic regression method and expressed as an odds ratio (OR) with a 95% confidence interval (CI).
The Kaplan-Meier method was performed for both groups where the 1-, 3-, and 5-year OS was determined for both patient and graft survival. Further comparison of OS between the groups was performed with the log-rank test.
Statistical analysis was performed using SPSS, version 21.0 (IBM, Armonk, NY), and a P value of <0.05 was considered to be significant.
RESULTS
Incidence, Demographics, and Baseline Characteristics
In this 5-year review, there were 58 patients who were identified with PVT with an incidence of 15.7%. Among the 58 patients, 28 (48.3%) patients had PVT identified preoperatively via routine imaging (group 1), and 30 (51.7%) patients had PVT diagnosed incidentally during surgery (group 2). The remaining 311 patients who underwent LT had no PVT (group 0).
Table 1 shows the demographics and baseline characteristics between groups 0, 1, and 2. All the groups were almost identical and comparable. There was no significant difference in terms of sex, age, body weight, Child-Pugh grading, liver cirrhosis status, and etiology. Only the Model for End-Stage Liver Disease (MELD) score (P = 0.003) and whether a LT was performed for tumor-related diseases (P < 0.001) showed significance. The majority of LTs performed for tumors were hepatocellular carcinoma (HCC). Only 1 patient had LT performed for cholangiocarcinoma. Because there were more patients in group 2 having LTs done for HCC, it is not surprising that group 2 also had a lower median MELD score.
| Non-PVT Group, (Group 0), n = 311 (%) | Preoperative PVT Group (Group 1), n = 28 (%) | Incidental PVT Group (Group 2), n = 30 (%) | P Value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 224 (72.0) | 20 (71.4) | 24 (80.0) | 0.64 |
| Female | 87 (28.0) | 8 (28.6) | 6 (20.0) | |
| Agea | 53.0 (16.0-72.0) | 54.0 (41.0-65.0) | 54.0 (40.0-64.0) | 0.11 |
| Body weight, kga | 66.0 (35.3-116.0) | 61.0 (45.5-82.0) | 68.5 (50.0-101.0) | 0.21 |
| MELD scorea | 22.84 (6.00-53.00) | 17.09 (7.00-43.00) | 14.93 (9.00-45.00) | 0.003 |
| Child-Pugh grade | ||||
| A | 60 (19.3) | 2 (7.1) | 7 (23.3) | 0.16 |
| B | 71 (22.8) | 7 (25.0) | 11 (36.7) | |
| C | 180 (57.9) | 19 (67.9) | 12 (40.0) | |
| Cirrhosis status | ||||
| Yes | 214 (68.8) | 27 (96.4) | 29 (96.7) | 0.69 |
| No | 97 (31.2) | 1 (3.6) | 1 (3.3) | |
| Etiology of cirrhosis | ||||
| Idiopathic/cryptogenic | 8 (2.6) | 3 (10.7) | 3 (10.0) | 0.51 |
| Hepatitis B | 190 (61.1) | 20 (71.4) | 24 (80.0) | |
| Hepatitis C | 29 (9.3) | 2 (7.1) | 0 (0) | |
| Hepatitis B + C | 4 (1.3) | 0 (0) | 1 (3.3) | |
| Alcohol | 12 (3.9) | 0 (0) | 1 (3.3) | |
| Autoimmune | 23 (7.4) | 3 (10.7) | 1 (3.3) | |
| Others | 45 (14.5) | 0 (0) | 0 (0) | |
| Tumor related | ||||
| No | 224 (72.0) | 19 (67.9) | 11 (36.7) | <0.001 |
| Yes | 87 (28.0) | 9 (32.1) | 19 (63.3) | |
| Preoperative blood test | ||||
| Platelet × 109/La | 73.0 (14.0-778.0) | 49.0 (24.0-119.0) | 76.0 (21.0-328.0) | 0.04 |
| Urea, mmol/La | 4.7 (0.9-53.9) | 4.80 (2.80-24.30) | 4.90 (0.90-29.10) | 0.28 |
| Creatinine, µmol/La | 79.0 (30.0-774.0) | 74.0 (38.0-456.0) | 77.0 (45.0-266.0) | 0.74 |
| Albumin, g/La | 33.0 (14.0-52.0) | 31.0 (20.0-45.0) | 33.0 (20.0-45.0) | 0.52 |
| Bilirubin, µmol/La | 179.0 (3.0-1022.0) | 63.0 (17.0-781.0) | 45.5 (10.0-444.0) | 0.01 |
| INRa | 1.8 (0.9-10.1) | 1.6 (1.1-2.8) | 1.4 (1.1-3.5) | 0.13 |
| Type of graft | ||||
| Deceased donor graft | 147 (47.3) | 15 (53.6) | 20 (66.7) | 0.11 |
| Living donor graft | 164 (52.7) | 13 (46.4) | 10 (33.3) | |
| Graft weight to estimated standard liver, volume (%) for deceased donor grafta | 94.9 (31.9-180.3) | 99.0 (79.1-128.9) | 95.5 (54.0-145.0) | 0.62 |
| Graft weight to estimated standard liver volume (%) for living donor grafta | 49.2 (29.5-110.4) | 50.0 (34.5-89.2) | 48.8 (36.7-93.4) | 0.92 |
| Waiting time to LT, daysa | ||||
| DDLT | 41.0 (0-3849.0) | 266.0 (1.0-3668.0) | 325.5 (1.0-2845.0) | 0.002 |
| LDLT | 9.0 (1.0-1918.0) | 30.0 (3.0-262.0) | 41.5 (1.0-486.0) | 0.06 |
- NOTE: P value in Pearson chi-square test unless otherwise stated.
- a Values in median (range) and Kruskal-Wallis test used for P value.
Preoperative blood tests before LT were also analyzed and showed no significant difference in blood urea, serum creatinine, albumin, and international normalized ratio (INR). There was a significant difference in platelet count (P = 0.04) and serum bilirubin (P = 0.01) among the groups.
There was also no significant difference in the type of LT performed for these groups, whether it was a DDLT or LDLT. However, the waiting time to LT for DDLT was significant with a shorter median time (days) in the non-PVT group compared with both groups with PVT (P = 0.002) but was not shown with all the groups subjected to LDLT as the waiting time to LT in LDLT is expected to be short. The median for graft weight to estimated standard liver volume received by the recipients of LT either DDLT or LDLT also showed no significant difference.
Likelihood of PVT
A multivariate logistic regression was performed identifying the variables between the groups. Only a high serum bilirubin level of >131 µmol/L was found to be significant (P = 0.004; OR = 3.395; 95% CI; 1.467-7.861).
Yerdel Classification and Comparisons Between Groups 1 and 2
In the preoperatively diagnosed PVT group (group 1; n = 28), there were 5 (17.9%), 18 (64.3%), 4 (14.3%), and 1 (3.6%) patients with grades 1, 2, 3, and 4, respectively. In the incidental PVT group or group 2, there were 30 patients who had either grades 1 or 2. There were no patients with grades 3 or 4 in group 2 from this study. Because PVT was identified intraoperatively, it was difficult to specifically determine either grade 1 or 2 on the basis of the operative documentation.
For comparison between groups on the basis of Yerdel classification, grades 1 and 2 were compared with grades 3 and 4 as shown in Table 2, which showed group 1 having more patients with Yerdel grades 3 or 4 than group 2 where there was none. This suggests that PVT unsuspected prior to surgery may be more likely to be grade 1 or 2. Hence, the type of intervention performed might just be a simple thrombectomy.
| Preoperative PVT Group (Group 1), n = 28 (%) | Incidental PVT Group (Group 2), n = 30 (%) | P Value | |
|---|---|---|---|
| Yerdel class (A)a | |||
| Class 1 and 2 | 23 (82.1) | 30 (100.0) | 0.02 |
| Class 3 and 4 | 5 (17.9) | 0 (0) | |
| Imaging done | |||
| <1 month preoperative | 16 (57.1) | 15 (50.0) | 0.59 |
| ≥1 month preoperative | 12 (42.9) | 15 (50.0) | |
- NOTE: P value in Pearson chi-square test unless otherwise stated.
- a Fisher's exact test used.
The timing of the imaging performed before LT for PVT in both groups was equally matched as well (P = 0.59; Table 2). This showed that even if imaging was performed just before LT (less than 1 month), PVT might still remain undiscovered.
On the basis of the Yerdel classification, the type of surgical intervention was determined where those in group 1 tended to have more extensive intervention than just a conventional thrombectomy because there were more patients with Yerdel 3 or 4 (P = 0.02; Table 3). The nonthrombectomy interventions that were performed for patients, especially those with Yerdel 3 or 4 included thromboendovenectomy (n = 3), coronary vein to portal vein anastomosis (n = 1), and SMV graft to native SMV anastomosis (n = 1).
| Preoperative PVT Group (Group 1), n = 28 (%) | Incidental PVT Group (Group 2), n = 30 (%) | P Value | |
|---|---|---|---|
| Conventional thrombectomy | |||
| Class 1 and 2 | 22 (78.6) | 30 (100.0) | 0.43 |
| Class 3 and 4 | 1 (3.6) | 0 (0) | |
| Other proceduresa | |||
| Class 1 and 2 | 1 (3.6) | 0 (0) | — |
| Class 3 and 4 | 4 (14.2) | 0 (0) | |
| Total | |||
| Class 1 and 2 | 23 (82.1) | 30 (100.0) | 0.02 |
| Class 3 and 4 | 5 (17.9) | 0 (0) | |
- NOTE: P value in Fisher's exact test used.
- a For “other procedures” include thromboendovenectomy (n = 3), coronary vein anastomosis (n = 1), and SMV graft to native SMV anastomosis (n = 1).
Postoperative Outcome
Table 4 showed that groups 0, 1, and 2 had similar intraoperative and postoperative outcomes in terms of operation time (P = 0.18), hospital stay (P = 0.07), intensive care unit stay (P = 0.31), and intraoperative blood loss (P = 0.60).
| Non-PVT Group (Group 0), n = 311 (%) | Preoperative PVT Group (Group 1), n = 28 (%) | Incidental PVT Group (Group 2), n = 30 (%) | P Value | |
|---|---|---|---|---|
| Operation time, minutesa | 631 (306.0-1273.0) | 685.5 (430.0-1101.0) | 603.0 (410.0-935.0) | 0.18 |
| Hospital stay, daysa | 18.0 (0-325.0) | 21.0 (10.0-103.0) | 14.5 (8.0-135.0) | 0.07 |
| Intensive care unit stay, daysa | 4.0 (0-169.0) | 4.0 (1.0-24.0) | 3.0 (2.0-11.0) | 0.31 |
| Blood loss, mLa | 3450.0 (156.0-52,500.0) | 3500.0 (1000.0-25,000.0) | 3250.0 (800.0-15,666.0) | 0.60 |
| Post-operative complication (Early) | ||||
| Intra-abdominal bleeding† | 7 (2.3) | 3 (10.7) | 0 (0) | 0.11 |
| Hepatic artery thrombosis | 6 (1.9) | 0 (0) | 0 (0) | 0.88 |
| PVT† | 3 (1.0) | 1 (3.6) | 1 (3.3) | 0.51 |
| HV and IVC stenosis/thrombosis | 1 (0.3) | 0 (0) | 0 (0) | 0.91 |
| Bile leak | 5 (1.6) | 0 (0) | 0 (0) | 0.92 |
| Bile duct stricture | 11 (3.5) | 0 (0) | 0 (0) | 0.72 |
| Intra-abdominal collection/abscess | 31 (10.0) | 5 (17.9) | 2 (6.7) | 0.36 |
| Chest infection | 29 (9.3) | 2 (7.1) | 1 (3.3) | 0.79 |
| Urinary tract infection | 3 (1.0) | 0 (0) | 0 (0) | 0.72 |
| IV line sepsis | 2 (0.6) | 0 (0) | 0 (0) | 0.98 |
| Wound infection† | 8 (2.6) | 1 (3.6) | 0 (0) | 0.89 |
| Postoperative complication (Late) | ||||
| HV and IVC stenosis† | 3 (1.0) | 0 (0) | 1 (3.3) | 0.66 |
| Hepatic artery thrombosis | 2 (0.6) | 0 (0) | 0 (0) | 0.98 |
| Portal vein stenosis | 7 (2.3) | 5 (17.9) | 3 (10.0) | 0.01 |
| PVT† | 0 (0) | 2 (7.1) | 0 (0) | 0.23 |
| Bile duct stricture† | 42 (13.5) | 7 (25.0) | 4 (13.3) | 0.28 |
| PTLD | 2 (0.6) | 0 (0) | 0 (0) | 0.74 |
- NOTE: P value in Pearson chi-square test unless otherwise stated.
- a Values in median (range) and Kruskal-Wallis test used for P value.
- b Fisher's exact test used.
There was no significant difference with early postoperative complication faced by all the groups where early is defined by a complication arising within a 30-day postoperative period. However, with late complications (>30-day postoperative period), only portal vein stenosis occurrence showed a significant difference, with group 1 being more frequent at 17.9% followed by group 2 at 10.0% (P = 0.01; Table 4).
Survival Analysis of Graft and Patient Survival
The median graft survival times were 41.3 (0-75.8), 38.1 (5.0-75.0), and 33.6 (12.3-75.5) months in group 0, group 1, and group 2, respectively (P = 0.65). The 1-, 3-, and 5-year overall graft survival rates were 91.3%, 88.2%, and 86.8% in group 0; 96.4%, 84.6%, and 84.6% in group 1; and 96.9%, 91.8%, and 91.8% in group 2, respectively (Fig. 1).
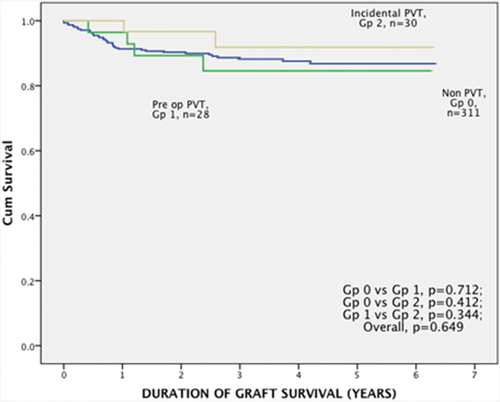
Graft survival between group 1, 2, and 0.
The median patient survival times were 41.3 (range, 0-75.8), 38.1 (range, 5.0-75.0), and 33.6 (range, 12.3-75.5) months in group 0, group 1, and group 2, respectively (P = 0.66). The 1-, 3-, and 5-year overall recipient survival rates were 92.0%, 88.8%, and 87.4% in group 0; 96.4%, 84.6%, and 84.6% in group 1; and 96.9%, 91.8%, and 91.8% in group 2, respectively (Fig. 2).
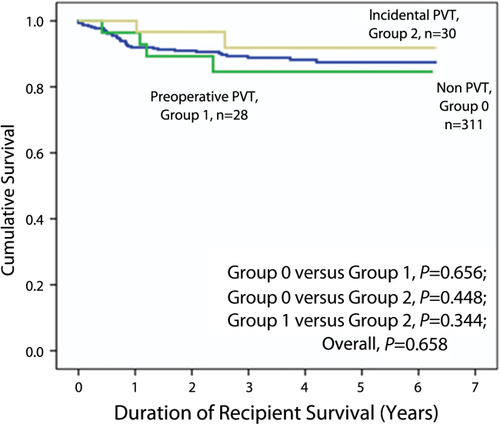
OS of patients between group 1, 2, and 0.
DISCUSSION
PVT in LT has always been a challenge, but it is no longer considered a hindrance to LT since the first reported case of PVT in LT by Shaw et al.6 in 1985. Our experience with patients with PVT having LTs in this center showed that the incidence of PVTs was 15.7%, which was similar to the reported incidence worldwide.2
The study also showed that all the groups had similar preoperative characteristics except for their MELD scores, tumor diagnoses, preoperative platelet levels, serum bilirubin levels, and waiting times, especially for those undergoing DDLT.
With regard to tumor diagnosis, a higher incidence was seen in the incidental PVT group. The presence of PVT in HCC is generally from clots due to a procoagulable state from HCC, which can be easily removed by thrombectomy as seen mainly in the incidental group. Evidence of HCC with vascular involvement would have been contraindicated for LT in our center. The tumors that were found were generally peripheral and not close to any vessel.
In this series, it was also found that the waiting time to LT especially in DDLT showed a shorter median time for the non-PVT group. We have found that the non-PVT group contained a higher number of patients with acute fulminant liver failure, whereas there was none in both PVT groups; hence, these patients did not wait long for LT because a graft was provided as a result of a lifesaving situation where some patients were not part of the LT waiting list. With PVT, both group 1 and group 2 had comparable waiting times, but the population in this group was smaller in numbers than the non-PVT group.
Only a high serum bilirubin level was found to be significant on multivariate analysis when an analysis for the risk factors for developing PVT was made. However, this finding is not consistent or is paradoxical in our series because the non-PVT group was noted to show a higher median score than both PVT groups. Most likely, the role of serum bilirubin is not clinically useful in predicting PVT. The finding of more patients with acute fulminant liver failure in the non-PVT group and none in the PVT group probably contributed to a higher preoperative serum bilirubin level in the non-PVT group. Our risk factor differs with some studies, which showed age, sex, MELD score, Child-Pugh score, liver cirrhosis, or low platelet counts as a risk for PVT.2, 7 Another potential risk factor for PVT development was the association with interventions used for the treatment of portal hypertension such as splenectomy, shunt operation, transjugular intrahepatic portosystemic shunts, and sclerotherapy for varices but none of our patients had undergone similar interventions; hence, PVT could not be evaluated in this aspect.3
This study was able to show that the outcome in OS in patients and grafts was similar to patients without PVT. Before the year 2000, most literature showed that despite LT in PVT being a possibility, the perioperative mortality was high and the 1-year survival was below 80%, which differed from our recent findings.8-10 With recent advances and knowledge of the disease as well as with improved surgical techniques, the outcomes and survival in patents with PVT undergoing LT have seen improvement as is evident in this series as well.11 However, Fouzas et al.12 noted that outcome and survival remained poor despite the technical innovations but that it was related to the grade of PVT instead.
In this study, further investigations and comparisons on the basis that PVTs were identified preoperatively (group 1) or incidental (group 2) were made, of which there was a scarcity of data comparing both situations. It is imperative that careful preoperative imaging to identify PVT before LT is determined when deciding the surgical strategy to be employed during the operation.13, 14 PVT discovered intraoperatively might increase the complexity of the surgery. The incidence of incidental PVT was reported to be 12%-64%, and this study revealed that incidental PVT accounted for 51.7% in our center.5, 7, 15
Early imaging before transplantation might be able to identify PVT, but as shown in this study, it bears no significance as incidental PVTs were still encountered intraoperatively despite imaging being done less than 1 month before surgery.
However, preoperative imaging still plays a role in determining the surgical strategy to be employed. If PVT was encountered before LT especially for those with Yerdel grades 3 or 4, this group of patients was in need of more advanced surgical techniques rather than a conventional thrombectomy, as the study had shown in instances where thromboendovenectomy, coronary vein anastomosis, and SMV graft to SMV native anastomosis were performed for patients at this grade. We could also safely determine that if preoperative imaging did not show PVT but PVT was discovered incidentally during surgery, then the PVT might not be as extensive as PVT is more likely of Yerdel grades 1 or 2; hence, a conventional thrombectomy or occasionally thromboendovenectomy would suffice, which would not require a highly complex intervention.
Although this study did not describe in length which of the various surgical strategies to use in relation to the various Yerdel grades encountered, for grade 1 or 2, a conventional thrombectomy or thromboendovenectomy is usually adequate.11, 16, 17 Occasionally, a resection of an affected short segment with primary anastomosis of the portal vein can be performed as well.7 For Yerdel grades 3 or 4, a more extensive and technical surgical strategy is often needed. Although we have described our techniques used for Yerdel grades 3 or 4, other sources in the literature have also reported various techniques, such as an interposition graft using a native or jump graft, renoportal anastomosis, portocaval hemitransposition, portal arterialization, or multivisceral transplant.15, 18-22
In the case of LDLT, the donor graft usually has a short portal vein end, which may present further challenge in the event of a recipient having a PVT especially when a simple thrombectomy or thromboendovenectomy cannot be performed. In such instance, a few approaches have been described to overcome this, such as recipient portal vein plasty, portal vein stenting, interposition graft use, or anastomosis to venous collaterals, where inflow anastomosis using paracholedochal veins had been described.23-25 Although they did not classify PVT according to Yerdel classification in their series of PVT in LDLT, Egawa et al.26 described techniques to overcome extensive PVT involving the portal trunk with SMV or splenic vein involvement where the “pull-through technique” was applied with subsequent eversion or incision technique. However, when the “pull-through technique” was not feasible, the SMV can be transected with an interposition graft using the recipient's left portal vein in right lobe LDLT.
Unlike LDLT, in DDLT, the long donor PV in DDLT gives surgeons an added benefit for the ease of portal vein anastomosis during the recipient's surgery. However, this study reports the discovery of PVT during the recipient LT where most PVs are isolated and transected close to the liver hilum with thrombosis being discovered and removed by a conventional thrombectomy. Having a long PV from the donor would probably allow a short segment of the recipient's PV to be transected at any point where it was deemed unhealthy.
When the postoperative outcomes in all 3 groups were compared, they were comparatively similar as well. Of interest would be the early postoperative portal vein rethrombosis where both groups 1 and 2 had slightly higher incidences than the non-PVT group although it remained <5%. This is less than most published studies, which have a reported incidence of 6%-40%.9, 27-29 In our series, we do not practice the use of anticoagulants in either the preoperative or postoperative period in our patients with PVT going for LT. Although several published series on the use of anticoagulants in PVT have been published to date, there is still no sufficient evidence for its use in clinical practice or as part of the standard guidelines.20, 30 The need to weigh between the risk of bleeding and a procoagulable state in patients with PVT for LT should be considered carefully before deciding to start such patients on anticoagulants as more evidence or randomized controlled trials are still required.
Our late complication also showed a high incidence of portal vein stenosis in group 1, which can be attributed to the group having a number of subjects with a higher Yerdel grade. However, in this series, despite reports of portal vein stenosis based on radiological imaging, very few patients required interventions, such as an angioplasty or reconstruction, where only 1 patient from either group had angioplasty with the rest requiring close follow-up as good venous patency and graft function continued throughout their follow-up.
This study has a limitation where we encountered difficulty in stratifying PVT with Yerdel grades 1 or 2 in the group where PVT was discovered incidentally during surgery. This was because it was difficult to differentiate between either Yerdel grades intraoperatively.
We hope that the study conducted would provide clinicians with further information for the management of PVT especially when incidental PVT is involved. An algorithm with regards to the management of PVT inclusive of incidental PVT could be determined as shown in Fig. 3 on the basis of the strategies employed in our institution, with further additional strategies for Yerdel grades 3 or 4 being described in other literature.26
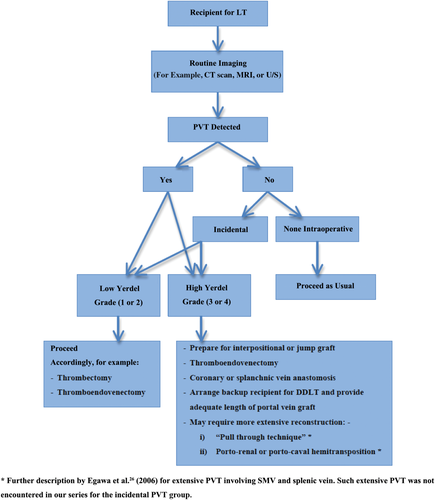
Management of PVT in LT in Queen Mary Hospital.
In conclusion, the study has shown that patients with PVT undergoing LT can have similar postoperative outcomes and OS as compared to those with non-PVT including instances of where PVT is discovered incidentally during the intraoperative period. The discovery of incidental PVT only requires thrombectomy, and it added little to the complexity of the transplantation where the outcome was not adversely affected because most will be of a lower Yerdel grade. Preoperative imaging is very useful to identify those with a higher Yerdel grade in order to allow proper planning of the surgical strategy during transplantation. With recent advances, the outcome of patients with PVT has improved in the last 5 years as shown by this data.