Rho-kinase inhibitor targeting the liver prevents ischemia/reperfusion injury in the steatotic liver without major systemic adversity in rats
Potential conflict of interest: Nothing to report.
This work was supported in part by the Japan Society for the Promotion of Science (Grant-in-Aid for Scientific Research 21591748 to Hirotaka Tashiro). This work was carried out in part at the Analysis Center of Life Science in Hiroshima University.
Abstract
Rho-kinase (ROCK) inhibitors improve liver blood flow after ischemia/reperfusion (IR) injury, especially in the setting of steatosis, by decreasing the resistance of intrahepatic microcirculation through hepatic stellate cell (HSC) relaxation. However, the systemic administration of ROCK inhibitors causes severe hypotension; therefore, liver-specific ROCK inhibition is required. Here, we tested vitamin A (VA)–coupled liposomes carrying the ROCK inhibitor Y-27632 for targeted HSCs in steatotic rats. Rat livers with steatosis induced by a choline-deficient diet were subjected to IR injury. The delivery site and effect of the ROCK inhibitor were investigated. After liposomal Y-27632 injection, the survival rate after IR, the liver blood flow, the portal perfused pressure, and the hemodynamics were investigated. Immunohistochemical studies showed VA–coupled liposome accumulation in livers. Liposomal Y-27632 was 100-fold more effective in inhibiting HSC activation than free Y-27632. Liposomal Y-27632 improved the survival rate after IR injury, the liver blood flow, and the portal perfusion pressure without severe hypotension. In contrast, untargeted Y-27632 elicited severe systemic hypotension. We conclude that VA–coupled liposomes carrying the ROCK inhibitor yield enhanced drug accumulation in the liver and thus mitigate IR injury in the steatotic liver and reduce major systemic adversity. Liver Transpl 21:123-131, 2015. © 2014 AASLD.
Abbreviations
-
- AST
-
- aspartate aminotransferase
-
- empty Lip
-
- liposome with no drug
-
- empty VA-Lip
-
- vitamin A–coupled liposome with no drug
-
- GFP
-
- green fluorescence protein
-
- HSC
-
- hepatic stellate cell
-
- IR
-
- ischemia/reperfusion
-
- Lip-GFP
-
- liposome carrying green fluorescence protein
-
- Lip-Y
-
- liposome carrying Y-27632
-
- MAP
-
- mean arterial pressure
-
- MLC
-
- myosin light chain
-
- P-MLC
-
- phosphorylated myosin light chain
-
- ROCK
-
- Rho-kinase
-
- SD
-
- standard deviation
-
- SEC
-
- sinusoidal endothelial cell
-
- VA
-
- vitamin A
-
- VA-Lip-GFP
-
- vitamin A–coupled liposome carrying green fluorescence protein
-
- VA-Lip-Y
-
- vitamin A–coupled liposome carrying Y-27632
Liver steatosis increases the risk of postoperative morbidity and mortality after liver surgery, including liver transplantation.1-3 Ischemia/reperfusion (IR) injury is one of the most critical complications commonly associated with liver surgery.4, 5 Compared with healthy livers, steatotic livers are vulnerable to IR.
Hepatic stellate cells (HSCs) are located in the space of Disse surrounding sinusoidal endothelial cells (SECs). HSCs are thought to play a role in modulating intrahepatic vascular resistance because of their capacity to contract through a Rho signaling mechanism. HSCs undergo contraction or relaxation in response to certain stimuli and, consequently, regulate the microcirculation by increasing or decreasing the diameter of the sinusoidal lumen.6 Rho-kinase (ROCK) is a key player in HSC contraction and is activated by active Rho A, a small guanosine triphosphatase coupled with many vasoconstrictor receptors such as endothelin that are involved in regulating portal hypertension.7-12 The inhibition of intrahepatic ROCK by Y-27632 attenuates resistance in cirrhotic rats, as shown by in situ liver perfusions.7, 12, 13
In our previous study, activation of Rho-ROCK signaling increased HSC susceptibility in rat steatotic livers to IR injury, and this indicated that HSC activation in the steatotic liver is associated with ROCK2 up-regulation and that enhanced ROCK2 activation is involved in the induction of IR injury in steatotic livers. Additionally, we found that an intra-abdominal infusion of fasudil, a specific ROCK inhibitor, significantly alleviated IR injury in steatotic livers.14 However, ROCK inhibitors have been reported to lead to several detrimental adverse effects, such as hypotension and ileus, via the inhibition of migration, contraction, adherence, and division of systemic cells of various organs.15, 16 To avoid these adverse effects, a more selective drug delivery system for HSCs is required. Recently, a new drug delivery system using liposomes that target HSCs has been reported. Vitamin A (VA)-coupled liposomes preferentially target HSCs, which have a remarkable capacity for VA uptake, most likely through receptors for retinol-binding protein.17
In this study, we tried to deliver an encapsulated ROCK inhibitor in VA-coupled liposomes to the liver in order to reduce hepatic IR injury on the basis of a study by Sato et al.17 We also examined whether this new drug reduced any detrimental adverse effects of the ROCK inhibitor (eg, hypotension).
MATERIALS AND METHODS
Animals
Four-week-old male Wistar rats were purchased from Charles River Breeding Laboratories (Osaka, Japan). The rats were fed either a choline-deficient diet (Hiroshima Institute for Experimental Animals, Hiroshima, Japan) for 6 weeks to encourage the development of steatosis in the liver or a normal diet that caused normal liver development. All animal experiments were performed according to the guidelines set by the US National Institutes of Health (1985). This experimental protocol was approved by the ethics review committee for animal experimentation of the Graduate School of Biomedical Sciences at Hiroshima University.
Liver IR
The rats were placed under anesthesia, and whole rat livers were subjected to warm ischemia via the clamping of the hepatic artery and the portal vein with microvascular clips. The specific ROCK inhibitor (Y-27632, Wako, Osaka, Japan) or a liposomal drug for Y-27632 was used to investigate the effect of ROCK inhibition on liver IR injury. The selected rats were pretreated with different quantities of drugs (via an intravenous bolus injection) 30 minutes before the induction of ischemia.
Preparation of VA-Coupled Liposomes Carrying the ROCK Inhibitor
Three types of empty liposomes (Coatsome EL-C-01, EL-N-01, and EL-A-01; NOF Corp., Tokyo, Japan), were used to deliver Y-27632. VA-coupled liposomes carrying the ROCK inhibitor were prepared as previously described with some modifications.17, 18 Briefly, 25 mg of the empty liposome product was added to 400 μL of a Y-27632 solution [20 mg/mL; liposome carrying Y-27632 (Lip-Y)]. To prepare VA-coupled liposomes, 200 nmol of VA (retinol; Sigma-Aldrich, St. Louis, MO) dissolved in dimethyl sulfoxide was mixed with the liposome suspensions (100 nmol as liposome) via vortexing in a 1.5-mL tube at 25°C [vitamin A–coupled liposome carrying Y-27632 (VA-Lip-Y)]. The quantities of Y-27632 taken up by the liposome were determined with a high-performance liquid chromatography system (LC-2010A; Shimadzu, Kyoto, Japan; Fig. 1A-C). Any free VA or Y-27632 that was not taken up by liposomes was separated from the liposomal preparations with a micropartition system (Vivaspin 2 concentrator; molecular weight cutoff = 30,000; polyethersulfone; Vivascience, Sigma-Aldrich, St. Louis, MO). The liposomal suspension was added to the filters and centrifuged 3 times at 1500g for 5 minutes each at 25°C. Fractions were collected, and the material trapped in the filter was reconstituted with phosphate-buffered saline to achieve the desired dose for in vitro or in vivo use. For the Lip-Y and VA-Lip-Y control groups for each experiment, we used liposomes without Y-27632 (empty Lip) or VA-coupled liposomes without Y-27632 (empty VA-Lip).
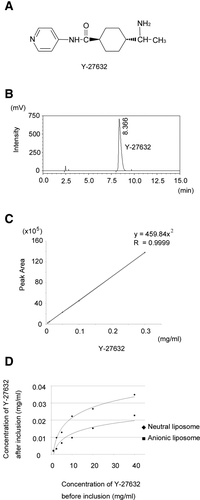
Preparation of VA-coupled liposomes carrying the ROCK inhibitor. (A) Chemical structure of Y-27632. (B) Chromatogram of Y-27632 extracted from VA-Lip-Y or Lip-Y. The specific peak of Y-27632 was identified at approximately 8.4 minutes. (C) Calibration curve for the determination of coupled Y-27632 in the liposome. (D) Comparison of the ratio of inclusion of Y-27632 among the 3 liposomes. The ratio of inclusion of Y-27632 was highest for the neutral liposome. Y-27632 was almost not included in the cationic liposome (data not shown).
Isolation of HSCs
HSCs were isolated from rat steatotic livers according to previously described procedures.14, 19, 20 Purity was estimated with ordinal light and fluorescence microscopy examinations and via indirect enzyme immunoreactivity with an anti-desmin antibody (Dako, Versailles, France). HSCs were grown in standard tissue culture plastic flasks in Dulbecco's minimum essential medium with 10% fetal bovine serum and antibiotics.
Collagen Gel Contraction Assay
The contractility of HSCs was evaluated with hydrated collagen gel lattices on 24-well culture plates as described previously with some modifications.14, 19, 21
Immunofluorescence of HSCs and Tissue Sections
To assess the activation of HSCs, phalloidin staining of isolated HSCs was performed according to previously described procedures.14, 22 Samples were observed under a conventional fluorescence microscope (BZ-9000; Keyence, Osaka, Japan). The average brightness of the 5 random areas was measured by microscopy (×400) with BZ-H1C dynamic cell count software (Keyence). For the histological analysis, liver specimens were collected from the middle hepatic lobe. To assess the accumulation of green fluorescence protein (GFP), frozen sections of the liver, lungs, heart, kidneys, and spleen were observed. To identify HSCs in the frozen liver sections, desmin staining was performed. The specific antibody against desmin was purchased from Abcam (Tokyo, Japan).
Statistical Analysis
Survival rates were compared with the Kaplan-Meier method and analyzed with the log-rank test. Other data are expressed as average values and standard deviations (SDs). Statistical analyses of experimental groups were performed with the t test. A 1-way analysis of variance was used for multiple comparisons. P < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 16 (SPSS Japan, Inc., Tokyo, Japan). For other descriptions of the materials and methods used in this study, please see the supporting information.
RESULTS
Preparation of VA-Coupled Liposomes Carrying the ROCK Inhibitor
We examined the inclusion of Y-27632 in 3 empty liposome products with high-performance liquid chromatography system: Coatsome EL-C-01 as a cationic liposome, EL-N-01 as a neutral liposome, and EL-A-01 as an anionic liposome. The ratio of Y-27632 inclusion was highest with the neutral liposome. The concentration of included Y-27632 increased in a dose-dependent manner and plateaued at a concentration of approximately 40 mg/mL (Fig. 1D). We used this concentration for subsequent experiments.
Y-27632 Suppresses the Level of Phosphorylation of Myosin Light Chains (MLCs) in HSCs
ROCK mediates cytoskeleton-dependent cell functions by enhancing MLC phosphorylation. We examined whether the ROCK inhibitor Y-27632 suppresses the phosphorylation level of MLCs in HSCs. Y-27632–treated HSCs showed dose-dependent suppression of the phosphorylation level of MLCs, with almost complete suppression at 10 μM (Fig. 2A).
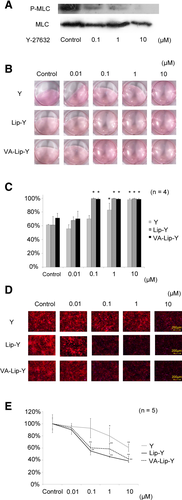
Effectiveness of VA-Lip-Y for activated HSCs in vitro. (A) Western blot analysis of the phosphorylation of MLCs in HSCs. The Y-27632 dose-dependently suppressed the phosphorylation level of MLCs in HSCs. (B) Collagen gel contraction assay. The contraction of collagen gels was induced by the activation of isolated HSCs that were untreated (control) or were treated with Y-27632, Lip-Y, or VA-Lip-Y. Without the addition of HSCs, the collagen gels did not contract during the observation period (not shown). (C) Changes in the collagen gel area induced by the contraction of HSCs. Average values (and SDs) of 4 independent experiments are shown. *P < 0.01 versus each control. (D) F-actin expression in isolated HSCs. HSCs were treated with Y-27632, Lip-Y, or VA-Lip-Y (24 hours). The cells were stained to show F-actin (red) and nuclei (blue). The control showed many stress fibers and high F-actin expression. In contrast, HSCs treated with Y-27632, Lip-Y, or VA-Lip-Y dose-dependently suppressed stress fiber formation and F-actin expression. (E) Levels of F-actin expression on HSCs. Average values (and SDs) of 5 independent experiments are shown. *P < 0.05 and **P < 0.01 versus each control. Y indicates Y-27632.
Contractility of HSCs Isolated From Steatotic Rat Livers
To evaluate the effectiveness of VA-Lip-Y with respect to contractility, HSCs isolated from rat steatotic livers were cultured on hydrated collagen gels. Contraction was measured by the reduction in the initial area of the gel. In the control group, the areas of the gels with HSCs were significantly lower than the areas of the gels without HSCs (data not shown). Empty Lip and empty VA-Lip as controls for Lip-Y and VA-Lip-Y did not effectively suppress these changes. In the presence of Y-27632 (10 μM), a reduction was not observed in the gel area with HSCs (P < 0.01). Similarly, a reduction in the area was not observed (P < 0.01) in the presence of Lip-Y (0.1 μM) or VA-Lip-Y (0.1 μM; Fig. 2B,C).
Effectiveness of VA-Lip-Y With Respect to the Morphological Features of HSCs
HSCs isolated from rat steatotic livers showed increased stress fiber formation and F-actin expression 24 hours after culture. The empty VA-Lip control for VA-Lip-Y and the empty Lip control for Lip-Y did not suppress the observed changes (Supporting Fig. 1). HSC stress fiber formation and F-actin expression were significantly suppressed by 0.1 μM VA-Lip-Y and Lip-Y (P < 0.01). In contrast, HSC activation was not suppressed by the same concentration of Y-27632 but was significantly suppressed at 10 μM (P < 0.01; Fig. 2D,E). Next, to confirm the toxicity of liposomes to HSCs, we examined the morphological changes of HSCs with time after the administration of VA-Lip-Y (Supporting Fig. 2). After administration, HSC stress fiber formation and F-actin expression were suppressed and gradually reactivated within 48 hours.
Delivery of GFP to the Liver by Liposomes
A histological analysis showed that GFP accumulation in the steatotic liver specimens injected with the vitamin A–coupled liposome carrying green fluorescence protein (VA-Lip-GFP) was significantly higher than that with the liposome carrying green fluorescence protein (Lip-GFP; P < 0.01; Fig. 3A,B). Additionally, there was no evidence of GFP accumulation in the heart, lungs, kidneys, or small intestine. Although there was slight GFP accumulation in the spleen, which was likely caused by nonspecific uptake by macrophages (Fig. 3C,D), GFP accumulation was significantly enhanced in the steatotic liver versus the spleen (P < 0.01). GFP accumulation was also enhanced in the normal liver. Furthermore, GFP accumulation in normal liver tissue sections included the desmin-stained area (Fig. 4A,B).
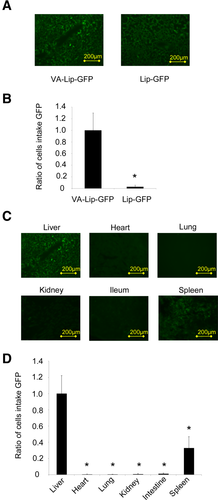
Liposome accumulation in steatotic livers. (A) Liver tissue sections of rats with steatotic livers treated with Lip-GFP or VA-Lip-GFP. (B) Ratio of the cell intake of GFP in liver tissue sections treated with VA-Lip-GFP to that in sections treated with Lip-GFP. (C) Tissue sections of each organ treated with VA-Lip-GFP. Liver, heart, lung, kidney, small intestine, and spleen sections were observed under a conventional fluorescence microscope. (D) Ratio of the cell intake of GFP in liver tissue sections versus other organs. Average values (and SDs) for individual groups are shown (n = 5 for all groups). *P < 0.01 versus the control group.
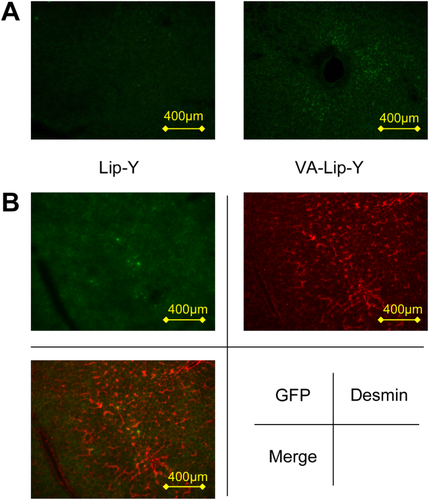
Accumulation of liposomes in liver tissue sections of rats with normal livers. (A) Liver tissue sections of rats treated with Lip-GFP or VA-Lip-GFP. (B) Desmin-stained liver tissue section of a rat treated with VA-Lip-GFP. The area showing an accumulation of GFP in the liver tissue section includes the desmin-stained area.
VA-Lip-Y Protects Steatotic Livers against IR Injury
We examined the effect of Y-27632 on the survival of rats with steatotic livers after 45 minutes of hepatic ischemia. None of the 10 untreated rats with steatotic livers survived more than 4 days. The survival rate of rats with steatotic livers was not improved by Y-27632 (0.1 mg/kg); the 1-week survival rate was 20% (2/10 rats). However, the survival of rats with steatotic livers was significantly prolonged by Y-27632 (10 mg/kg), Lip-Y (0.1 mg/kg), and VA-Lip-Y treatment (0.1 mg/kg); the 1-week survival rates were 60% (6/10 rats), 60% (6/10 rats), and 70% (7/10 rats), respectively (P < 0.05, P < 0.01, and P < 0.01, respectively). The survival rates did not significantly differ for Y-27632 (10 mg/kg), VA-Lip-Y (0.1 mg/kg), and Lip-Y (0.1 mg/kg; Fig. 5A).
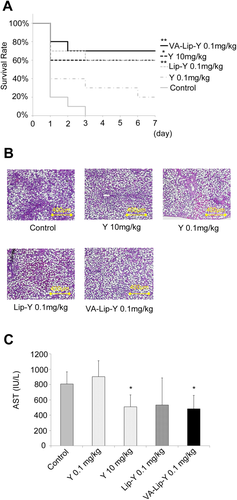
VA-Lip-Y protects steatotic livers against IR injury. Rats with steatotic livers were treated with IR. Each drug was administered 30 minutes before IR. (A) Survival rates of rats with steatotic livers after IR. The rats underwent 45 minutes of ischemia (n = 10). *P < 0.05 and **P < 0.01 versus the control group. (B) Histological examination of steatotic livers after IR. The rats underwent 30 minutes of ischemia followed by 3 hours of reperfusion (representative hematoxylin and eosin–stained liver sections). (C) Serum AST levels in rats with steatotic livers. The rats underwent 30 minutes of ischemia followed by 3 hours of reperfusion. Average values (and SDs) for individual groups are shown (n = 5 for all groups). *P < 0.05 versus the control group. Y indicates Y-27632.
Serum aspartate aminotransferase (AST) levels and histological changes in steatotic livers were measured after 30 minutes of ischemia followed by 3 hours of reperfusion. Sinusoidal congestion and massive necrosis were observed in the steatotic livers from the control, whereas no significant necrosis was observed in steatotic livers from rats treated with VA-Lip-Y (0.1 mg/kg; Fig. 5B). The serum AST levels in rats treated with a 10 mg/kg injection of Y-27632 and a VA-Lip-Y injection (0.1 mg/kg) were significantly lower than those of the control and Y-27632 rats at the 0.1 mg/kg concentration (P < 0.01 and P < 0.01, respectively; Fig. 5C).
VA-Lip-Y Improves Hepatic Tissue Blood Flow in Steatotic Livers After IR
Liver blood flow was measured during drug injection, ischemia, and reperfusion. In untreated rats with steatotic livers, liver blood flow decreased after ischemia and did not recover after reperfusion. The liver blood flow for the empty VA-Lip-Y group was the same as that for untreated rats (Supporting Fig. 3). In the rats treated with Y-27632 at 10 mg/kg, the liver blood flow after ischemia was significantly less than that in untreated rats (P < 0.05). In contrast, blood flow recovered in the VA-Lip-Y (0.1 mg/kg)–treated rats after reperfusion; the blood flow 10 minutes after reperfusion was significantly higher than that in the untreated rats (P < 0.05), and the blood flow at 15 minutes after reperfusion was significantly higher than that in rats treated with 0.1 mg/kg Lip-Y (P < 0.05; Fig. 6A,B).
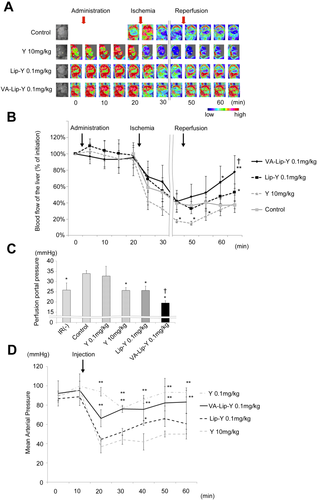
VA-Lip-Y improves the hepatic microcirculation after IR. (A) Change in the liver blood flow after IR. Pseudocolor images of the liver are shown; the color bar indicates blood flow. (B) Comparison of changes in the liver blood flow over time. Each drug was administered 15 minutes before ischemia. After 30 minutes of ischemia, the liver blood flow was measured for 15 minutes. Average values (and SDs) for individual groups are shown (n = 4 for all groups). *P < 0.05 and **P < 0.01 versus the control group; †P < 0.05 versus the Lip-Y group. (C) Portal pressures in isolated perfused livers. Livers were untreated, treated with 45 minutes of ischemia followed by 15 minutes of reperfusion, or pre-injected with each drug 30 minutes before IR. Average values (and SDs) for individual groups are shown (n = 5 for all groups). *P < 0.01 versus the control group; †P < 0.05 versus the Y-27632 (10 mg/kg) and Lip-Y groups (0.1 mg/kg). (D) MAP in rats. MAP was measured in rats during drug administration. Average values (and SDs) for individual groups are shown (n = 5 for all groups). *P < 0.05 and **P < 0.01 versus the Y-27632–treated group. Y indicates Y-27632.
Next, to determine the influence of IR on microvascular blood flow in the hepatic lobule, the portal perfusion pressure was assessed in isolated rat steatotic livers. Perfusion pressures were measured after 45 minutes of total hepatic ischemia followed by 15 minutes of reperfusion. The portal perfusion pressures of rats after IR were significantly higher than those of rats that did not undergo IR (P < 0.01), and Y-27632 did not decrease the portal perfusion pressure at the concentration of 0.1 mg/kg. However, the portal perfusion pressure was significantly suppressed 30 minutes before the pre-injection of Y-27632 at 10 mg/kg, Lip-Y at 0.1 mg/kg, and VA-Lip-Y at 0.1 mg/kg (P < 0.01, P < 0.01, and P < 0.01, respectively). The portal perfusion pressures of the VA-Lip-Y group were significantly lower than those with Y-27632 at 10 mg/kg and Lip-Y at 0.1 mg/kg (P < 0.01 and P < 0.01, respectively; Fig. 6C).
Mean Arterial Pressure (MAP) Measurement in Rats During Drug Administration
To determine the effect of drug injection on blood pressure, the MAP of rats was measured during drug administration. The MAP of the rats that received bolus injections of VA-Lip-Y at a concentration of 0.1 mg/kg exhibited a decrease in MAP to 66.3 mm Hg, but the MAP in the VA-Lip-Y group was significantly higher than that for the Y-27632 (10 mg/kg) and Lip-Y groups. Subsequently, the MAP of the VA-Lip-Y group improved to 83.3 mm Hg (P < 0.01 and P < 0.01, respectively; Fig. 6D).
DISCUSSION
We have developed a new drug system for delivering a ROCK inhibitor encapsulated in VA-coupled liposomes to the liver to ameliorate hepatic IR injury without the detrimental adverse effects of global ROCK inhibition (eg, hypotension). The current study shows that (1) VA-coupled liposomes carrying the ROCK inhibitor were predominantly taken up into livers, (2) VA-coupled liposomes carrying the ROCK inhibitor were approximately 100-fold more effective in suppressing the contractility of the activated HSCs than the nonliposomal ROCK inhibitor, (3) VA-coupled liposomes carrying the ROCK inhibitor significantly protected steatotic livers against IR injury in comparison with the nonliposomal ROCK inhibitor, and (4) VA-coupled liposomes carrying the ROCK inhibitor significantly ameliorated systemic hypotension in comparison with VA-free liposomes carrying the ROCK inhibitor. These results provide evidence that the VA-coupled liposomal ROCK inhibitor selectively delivers the ROCK inhibitor to the liver and can attenuate IR injury in steatotic livers without detrimental adverse effects.
To confirm the specific delivery of VA-coupled liposomes to the liver in vivo, we administered VA-Lip-GFP to rats with steatotic livers. Using immunohistochemical analysis, we observed that VA-coupled liposomes were predominantly taken up by the liver and that the nonspecific uptake of VA-Lip-Y observed in the spleen occurred in a VA receptor–independent manner mediated by the reticuloendothelial system. These results are consistent with previous studies indicating that VA-coupled cationic liposomes are specifically taken up by the liver.17 Furthermore, we showed that GFP accumulation in normal liver tissue sections included the desmin-stained area. These results suggest that VA-coupled cationic liposomes are specifically taken up by HSCs. However, the extent to which HSCs take up VA-Lip-Y remains unclear and requires further study.
The collagen gel contraction assay and the morphological studies showed that liposomal formation for Y-27632 produced biological effects at a dose of 0.1 μM liposome, whereas for nonliposomal Y-27632, a dose of 10 μM was necessary. These results indicated that liposomal Y-27632 was approximately 100-fold more effective than free Y-27632. The coupling of VA to the liposome as a ligand of HSCs had effects similar to those of the VA-free liposome in our in vitro study (Fig. 2B,C). Sato et al.17 reported that a transduction time of 30 minutes should be used to distinguish between the receptor-mediated transduction efficacy of vitamin A-coupled liposomes with small interfering RNA and the nonspecific transduction efficacy of liposomes with small interfering RNA because the nonspecific lipofection rate will increase and become more similar to receptor-mediated transduction with longer transduction times. These morphological and contraction assays lasted for 24 and 2 hours, respectively. This result suggests that HSCs can take up liposomes without involving VA receptors.
VA-free liposomes carrying the ROCK inhibitor showed protective effects against IR injury in steatotic livers similar to those of VA-coupled liposomes carrying the ROCK inhibitor. These results suggest that in the steatotic liver, the ROCK inhibitor works not only by direct inhibition of HSCs14, 19 but also by other mechanisms such as the activation of nitric oxide synthase in SECs23 or the inhibition of neutrophil infiltration.24 Although this new drug, VA-Lip-Y, increases selective HSC targeting, other mechanisms may also contribute to improve IR injury. Indeed, it has been shown that microcirculatory disturbances are induced in steatotic livers and that steatotic livers lack swollen SECs, an early marker of SEC injury.25
Impaired hepatic microcirculation has been proposed as the main contributing factor for the low tolerance of steatotic livers for IR injury.26 We have previously shown that improvements in microcirculatory disturbances by the ROCK inhibitor can aid in preventing IR injury in steatotic livers.14 In the current study, the blood flow in the steatotic liver after IR significantly recovered with VA-Lip-Y and Lip-Y treatments in comparison with Y-27632 treatment. Additionally, the portal perfusion pressure after IR was lowered by Y-27632 or Lip-Y and VA-Lip-Y treatments (Fig. 6C). These results indicated that the ROCK inhibitor prevented HSC activation and relaxed HSC contraction to decrease the resistance of intrahepatic microcirculation. Therefore, these improvements in hepatic microcirculation may contribute to a reduction in IR injury in the steatotic liver. Furthermore, a significant improvement in the hepatic blood flow and a significant lowering of the portal perfusion pressure were observed in VA-Lip-Y–treated rats versus Lip-Y–treated rats (Fig. 6A,B). These results suggest that VA-coupled liposomes increase Y-27632 accumulation in HSCs. On the other hand, the liver blood flow after ischemia in rats treated with 10 mg/kg Y-27632 was significantly lower than that in untreated rats. This reduction in the hepatic blood flow may be attributed to Y-27632–induced severe hypotension (Fig. 6A,B). After reperfusion, the liver blood flow in the 10 mg/kg Y-27632–treated rats gradually increased, and this may have ameliorated IR injury, whereas the liver blood flow in the untreated rats did not increase during the quantification of the hepatic blood flow.
Targeting HSCs is an elegant approach to bypassing the problem of extrahepatic effects caused by nonhepatic ROCK inhibition. In the current study, the degree of hypotension induced by VA-Lip-Y was significantly less than that induced by Y-27632 at 10 mg/kg or by Lip-Y (Fig. 6D). This result indicates that VA-coupled liposomes increased the selectivity for livers and reduced nonspecific delivery to systemic blood vessels. However, the systemic injection of VA-coupled liposomes still induced mild hypotension (Fig. 6D). This mild hypotension induced by the venous injection of VA-Lip-Y was also thought to be due partially to the bolus injection of the drug. This mild hypotension may be alleviated by a 1-hour venous drip injection of VA-Lip-Y. Similarly, when the ROCK inhibitor fasudil is administered by venous drip infusion for 1 hour, it does not induce systemic hypotension.23 Fasudil also induces detrimental systemic hypotension by bolus venous injection. Therefore, studies are required to investigate whether a 1-hour venous drip of the drug can prevent mild hypotension. In summary, VA-coupled liposomes used for the delivery of ROCK inhibitors can prevent IR injury in a rat model of a steatotic liver and ameliorate detrimental adverse effects.