Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts
Abstract
Hepatic steatosis continues to present a major challenge in liver transplantation. These organs have been shown to have increased susceptibility to cold ischemia/reperfusion (CIR) injury in comparison with otherwise comparable lean livers; the mechanisms governing this increased susceptibility to CIR injury are not fully understood. Endoplasmic reticulum (ER) stress is an important link between hepatic steatosis, insulin resistance, and metabolic syndrome. In this study, we investigated ER stress signaling and blockade in the mediation of CIR injury in severely steatotic rodent allografts. Steatotic allografts from genetically leptin-resistant rodents had increased ER stress responses and increased markers of hepatocellular injury after liver transplantation into strain-matched lean recipients. ER stress response components were reduced by the chemical chaperone taurine-conjugated ursodeoxycholic acid (TUDCA), and this resulted in an improvement in the allograft injury. TUDCA treatment decreased nuclear factor kappa B activation and the proinflammatory cytokines interleukin-6 and interleukin-1β. However, the predominant response was decreased expression of the ER stress cell death mediator [CCAAT/enhancer-binding protein homologous protein (CHOP)]. Furthermore, activation of inflammation-associated caspase-11 was decreased, and this linked ER stress/CHOP to proinflammatory cytokine production after steatotic liver transplantation. These data confirm ER stress in steatotic allografts and implicate this as a mediating mechanism of inflammation and hepatocyte death in the steatotic liver allograft. Liver Transpl 17:189–200, 2011. © 2011 AASLD
The epidemic of obesity in developed societies has led to increases in its attendant complications, including metabolic syndrome and nonalcoholic fatty liver disease (NAFLD).1 NAFLD is currently defined as >5% steatosis in the liver according to either light microscopy evaluation or magnetic resonance spectroscopy.2 NAFLD is recognized as a marker of metabolic syndrome1, 3 and is one of the most rapidly increasing and important causes of liver disease. Currently, hepatic steatosis is the most prevalent underlying condition affecting human liver donors1 and presents a particular challenge in the setting of liver transplantation. Steatosis is currently estimated to be present in up to 50% of deceased donor livers1 and is recognized as the key donor variable predicting posttransplant allograft function.4 Because of the current epidemic of obesity, the impact of hepatic steatosis on liver transplantation will likely continue to increase.
Steatosis is thought to affect liver allograft function and survival primarily by increasing the susceptibility of these organs to cold ischemia/reperfusion (CIR) injury.5, 6 Both the early clinical functional recovery and the regenerative capacity of steatotic allografts are significantly impaired in comparison with lean allografts, and this results in increased primary nonfunction and initial poor function rates in these organs.1, 6, 7 There is no universally accepted measurement of liver steatosis, and the majority of centers rely on estimates based on frozen section histological evaluation. Although many centers routinely use organs with mild macrosteatosis (usually defined as <30% macrosteatosis via histological estimation), the presence of moderate (31%-59% macrosteatosis) to severe steatosis (>60% macrosteatosis) has been estimated to account for up to 40% of unused and discarded liver allografts.8, 9
The mechanisms underlying CIR injury in steatotic livers differ significantly from those underlying similar injuries in lean livers.5, 10, 11 Steatotic livers have a predominance of rapid necrotic cell death, which may be related to altered energy homeostasis in steatotic livers.5, 10-12 However, much about the underlying cellular and molecular mechanisms affecting CIR injury in steatotic allografts remains poorly understood. NAFLD is strongly associated with metabolic syndrome, a combination of abnormalities related to insulin resistance and adipose tissue–derived proinflammatory factors that increase the hepatic inflammatory response.1, 13, 14 Endoplasmic reticulum (ER) stress is emerging as an important component of inflammatory responses in the liver associated with insulin resistance. ER stress in NAFLD is linked to alterations of hepatic lipid metabolism as well as hepatic insulin resistance.15-17
A multitude of insults, including ischemia/reperfusion, can induce an ER stress response, which is designed to dampen the injury and allow cellular adaptation. Following a cellular stressor, ER chaperones [glucose-related/binding immunoglobulin protein 78 (GRP-78)] recognize misfolded proteins and trigger downstream responses designed to globally reduce gene expression and alleviate the stress.18-20 Chaperone recognition of unfolded proteins activates 3 major ER stress mediators: pancreatic eukaryotic translation initiation factor kinase (PERK), inositol-requiring enzyme 1 (IRE1α), and activating transcription factor 6 (ATF6).19, 20 Although the initial ER stress response is adaptive, increased or sustained ER stress can result in cell death via several pathways. ER stress mediators can activate nuclear factor kappa B (NF-κB) and a proinflammatory response usually via the IRE1α/X-box protein 1 (XBP-1) pathway.21 In addition, the ER stress cell death responses are often associated with ATF4 activation of CCAAT/enhancer-binding protein homologous protein (CHOP), which is a cell death mediator.18, 22 In lean livers, ER stress has been implicated as a mediator of warm ischemia/reperfusion injury,23 and ER stress responses are known to occur after lean liver transplantation.24 However, little is known about the ER stress response and its effect on the steatotic liver after CIR.
We hypothesized that steatotic livers would have an increased ER stress response after CIR potentiating the injury response. In this report, we demonstrate an exaggerated acute ER stress response in severely steatotic allografts and postulate that this is involved in steatotic allograft injury.
Abbreviations: ALT, alanine aminotransferase; ATF, activating transcription factor; CHOP, CCAAT/enhancer-binding protein homologous protein; CIR, cold ischemia/reperfusion; ELISA, enzyme-linked immunosorbent assay; ER, endoplasmic reticulum; GRP-78, glucose-related/binding immunoglobulin protein 78; HMGB1, high-mobility group box protein 1; HOMA, homeostasis model assessment; HTK, histidine tryptophan ketoglutarate; IL, interleukin; IRE1α, inositol-requiring enzyme 1; mRNA, messenger RNA; NAFLD, nonalcoholic fatty liver disease; NF-κB, nuclear factor kappa B; PERK, pancreatic eukaryotic translation initiation factor kinase; qRT-PCR, quantitative real-time polymerase chain reaction; TUDCA, taurine-conjugated ursodeoxycholic acid; XBP-1, X-box protein 1.
MATERIALS AND METHODS
Steatotic Liver Transplant Model
Our laboratory has an established steatotic liver transplant model in rats that is approved by the Animal Studies Committee of Washington University (St. Louis, MO). Our method is a modification of the cuff technique described by Kamada and Calne25 and others.26, 27 The donor operation was carried out under isoflurane anesthesia in obese (leptin missense) and lean (control) Zucker rats after systemic heparinization. The hepatic artery was ligated, the portal vein was cannulated, and the liver was flushed with a cold (4°C) histidine tryptophan ketoglutarate (HTK) solution. The liver was subsequently extirpated with the vena cava and stored in cold HTK for 2 hours. Vascular cuffs were prepared for the portal vein and the infrahepatic vena cava. While the donor liver was being cold-stored, the lean Zucker recipient was prepared under isoflurane anesthesia. Via a midline incision, the recipient hepatectomy was performed by ligation and division of the hepatic artery and clamping of the infrahepatic and suprahepatic vena cava as well as the portal vein. The vena cava and portal veins were then divided, and the liver was removed. The donor liver was brought to the operative field, and the suprahepatic vena cava anastomosis was performed with a 9-0 monofilament suture. The infrahepatic and portal vein anastomoses were performed with the previously placed cuffs. The bile duct was anastomosed over a cannula. After reperfusion of the liver, the recipient received 1 mL of saline intraperitoneally. The abdomen was closed, and the animal was allowed to recover under close observation. At the appropriate time points, the animals were euthanized, and the allografts were recovered for analysis.
Sham obese and lean animals underwent laparotomy with an anesthesia time similar to that for the transplant procedure. The animals were euthanized at the appropriate time points for analysis.
Chemical Chaperone Treatment
Previous studies have suggested that the ER stress response can be modulated with the chemical chaperone taurine-conjugated ursodeoxycholic acid (TUDCA).15 In the current study, we first investigated the effect of treating obese donor animals with TUDCA (0.5 mg/kg in saline) daily for 7 days. Control obese donor animals received the vehicle (saline). Serum was obtained on days 2, 4, 6, and 7, and the animals underwent liver procurement on day 7. These organs were flushed and cold-preserved in HTK.
Next, we investigated the effect of TUDCA treatment of steatotic donor organs during cold storage and reperfusion. This was carried out by the addition of TUDCA (10mM) to the HTK solution in which the donor organs were flushed and cold-stored. Immediately after reperfusion, the lean recipient animals received TUDCA (0.5 mg/kg) or saline intraperitoneally.
At the time of euthanasia of the recipient animal, serum was obtained, and aliquots of the liver allograft were paraffin-embedded for histological analysis or flash-frozen for RNA, protein, and lipid extraction.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
RNA was extracted from frozen liver tissue with RNA-Bee (Tel-Test, Inc., Friendswood, TX) and treated with deoxyribonuclease. Reverse transcription was performed with the SuperScript II first-strand synthesis system (Invitrogen, Carlsbad, CA) with 1 μg of total RNA and random hexamers to generate complementary DNA. qRT-PCR assays were performed in triplicate on an ABI-Prism 7000 sequence detection system with SYBR Green polymerase chain reaction master mix (Applied Biosystems, Foster City, CA). The messenger RNA (mRNA) level of individual genes was quantified and normalized against a control reaction for rat 18S mRNA. After normalization to 18S, the relative mRNA abundance of individual genes in experimental groups was determined by comparison to sham-operated livers and was then expressed as the mRNA fold change by comparison to different groups. The primer sequences are summarized in Table 1.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ATF4 | 5′-tggccatctcccagaaagtg-3′ | 5′-gggaagaggctgcaagaatg-3′ |
| GRP-78 | 5′-acgtccaacccggagaaca-3′ | 5′-ttccaagtgcgtccgatga-3′ |
| IRE1α | 5′-ctctcctgtggtggccttcta-3′ | 5′-acgttgatgtgcaccaccttt-3′ |
| CHOP | 5′-ttgaccctgcatccctagct-3′ | 5′-gggctttgggaggtgctt-3′ |
| 18S | 5′-ttgattaagtccctgccctttg-3′ | 5′-cgatccgagggcctcacta-3′ |
Determination of Lipids and Insulin Resistance
Approximately 100 mg of frozen rat liver was homogenized to prepare lipid extracts for an enzymatic assay of triglycerides.28 The determination of serum and hepatic triglyceride and free fatty acid contents was carried out according to methods described by the supplier (Wako Diagnostics, Richmond, VA). Insulin was quantified in the rat serum with a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia AB, Uppsala, Sweden). The quantification of serum glucose was performed with a commercially available assay (Wako Diagnostics). Insulin resistance was calculated with the homeostasis model assessment (HOMA) model.29
Western Blot Analysis
Western blot analysis was carried out as previously reported.30-32 Briefly, cytoplasmic extracts (20-60 μg of protein) were subjected to polyacrylamide gel electrophoresis and were transferred overnight to nitrocellulose membranes (Invitrogen). Rat anti–XBP-1 (1-2 μg/mL; Pro Sci, Inc., Poway, CA), anti-CHOP (1:1000 dilution; Cell Signaling Technology, Danvers, MA), anti–activated caspase-11 (p20, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), anti–caspase-11 (1:200; Santa Cruz Biotechnology), anti-actin (1:1000; Santa Cruz Biotechnology), and antibody to high-mobility group box protein 1 (anti-HMGB1; 1:1000 dilution; Sigma-Aldrich, St. Louis, MO) were used as primary antibodies along with respective horseradish peroxidase–conjugated secondary antibodies. Semiquantitation via densitometry estimation was performed with ImageJ (ImageJ 1.41o, Wayne Rasband, National Institutes of Health). All experiments were performed in triplicate.
Determination of NF-κB Activation by Immunoprecipitation
The change in NF-κB activation upon treatment with TUDCA was determined with an immunoprecipitation method. Nuclear extracts were prepared from the liver as described by Burstein et al.33 Briefly, the 5′ biotinylated canonical oligonucleotide sequence (5′-TGCA AGGGACTTTCCGCTGGGGACTTTCCTGCA-3′) was coated onto streptavidin beads and used to pull down the activated NF-κB. After overnight incubation, the beads were washed and heat-denatured at 70°C. The pull-down protein was loaded onto 4% to 12% bis(2-hydroxyethyl)amino-tris(hydroxymethyl)methane gel and was probed with a p50 antibody (Santa Cruz Biotechnology).
Histopathology
Paraffin-embedded liver tissue sections were stained with hematoxylin and eosin and were examined in a blinded fashion by an experienced hepatopathologist (E.M.B.) for macrovesicular steatosis, inflammation, evidence of apoptosis, ischemic necrosis, and other lesions of hepatic injury.
Determination of Proinflammatory Cytokines
Rat cytokine [tumor necrosis factor α, interleukin-6 (IL-6), and IL-1β] assays were performed according to the ELISA protocol given by the supplier (Invitrogen). Rat serum (25 or 50 μL) and 50 μg of whole liver protein extracts were used for ELISA.
Statistical Analysis
A direct comparison of study cohorts was carried out with the Student t test. P values less than 0.05 were considered significant.
RESULTS
CIR Injury Is Associated With an Increased ER Stress Response in the Steatotic Liver
The obese Zucker rat is a model of hepatic steatosis in the context of metabolic syndrome. These animals served as donors, and the allografts were transplanted into lean Zucker animals as a model of severely steatotic liver transplantation. The steatotic allografts demonstrated severe (>60%) macrovesicular steatosis histologically and had a hepatic triglyceride content 4-fold greater than that of the lean allograft controls (P < 0.001; Fig. 1A). The obese donor animals also exhibited increased systemic insulin resistance (P < 0.005) and a significant increase in both serum free fatty acids (P < 0.02) and triglycerides (P < 0.001) that was consistent with metabolic syndrome (Fig. 1B-D). After the CIR injury from liver transplantation, the severely steatotic allografts had significantly increased injury in comparison with the lean control (P < 0.001; Fig. 2).
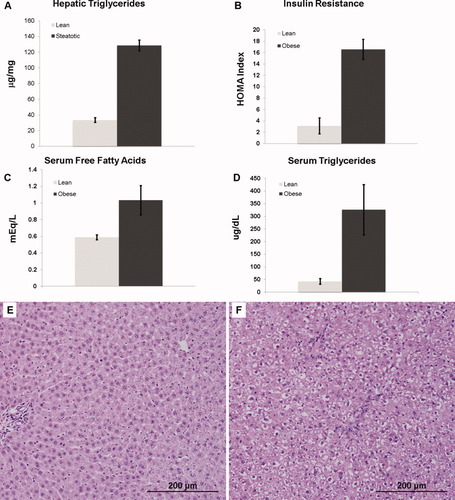
Livers of obese Zucker rats (donors) had (A) a 4-fold higher triglyceride content (P < 0.001), (B) increased insulin resistance (P < 0.005), (C) increased serum free fatty acids (P < 0.02), and (D) increased serum triglycerides (P < 0.001) in comparison with the livers of lean Zucker rats. Representative photomicrographs (magnification ×200) of livers from (E) lean and (F) obese donor Zucker rats are shown. The liver from the obese animal had severe macrovesicular steatosis. The macrovesicular steatosis was predominantly located in zone 1 with relative sparing of zone 3.
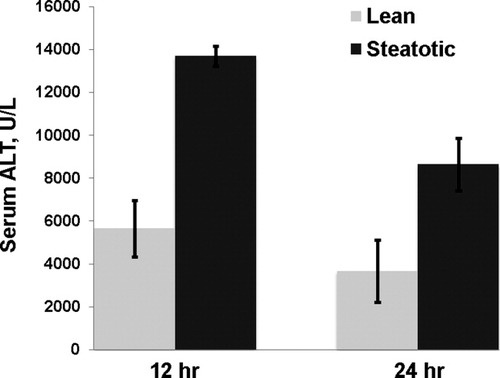
Allograft injury (measured by serum ALT levels) was significantly increased in the steatotic allograft at 12 and 24 hours in comparison with the lean allograft (P < 0.001 for each).
To investigate the ER stress response after CIR injury, we used qRT-PCR to determine the hepatic mRNA expression of GRP-78, IRE1α, ATF4, and CHOP in transplanted allografts. Sham-operated animals were evaluated to determine the chronic expression of these ER stress genes in the steatotic and lean livers. ATF4 was significantly up-regulated in the steatotic liver (1.5-fold, P < 0.04), and there was a trend toward increased expression of CHOP (1.37-fold, P > 0.05; Fig. 3A). Transplantation triggered an ER stress response in both the lean and steatotic allografts. In the lean allograft, there was a significant increase in ATF4 and CHOP mRNA (P < 0.04 for both; Fig. 3B) in comparison with the lean sham. The steatotic allograft, however, showed significant increases in all ER stress genes in comparison with the steatotic sham (P < 0.03 for all; Fig. 3C). The ER stress response after transplantation was more robust in the steatotic allograft than in the lean allograft. A direct comparison of the cohorts by normalization to the lean sham values showed that the magnitude of the acute increase of ER stress gene expression after CIR was significantly greater in the steatotic allograft (P < 0.03 for all; Fig. 3D) versus the lean one. The greatest example of this difference was the acute 23-fold increase in CHOP mRNA at 12 hours in the steatotic allograft versus the 2.9-fold increase in the lean group.
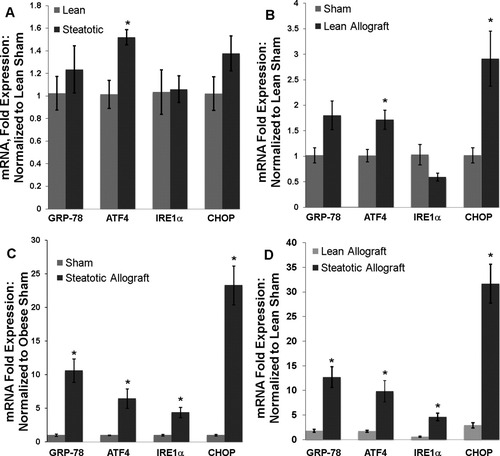
(A) Hepatic mRNA expression of GRP-78, ATF4, IRE1α, and CHOP in sham-operated lean and obese Zucker rats (n = 3 in each group). ATF4 was chronically expressed at higher levels (*P < 0.04) in the steatotic liver. In this panel, all samples are normalized to lean sham mRNA expression. (B) Twelve hours after liver transplantation, hepatic mRNA levels of ATF4 and CHOP were significantly elevated in the lean allograft (*P < 0.04 for both, n = 3). The changes in GRP-78 and IRE1α were not significant (P > 0.07 for each). (C) In the steatotic allograft, transplantation triggered a significant increase in mRNA levels for all ER stress genes (*P < 0.03 for all, n = 5). (D) Normalizing the mRNA expression of both lean and steatotic allografts to the lean sham allowed a direct comparison of the cohorts. The magnitude of the ER stress response in the steatotic allograft after CIR was significantly greater for all markers (P < 0.03 for all).
After reperfusion, the acute ER stress response genes in the steatotic allograft were increased significantly at 6 hours, they peaked at 12 hours, and they remained elevated significantly at 24 hours (P < 0.05 for all; Fig. 4A). ATF4, which was the most highly expressed marker 6 hours after reperfusion, is a downstream mediator of the PERK pathway that can subsequently stimulate CHOP expression. As expected, CHOP expression peaked after ATF4 12 hours after reperfusion. IRE1α mRNA showed a more gradual increase in expression, which peaked at 4.4-fold 12 hours after reperfusion. By performing western analysis for XBP-1, we next measured downstream activation of the IRE1α pathway. In sham-operated animals, total XPB-1 protein expression was similar in lean and steatotic animals (normalized ratios of 1.0 and 0.8, respectively, P > 0.05). In addition, the ratios of spliced (active) XBP-1 protein to unspliced XBP-1 protein were similar in the sham lean and steatotic livers (normalized ratios of 1 and 1.11, respectively, P > 0.05). The lean and steatotic allografts showed similar significant increases in the ratio of the spliced form of XBP-1 after CIR (Fig. 4B). However, transplantation caused an increase in total XBP-1 protein in the steatotic allograft (normalized ratios of 0.8 and 1.87, respectively, P < 0.005) but not in the lean allograft (normalized ratios of 0.79 and 1.0, respectively, P > 0.05).
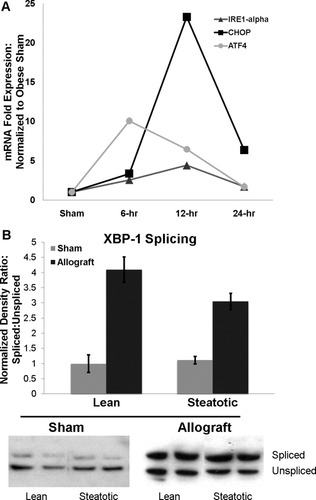
(A) Time course of ER stress mRNA expression in the steatotic allograft. The mRNA expression levels are shown for IRE1α, CHOP, and ATF4 in sham rats and in rats 6, 12, and 24 hours after transplantation. ATF4 expression peaked at 6 hours and was followed by the downstream up-regulation of CHOP, which showed peak expression at 12 hours. All 3 genes remained up-regulated 24 hours after transplantation. (B) Western analysis of XBP-1 splicing with representative images and densitometry analysis. In the sham-operated lean and steatotic livers, the ratios of spliced XBP-1 protein (upper band) to unspliced XBP-1 protein (lower band) were similar. CIR increased the ratio of XBP-1 splicing in both lean and steatotic allografts. When densitometry ratios were normalized to the lean sham liver, there were no significant differences in XBP-1 splicing between the lean and steatotic livers before or after transplantation.
Decreased Chronic ER Stress in the Donor Steatotic Liver Does Not Alter the Acute Post-CIR ER Stress Response
Chronic administration of TUDCA has been shown to decrease hepatic ER stress in animals with metabolic syndrome.15 To determine if short-term TUDCA therapy in obese donor animals could alter posttransplantation ER stress induction, we treated obese donor animals with TUDCA (0.5 mg/kg daily) or vehicle (saline) for 7 days. The findings were similar to those of previous reports: the TUDCA-treated donors exhibited decreased expression of IRE1α and CHOP (Fig. 5A). Interestingly, GRP-78 was induced in the TUDCA group versus the vehicle group. As expected, these changes resulted in an improved insulin resistance profile in the donor animals. The insulin resistance in the TUDCA group was significantly lower than that in the saline group by day 7 (P < 0.03; Fig. 5B). However, when the organs from these donor animals were used for transplantation, there was no change in the acute ER stress response between the TUDCA and vehicle groups (Fig. 5C). Furthermore, the post-CIR serum alanine aminotransferase (ALT) levels were unchanged between the 2 groups (9900 versus 8600 U/L, P > 0.5).
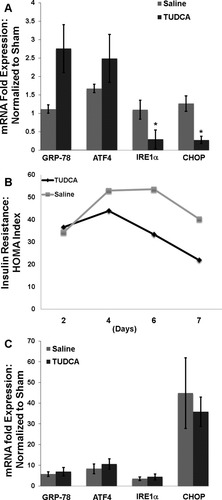
(A) Obese donor rats showed decreased expression of IRE1α and CHOP (*P < 0.05 for each) after 7 days of TUDCA therapy. (B) TUDCA therapy also decreased insulin resistance in the donor animals by day 7 (P < 0.03). (C) After 7 days of TUDCA therapy, the steatotic allografts were procured and used for transplantation. After CIR, there was no difference in the ER stress response between the TUDCA therapy group and the vehicle group.
Chemical Chaperone Treatment Decreases the Acute ER Stress Response to CIR in the Steatotic Allograft
To determine whether the delivery of TUDCA during cold storage and reperfusion could alter the posttransplant acute ER stress response in the steatotic allografts, we added TUDCA (10mM) to the HTK storage solution in which the steatotic donor livers were cold-preserved. In addition, we injected the recipients of these allografts with TUDCA (0.5 mg/kg in saline intraperitoneally) at the time of reperfusion. This cohort was compared to steatotic livers stored in HTK alone whose recipients were injected with the vehicle (saline) at reperfusion. After 6 hours of reperfusion, ATF4 expression and CHOP expression were lower (P < 0.03) in the TUDCA-treated cohort (Fig. 6A). Twelve hours after reperfusion, hepatic expression of all of the ER stress markers was significantly decreased (P < 0.04) in the TUDCA-treated cohort (Fig. 6B). In the TUDCA-treated cohort, hepatic expression of GRP-78, ATF4, and IRE1α peaked at 6 hours. CHOP expression continued to increase and peaked 12 hours after reperfusion but was reduced by approximately 50% (13-fold versus 23-fold, P < 0.02) by TUDCA treatment. The decrease in CHOP mRNA at 12 hours correlated with a decrease in CHOP protein detected by western analysis (Fig. 6C). However, despite the significant down-regulation of IRE1α, the total expression of XBP-1 protein was not altered by TUDCA (normalized ratios of 1.87 and 2.15, P > 0.05). Similarly, the splicing of XBP-1 was not inhibited by TUDCA (Fig. 6D).
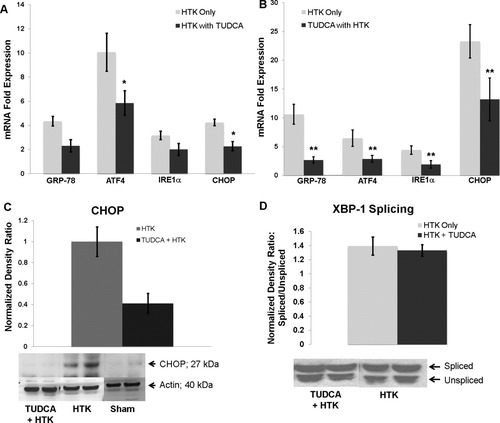
(A) Delivering TUDCA to the steatotic allograft during cold storage and at the time of reperfusion decreased ATF4 and CHOP mRNA levels 6 hours after reperfusion (*P < 0.03 for each, n = 4). (B) Twelve hours after reperfusion, the mRNA levels of all ER stress markers were decreased (**P < 0.04 for all, n = 5). (C) Western analysis of hepatic extracts for CHOP revealed decreased CHOP protein expression in the TUDCA cohort. One representative western blot is shown (n = 5 for TUDCA and HTK and n = 4 for HTK). Densitometry demonstrated a decrease in CHOP protein expression (P < 0.05). (D) Representative western blots of XBP-1 in steatotic allografts. There was no change in the ratio of the spliced form of XBP-1 (upper band) to the unspliced form (lower band) in the steatotic allografts treated with TUDCA.
TUDCA Treatment Decreased Steatotic Allograft Injury and Inflammation
TUDCA treatment decreased allograft injury as measured by serum ALT levels (Fig. 7A). In addition, recipients of the TUDCA-treated allografts had no increase in their serum ALT levels from 6 to 12 hours after reperfusion in comparison with controls. There was less HMGB1 in the TUDCA-treated steatotic allografts 12 hours after reperfusion (Fig. 7B); this was consistent with decreased hepatic cell death and injury. Six and 12 hours after reperfusion, there was no quantifiable change in ischemic necrosis between the cohorts (data not shown).
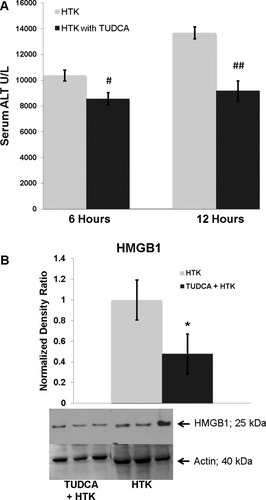
(A) Serum ALT levels decreased significantly in the TUDCA cohort 6 and 12 hours after reperfusion (#P < 0.05 and ##P < 0.05). (B) HMGB1 decreased in the TUDCA cohort 12 hours after reperfusion (*P < 0.03); this was consistent with decreased cell death and injury.
A histological examination of the nonnecrotic liver revealed that the macrovesicular steatosis in the HTK cohort had a zone 3 predominance (Fig. 8A). In the TUDCA cohort, the zone 1 predominance observed in the sham animals remained (Fig. 8B). There were increased numbers of apoptotic hepatocytes and mitotic figures in the HTK group versus the TUDCA cohort (Fig. 8C,D). Twelve hours after reperfusion, a pattern of decreased inflammation could be seen between the TUDCA and HTK groups. There was a decrease in both portal and lobular inflammation, and decreased neutrophil infiltration was documented in the TUDCA group (Fig. 8E,F). There was not a significant difference in the hepatic content of triglycerides between the HTK and the TUDCA cohorts 12 hours after transplantation (112.2 ± 17.3 versus 86.5 ± 7.4 μg/mg, P = 0.2).
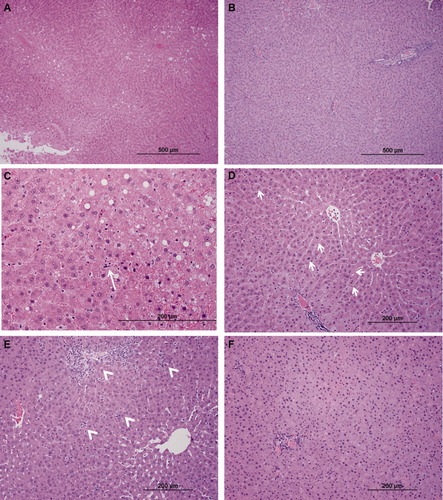
Representative photomicrographs of nonnecrotic allograft parenchyma. (A) A steatotic allograft 6 hours after reperfusion showed a predominance of zone 3 macrosteatosis with global evidence of early injury changes (magnification ×100). (B) The TUDCA cohort had decreased histological evidence of allograft injury and maintained a zone 1 distribution of macrosteatosis, which was the distribution observed in the sham animal. (C) A control allograft at 6 hours demonstrated apoptotic changes (long arrow) that were absent in the TUDCA-treated allografts (magnification ×400). (D) Mitotic bodies (arrows) in hepatocytes were seen in the controls but not in the TUDCA cohort after 6 hours of reperfusion (magnification ×200). (E) A steatotic allograft 12 hours after reperfusion showed marked portal tract edema and inflammatory infiltrates (arrowheads; magnification ×100). Lobular inflammation was also widely present. (F) In the TUDCA cohort, after 12 hours of reperfusion, the changes in allograft inflammation were reduced (magnification ×100).
TUDCA Treatment Decreased NF-κB Activation and IL-6, IL-1β, and Caspase-11 Activation
Because of the association of decreased histological allograft inflammation with decreased CHOP expression, we examined the expression of inflammatory mediators after steatotic liver transplantation. TUDCA decreased NF-κB activation in the steatotic allograft 6 and 12 hours after reperfusion in comparison with HTK alone (Fig. 9A). There was no change in the hepatic levels of tumor necrosis factor α protein between groups (ELISA, data not shown). However, TUDCA treatment significantly decreased hepatic expression of IL-1β and IL-6 (Fig. 9B,C). Because caspase-11 activation is known to link CHOP to both the proinflammatory mediator IL-1β and apoptosis,34-37 we examined caspase-11 activation in the steatotic allograft. Twelve hours after reperfusion, the p20 form of caspase-11 was present in the HTK cohort of severely steatotic allografts. The presence of this cleavage product was diminished in the TUDCA cohort (Fig. 9D).
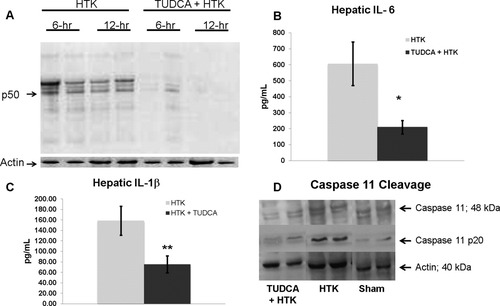
(A) Representative immunoprecipitation assay demonstrating decreased NF-κB p50 activation in the TUDCA-treated steatotic allograft both 6 and 12 hours after reperfusion in comparison with the allograft treated with HTK alone. (B) TUDCA treatment significantly decreased the hepatic levels of IL-6 (*P < 0.05, n = 5). (C) TUDCA treatment significantly decreased the hepatic levels of IL-1β (P < 0.05, n = 5). (D) Representative western blot demonstrating the presence of activated caspase-11 (p20) 12 hours after reperfusion. This was diminished in the TUDCA cohort.
DISCUSSION
NAFLD in donor organs is an increasingly important problem in liver transplantation. Hepatic steatosis increases the susceptibility of allografts to CIR injury, which leads to increased rates of allograft failure, slow functional recovery, or both. Many centers currently use mildly steatotic livers for transplantation with good results; however, even with mild steatosis, there are increases in primary delayed function and resource utilization post-transplant in comparison with lean donor allografts.4, 7, 38, 39 It has been demonstrated that the pathophysiology of CIR injury in the steatotic liver differs from that in the lean organ, and few successful strategies have been developed to attenuate this injury.5, 10, 11, 40, 41 Because hepatic steatosis is closely linked to metabolic syndrome in the donor,1 the exploration of factors linking steatosis to systemic insulin resistance and inflammation could yield important information about the behavior of the steatotic allograft after CIR injury.
Hepatic ER stress is recognized as an important mechanistic link between obesity, insulin resistance, and hepatic steatosis.15-17 Although ER stress is, in general, an adaptive response, prolonged or sudden increases in ER stress can initiate pathways that result in cell death.22, 42, 43 There are multiple pathways through which ER stress is known to mediate cell death.21, 22, 43-46 Although their mechanisms are not entirely clear, drugs such as TUDCA are classified as chemical chaperones because of their ability to enhance ER protein folding and thus decrease the ER stress response.43 Studies using chemical chaperones to target the PERK and IRE1α pathways of hepatic ER stress have demonstrated improvements in the insulin resistance of obese rodents.15, 17 In this study, we investigated the ER stress response in the steatotic liver allograft as a component of the postreperfusion injury observed in severely steatotic livers. Our results demonstrate that acute ER stress plays an important role in the CIR injury of the steatotic allograft.
An ER stress response occurs after liver transplantation in both lean and steatotic allografts. Although lean allografts showed an ER stress response, the magnitude of the response seen in the steatotic liver was far greater and more prolonged. The ER stress response in the steatotic allograft peaked at 12 hours but remained significantly elevated 24 hours after reperfusion. The magnitude of ER stress gene induction and the prominence of CHOP expression suggested the hypothesis that ER stress–associated cell death pathways may play a mediating role in the CIR injury incurred by the steatotic allograft. Because chemical chaperones are well-accepted ER stress inhibitors, we initially treated the obese donor animals to determine whether decreasing chronic hepatic ER stress in the steatotic liver would affect the post-CIR injury. When TUDCA was given to donor animals for 7 days, we observed an improvement in the animals' insulin resistance that was accompanied by a decrease in the hepatic expression of IRE1α and CHOP. These findings are similar to those of previous reports of TUDCA administration in ob/ob mice.15 Despite the successful decrease in the chronic hepatic IRE1α and CHOP levels, the steatotic allografts procured from the TUDCA-treated donors had posttransplant ER stress responses and allograft injuries similar to those of the allografts from donors treated with the vehicle. These results suggest that the acute ER stress response in the steatotic liver is not directly related to chronic ER stress levels.
In contrast, the addition of TUDCA to the cold storage solution in addition to delivery at the time of reperfusion was a successful strategy for decreasing the acute posttransplant ER stress response in the steatotic allograft. Although this effect could be measured 6 hours after transplantation, the greatest improvement was seen at 12 hours. The effect on allograft injury followed the pattern of ER stress change, with a small improvement in the serum ALT level noted at 6 hours and a greater change noted at 12 hours. Although TUDCA was able to decrease HMGB1 production in the steatotic allograft, there was not a clear change in histological necrosis qualitatively. The histological changes following TUDCA treatment were decreased portal and lobular inflammation. This suggested that TUDCA decreased a secondary component of the CIR injury. The improvements in histological allograft inflammation were consistent with the observed decreased NF-κB activation and decreased IL-6 and IL-1β levels in the TUDCA-treated steatotic allografts. These data implicate ER stress as a mediator of the inflammatory component of CIR injury in the steatotic allograft.
Because the component of injury that seemed to be improved by TUDCA was inflammatory, we suspected that the IRE1α pathway with its well-described ability to stimulate NF-κB and proinflammatory cytokines was a mediating pathway. However, we observed no difference in downstream IRE1α pathway activation (XBP-1 splicing) between the lean and steatotic allografts. Furthermore, TUDCA had no effect on XBP-1 splicing. Together, these observations suggest that the IRE1α pathway may not be an important mediator of allograft injury in our model.
CHOP, a specific cell death mediator, was the most prominently up-regulated ER stress marker in the steatotic allograft after CIR injury, and the improvements observed in allograft injury were accompanied by an almost 50% reduction of CHOP expression 12 hours after reperfusion. The correlation of CHOP overexpression with CIR injury and the decrease in this expression with TUDCA suggested that CHOP is an important effector of ER stress–mediated cell death in the steatotic allograft. Our observation that caspase-11 activity was inhibited by TUDCA supports this hypothesis as caspase-11 links CHOP to IL-1β production.34, 35 This is also one of many pathways linking CHOP to cell death.36, 37 This observation, combined with the lack of support implicating the IRE1α pathway, suggests that the ER stress/CHOP pathway plays a mediating role in steatotic liver allograft injury via induction of inflammation-associated caspases.
The relationship of ER stress–mediated cell death and CIR injury in the steatotic liver will require further definition. However, this study implicates ER stress on a broad scale as an important factor in this injury. The rodent transplant model is a good clinical approximation of the clinical scenario of steatotic liver transplantation, but it has limitations in the definition of specific cellular mechanisms involved in this multifaceted problem. The nonarterialized model is useful for making observations about acute CIR injury such as those in this study, but it is less useful for more long-term survival studies. In addition, this is a severely steatotic model; the extent of the reperfusion injury can often obscure the potential benefit of an intervention. This potentially explains the lack of correlation between histological necrosis and decreased HMGB1 release. In this study, we used a leptin-deficient model of obesity, metabolic syndrome, and hepatic steatosis. Steatotic livers from leptin-deficient rodents and rodents treated with a high-fat diet have been shown to have similar ER stress responses, and it is unlikely that the leptin mutation in our model had any significant effect on the hepatic ER stress response following CIR injury.16
In summary, the steatotic liver allograft has an exaggerated ER stress response after CIR injury. These data implicate ER stress as a mediating factor in cell death and inflammation of the steatotic liver after transplantation. The definition of the exact mechanisms involved in ER stress–mediated steatotic allograft injury will require further investigation, but the results of this study suggest that the ER stress pathways and specifically the CHOP/caspase-11/IL-1β pathway are potential targets for improving steatotic liver allograft function after liver transplantation.