Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: A randomized, open-label trial†‡
This study and statistical analyses, and the editorial and project management services of Acumed involved in the preparation of this manuscript, were supported by Astellas Pharma Europe, Ltd., Staines, UK.
L. Fischer, B. Gridelli, A. Roy, A. Vitale, A. Valdivieso, E. Varo, D. Seehofer, B. Ericzon, and K. Boudjema have declared no conflicts of interest. P. Trunečka has acted as study investigator and advisory board member for Astellas and has received speaker's fees. S. Lynch has received honoraria for chairing and speaking at international meetings for Astellas and Janssen-Cilag, Ltd. D. Samuel has acted as a consultant and also received research grants from Astellas and Novartis and acted as a consultant for Roche. C. Karpf was an employee of Astellas at the time of the study, and N. Undre is currently an employee of Astellas.
Abstract
Tacrolimus, a cornerstone immunosuppressant, is available as a twice-daily formulation (tacrolimus bid). A once-daily prolonged-release formulation (tacrolimus qd) has been developed. This 6-week, randomized, phase 2, multicenter, open-label, prospective trial in primary liver transplant recipients investigated and compared the pharmacokinetics (PK) of tacrolimus for qd and bid formulations. All patients received tacrolimus-based immunosuppression (tacrolimus qd, n = 67; bid, n = 62). PK data were available for 77 patients (tacrolimus qd, n = 45; bid, n = 32). Tacrolimus area under the curve (AUC) from 0 to 24 hours (AUC0-24) at equivalent doses was approximately 50% lower for tacrolimus qd than for bid on day 1 (146 versus 264 ng·h/mL, respectively), but by day 14 was comparable between treatments (324 and 287 ng·h/mL, respectively) with higher tacrolimus qd doses. There was a strong correlation between AUC0-24 and concentration at 24 hours for tacrolimus qd and bid (r = 0.92 and r = 0.76, respectively). Furthermore, the relationship between these 2 parameters (ie, the slope of the line) was also similar for the 2 formulations. Efficacy endpoints were comparable for both formulations at 6 weeks with no marked differences in incidence, nature, or severity of adverse events between treatments (although the study was not powered to draw efficacy conclusions). These results suggest that targeting the same trough levels will achieve similar total AUC over 24 hours for both tacrolimus qd and tacrolimus bid in de novo liver transplant recipients. Liver Transpl 17:167–177, 2011. © 2011 AASLD.
Prograf (Astellas Pharma Europe, Ltd., Staines, UK) is an immediate-release formulation of the immunosuppressive agent tacrolimus, administered twice-daily (bid) for the prevention and treatment of allograft rejection in liver, kidney, and heart transplantation; hereafter, this formulation will be referred to as tacrolimus bid. A prolonged-release formulation of tacrolimus (Advagraf; Astellas Pharma Europe, Ltd., Staines, UK) has been developed to provide once-daily (qd) dosing while ensuring similar efficacy and safety to the standard bid formulation; hereafter, it will be referred to as tacrolimus qd. Tacrolimus is a medication exhibiting a narrow therapeutic index1, 2 and the oral bioavailability is highly variable between individuals.3 Systemic exposure to tacrolimus (area under the curve [AUC]) is a significant variable for efficacy and safety, and therefore, therapy is optimized on an individual patient basis by monitoring trough levels as surrogate markers of exposure (AUC).3 Therefore, the aim in the development of tacrolimus qd was to apply the same drug-level monitoring concept as is established for tacrolimus bid.
The AUC from 0 to 24 hours after dosing (AUC0-24) of tacrolimus qd has previously been shown to be approximately 10% lower in comparison to that of the bid formulation in stable liver transplant patients converted on a 1:1 (milligram:milligram) total daily dose basis (measured at steady-state 14 days after conversion).4 Furthermore, follow-up data in stable liver transplant patients converted from tacrolimus bid to tacrolimus qd demonstrate good efficacy and safety profiles at 2 years.5 However, the pharmacokinetics (PK) of tacrolimus qd in de novo–transplanted patients has not been investigated previously. The primary objective of this study was to evaluate and compare PK parameters of tacrolimus qd and tacrolimus bid in de novo liver transplant recipients. Assessments were made following the first dose and under steady-state conditions in patients undergoing primary liver transplantation.
Abbreviations:
AE, adverse event; AUC0-24, area under the curve from 0 to 24 hours; AZA, azathioprine; bid, twice daily; BPAR, biopsy-proven acute rejection; C24, concentration at 24 hours; Cmax, maximum concentration; Cmin, minimum blood concentration; MMF, mycophenolate mofetil; NEC, not elsewhere classified; PK, pharmacokinetics; qd, once daily; SD, standard deviation; Tmax, time to maximum concentration.
PATIENTS AND Methods
Study Design and Procedures
This was a 6-week, phase 2, multicenter, open-label, prospective, 1:1 randomized trial comparing the PK of tacrolimus qd and tacrolimus bid in patients undergoing primary liver transplantation. Patient enrollment began in January 2003 and the last evaluation was performed in January 2004. Patients were allocated to treatment groups, locally at each center, using sealed randomization envelopes provided by the Data Operations Department of Astellas Pharma, Ltd. Randomization was performed preoperatively on a 1:1 (tacrolimus qd:tacrolimus bid) basis stratified by center, with each center being allocated a unique sequence of patient numbers. As this was an open-label study, no blinding was necessary. Visits were scheduled at days 0, 1, 3, 7, 11, 14 and weeks 4 and 6. At the end of the 6-week study period, patients in the tacrolimus qd group had the option to continue treatment with tacrolimus qd as part of a long-term extension study. Patients treated with tacrolimus bid were not followed after 6 weeks. Also reported here are 1-year follow-up results from the tacrolimus qd group.
Patients
Eligible patients were aged between 18 and 65 years, undergoing primary whole or split liver transplantation, had provided written informed consent, had received a first dose of tacrolimus and corticosteroids within 6-12 hours (but not later than 18 hours, depending on time of surgery) of skin closure, and were expected to be maintained on tacrolimus and corticosteroid treatment throughout the study.
Patients were excluded if they had malignancy (except hepatic cell carcinoma fulfilling Milan criteria) or history of malignancy within the last 5 years, had systemic infections requiring treatment, had serum creatinine >175 μmol/L, required induction therapy, had received an ABO-incompatible graft, had previously received or were receiving an organ transplant other than liver, or were likely to be nonadherent to the study protocol. Pregnant women and nursing mothers were also excluded.
Patients could be withdrawn due to withdrawal of consent, intolerable adverse event, retransplantation, noncompliance with study protocol, loss to follow-up, pregnancy or initiation of prohibited concomitant medication known to affect the pharmacokinetics of tacrolimus (ketoconazole, fluconazole, itraconazole, clotrimazole, nifedipine, nicardipine, erythromycin, clarithromycin, josamycin, danazol, ethinyl estradiol, omeprazole, calcium antagonists such as diltiazem, nefazodone, phenobarbital, phenytoin, rifampicin).
The study was conducted at 21 centers: 16 in Europe, 3 in Canada, and 2 in Australia. The study was conducted in accordance with the Declaration of Helsinki, fifth revision (2000).
Study Treatment
The first dose of tacrolimus qd was administered within 6-12 hours (but not later than 18 hours, depending on time of surgery) after skin closure, in the morning following transplantation. The recommended first oral daily dose of tacrolimus for both groups was to be in the range of 0.10-0.15 mg/kg, with tacrolimus qd given as 1 dose in the morning. The first dose of tacrolimus bid was administered orally within 6-12 hours (but not later than 18 hours, depending on time of surgery) after skin closure, in the morning following transplantation. The second dose of tacrolimus bid was taken in the evening of the day following transplantation. Patients unable to swallow a capsule could receive the contents of the tacrolimus bid capsules via nasogastric tube. It was not permitted to administer the dose of tacrolimus qd by nasogastric tube. Doses of tacrolimus qd were taken orally in the morning, whereas tacrolimus bid was taken as 2 daily oral doses, on an empty stomach or at least 1 hour before or 2-3 hours after a meal. Capsules were to be swallowed intact, with fluid (preferably water). Doses of both tacrolimus qd and tacrolimus bid were adjusted on an individual patient basis according to clinical signs aided by whole-blood tacrolimus trough level monitoring. Whole-blood samples were taken daily during the first 2 weeks, at weeks 4 and 6, and whenever clinically indicated. Concentrations of tacrolimus were determined locally using microparticle enzyme immunoassay (MEIA; IMx) or enzyme multiplied immunoassay technique. The recommended target tacrolimus whole-blood trough level range was 10-20 ng/mL for all patients.
Patients received an intravenous bolus (500-1000 mg) of methylprednisolone (or equivalent) prior to reperfusion, with subsequent doses given as oral prednisone (or equivalent) at a dose of 15-20 mg/day during the first month and tapered doses thereafter. The use of antibodies for induction therapy was not permitted, but their use for the treatment of acute rejection was allowed.
Pharmacokinetic Profiles and Assay
PK profiles were obtained following the initial dose of tacrolimus qd or tacrolimus bid (day 1 posttransplantation), and twice under steady-state conditions (on day 14 and at 6 weeks ± 7 days after transplantation) (Fig. 1). For both tacrolimus qd and tacrolimus bid, the first blood sample was taken in the morning before the first dose of study treatment was administered (0) and at 0.5, 1, 2, 3, 4, 6, 8, 12, 12.5, 13, 14, 15, 16, 18, 20, and 24 hours after administration. The assays were based on serial whole-blood samples collected in ethylene diamine tetraacetic acid tubes and frozen at −20°C (−4°F) within 2 hours of collection, then stored until shipment to a central laboratory (AAI Deutschland GmbH & Co. KG) for analysis. Tacrolimus concentrations were determined using validated high-performance liquid chromatography tandem mass spectroscopy assay methods (lower limit of quantification = 0.1 ng/mL).6 Whole-blood calibration standards, quality control samples and study samples were thawed and 1-mL aliquots taken. Internal standard (20 μL, 50 ng/mL) was added and mixed briefly. Aliquots were extracted using protein precipitation and solid phase extraction using C18 200 mg/3-mL extraction cartridges. Elutes were evaporated to dryness under a stream of nitrogen at 40°C, and residues redissolved in a 50:50 mix (vol/vol) of acetonitrile and water, mixed and centrifuged, before being submitted for assay.
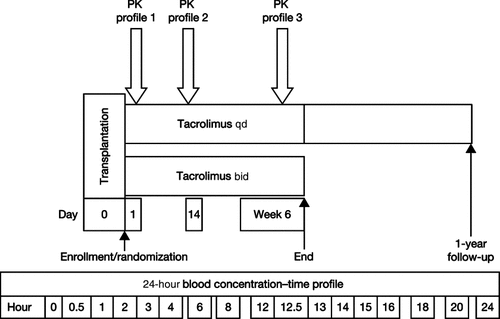
Study design and schedule of PK profiles.
As an additional aid for monitoring exposure, limited sampling AUC monitoring was performed on days 1, 3, 7, 11, 14, and as clinically indicated thereafter (for example, if signs of toxicity or lack of efficacy were observed within the recommended target ranges, and the clinician felt that a limited AUC sampling would be a helpful aid in optimizing dose). Blood samples were collected at 0 (predose) and at 2, 4, 8, and 12 hours postdose for both formulations, with an additional sample at 24 hours postdose for tacrolimus qd. Tacrolimus levels were assayed at the investigational site and the resulting data were sent to an external panel (Covance Clinical Research Unit, Ltd., Leeds, UK) to estimate the AUC using the linear trapezoidal method. The AUC values were immediately made available to the investigational site to aid in exposure estimation; the decision to make any dose adjustments was the responsibility of the site investigator. All procedures were performed in compliance with the principles of Good Laboratory Practice.
Objectives and Endpoints
The objective of this study was to evaluate and compare PK parameters following first administration and under steady-state conditions in de novo liver transplant recipients treated with tacrolimus qd and tacrolimus bid.
The primary endpoint was a comparison of AUC0-24 for tacrolimus qd and tacrolimus bid on day 1, day 14, and week 6 after transplantation. Secondary endpoints included the comparison between tacrolimus qd and tacrolimus bid of the maximum concentration of tacrolimus (Cmax), time to maximum concentration (Tmax), trough levels (minimum blood concentration [Cmin] at 12 hours for tacrolimus bid and at 24 hours for tacrolimus bid and tacrolimus qd), correlation between Cmin and AUC0-24, incidence of and time to biopsy-proven acute rejection (BPAR) events at 6 weeks, overall frequency of acute rejection episodes at 6 weeks, severity of BPAR at 6 weeks, graft and patient survival at 6 weeks, and incidence of adverse events (AEs) at 6 weeks.
All acute rejection episodes were classified as biopsy-proven or suspected acute rejection episodes (further classified as spontaneously resolving, corticosteroid-sensitive or corticosteroid-resistant acute rejections). The incidence of graft loss (defined as retransplantation or death) and duration of patient survival were also recorded. In addition, AEs and abnormal laboratory measurements for hematology and biochemistry (performed at baseline, at the time of PK profiling and at week 4) were recorded for both treatment groups. Clinical laboratory parameters (including red blood cell count, hemoglobin, sodium, potassium, creatinine, total bilirubin, and albumin) were also measured at baseline, at the same time as PK profile sampling, and at weeks 4 and 6.
Statistical Analyses
From previous early-phase studies with tacrolimus qd,8 a steady-state AUC0-24 ratio of 0.9 and a standard deviation (SD) of 0.35 for each treatment arm was assumed in this study and it was estimated that 32 patients with 3 complete PK profiles each would be adequate to obtain well-founded information on the primary parameter and its ratio. Taking into consideration potential dropout rates, it was planned to enroll approximately 40 patients per treatment arm.
PK analyses were conducted in the PK Evaluable Set (patients who received all doses of study medication, provided 3 complete evaluable PK profiles, and had no major protocol violations). Comparison of PK parameters for tacrolimus qd and tacrolimus bid (AUC0-24, Cmax, and Cmin) was performed using a 2-sided 90% confidence interval for the ratio of means, with an acceptance interval of 80%-125% for similarity between any 2 parameters. Descriptive statistics were calculated for Tmax. The correlation between Cmin and AUC0-24 was assessed for both tacrolimus qd and tacrolimus bid.
PK parameters were calculated from the complete 24-hour concentration–time profiles of tacrolimus in whole-blood following administration of tacrolimus qd or tacrolimus bid. AUC0-24 was estimated using the linear trapezoidal method. Cmax, Tmax, and minimum blood concentration at 12 or 24 hours were obtained directly from the 24-hour concentration–time profiles.
Efficacy and safety analyses were conducted for the Full Analysis Set (patients who received at least 1 dose of study medication). Incidence of acute rejection and BPAR were assessed using a chi-square test. The incidence of, and time to, first acute rejection and to first BPAR episode was calculated using Kaplan-Meier survival estimates. The difference between treatments was assessed using the Wilcoxon Gehan test. Differences between treatments in the severity of BPAR episodes were assessed using the Wilcoxon rank-sum test. Patient and graft survival were analyzed using Kaplan-Meier estimates (Wilcoxon Gehan test).
RESULTS
Study Population
A total of 133 patients were randomized to tacrolimus qd (n = 69) or tacrolimus bid (n = 64) treatment. Of these patients, 4 did not receive study medication. Therefore, 129 (tacrolimus qd, n = 67; tacrolimus bid, n = 62) treated patients were included in the efficacy and safety analyses (Full Analysis Set). Evaluable PK data (from patients with 3 evaluable PK profiles without any major PK relevant protocol violations) were available for 77 patients (tacrolimus qd, n = 45; tacrolimus bid, n = 32). Patient disposition (Fig. 2) was generally comparable between the tacrolimus qd and tacrolimus bid groups; however, slightly more patients were withdrawn following administration of tacrolimus bid.
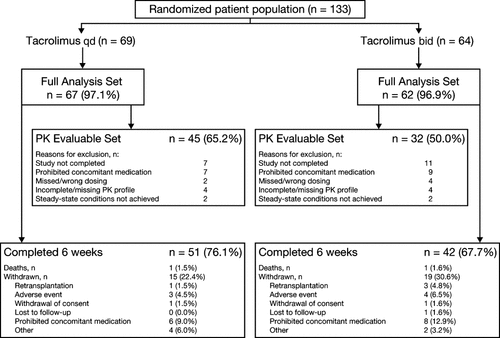
Patient disposition.
Baseline demographics and disease characteristics for all randomized and treated patients (Full Analysis Set; Table 1) were similar for patients who received tacrolimus qd or tacrolimus bid. Patient demographics for the PK Evaluable Set were also similar for the 2 treatment groups (Table 1), with the exception of mean patient age, which was higher for the tacrolimus bid treatment group (52.7 years, range 35-65 years) compared with the tacrolimus qd treatment group (48.6 years, range 26-64 years, P = 0.044; Student t test).
| Characteristics | Tacrolimus qd (n = 67) | Tacrolimus bid (n = 62) |
|---|---|---|
| Full Analysis Set | ||
| Male patients (n [%]) | 49 (73.1) | 45 (72.6) |
| Age (years), mean (range) | 49.4 (24-65) | 52.4 (27-68) |
| Weight (kg), mean (range) | 78.0 (40-127) | 77.5 (48-142) |
| Race, n (%) | ||
| Caucasian | 65 (97.0) | 61 (98.4) |
| Black | 1 (1.5) | 0 |
| Asian | 0 | 1 (1.6) |
| Other | 1 (1.5) | 0 |
| Viral status, n (%) | ||
| Hepatitis B surface antigen–positive | 6 (9.0) | 5 (8.1) |
| Anti-hepatitis C–positive | 21 (31.3) | 19 (30.6) |
| Existing glucose metabolism disorder | 19 (28.4) | 21 (33.9) |
| Primary diagnosis, n (%) | ||
| Cirrhosis* | 48 (71.6) | 47 (75.8) |
| Primary biliary | 2 (3.0) | 2 (3.2) |
| Secondary biliary | 1 (1.5) | 1 (1.6) |
| Posthepatitic | 19 (28.4) | 19 (30.6) |
| Hepatitis B | 3 (4.5) | 4 (6.5) |
| Hepatitis B delta | 2 (3.0) | 1 (1.6) |
| Hepatitis C | 14 (20.9) | 15 (24.2) |
| Autoimmune | 3 (4.5) | 2 (3.2) |
| Alcoholic | 23 (34.3) | 17 (27.4) |
| Cryptogenic | 3 (4.5) | 4 (6.5) |
| Other | 2 (3.0) | 3 (4.8) |
| Carcinoma† | 10 (14.9) | 6 (9.7) |
| Budd–Chiari | 2 (3.0) | 0 |
| Metabolic disease | 2 (3.0) | 1 (1.6) |
| Sclerosing cholangitis | 3 (4.5) | 6 (9.7) |
| Other | 2 (3.0) | 2 (3.2) |
| PK Evaluable Set | Tacrolimus qd (n = 45) | Tacrolimus bid (n = 32) |
| Male patients, n (%) | 34 (75.6) | 24 (75.0) |
| Age (years), mean (range) | 48.6 (26-64) | 52.7 (35-65)‡ |
| Weight (kg), mean (range) | 78.2 (40-127) | 78.9 (48-142) |
| Caucasian | 45 (100) | 32 (100) |
| Viral status | ||
| Hepatitis B–positive | 5 (11.1) | 4 (12.5) |
| Anti-hepatitis C–positive | 13 (28.9) | 8 (25.0) |
| Existing glucose metabolism disorder | 13 (28.9) | 6 (18.8) |
| Donor Characteristics | Tacrolimus qd (n = 67) | Tacrolimus bid (n = 62) |
| Male donor, n (%) | 43 (64.2) | 35 (56.5) |
| Donor age (years), mean (range) | 41.2 (6-78) | 45.2 (15-81) |
| Partial liver transplant, n (%) | 7 (10.4) | 8 (12.9) |
| Assessment of liver, n (%) | ||
| Very good/good | 61 (91.0) | 56 (90.3) |
| Fair/poor/not recorded | 6 (9.0) | 6 (9.7) |
| Total ischemia time (hours), mean (range) | 8.2 (2.2-16.3)§ | 8.2 (2.0-16.0)e |
- * Multiple reports possible
- † All hepatocellular carcinoma
- ‡ P = 0.044; all other demographic parameters were not significantly different
- § n = 63
- e n = 58.
Donor characteristics and surgical details were comparable for the tacrolimus qd and tacrolimus bid treatment groups (Table 1).
Drug Administration and Exposure
In the Full Analysis Set, all patients received maintenance corticosteroid therapy. Median daily starting dose was 750 mg/day in both treatment arms (with ranges of 20-1500 mg for tacrolimus qd and 25-1500 mg for tacrolimus bid). Maintenance daily doses were gradually tapered to a median of 15 mg/day in both treatment arms (with ranges of 10-25 mg for tacrolimus qd and 5-20 mg for tacrolimus bid) by week 6.
Other immunosuppressive medication, generally administered as prophylaxis, was received by 16/67 (23.9%) patients in the tacrolimus qd group and 17/62 (27.4%) patients in the tacrolimus bid group. In the tacrolimus qd group, 1 (1.5%) patient received mycophenolate mofetil (MMF), 14 (20.9%) received azathioprine (AZA), and 1 (1.5%) received both MMF and AZA. In the tacrolimus bid group, 4 (6.5%) patients received MMF, 12 (19.4%) received AZA, 1 (1.6%) received both MMF and AZA and 1 (1.6%) received sirolimus. Of these patients, 2 patients in the tacrolimus qd group and 4 patients in the tacrolimus bid group had MMF or AZA added at the time of treatment of a rejection episode.
The mean total daily doses of both tacrolimus qd and tacrolimus bid increased during the early posttransplant period (Fig. 3A). Apart from the first dose (day 1), the mean tacrolimus qd dose (0.117 mg/kg on day 1 to 0.226 mg/kg at week 6) was higher than the corresponding tacrolimus bid dose (0.110-0.162 mg/kg). Trough levels were similar for patients treated with tacrolimus qd and tacrolimus bid (Fig. 3B).
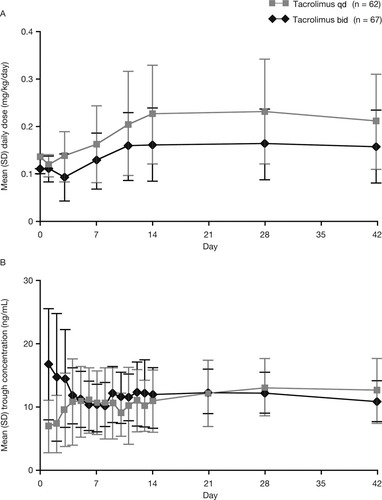
Summary of (A) daily tacrolimus doses and (B) trough concentrations (Full Analysis Set).
During the study, patients could receive concomitant medication (Table 2).
| Full Analysis Set | Tacrolimus qd (N = 67) | Tacrolimus bid (N = 62) |
|---|---|---|
| n (%) | n (%) | |
| Furosemide | 56 (83.6) | 49 (79.0) |
| Ranitidine | 43 (64.2) | 37 (59.7) |
| Bactrim | 36 (53.7) | 37 (59.7) |
| Insulin | 34 (50.7) | 32 (51.6) |
| Ursodeoxycholic acid | 31 (46.3) | 28 (45.2) |
| Heparin-fraction | 29 (43.3) | 27 (43.5) |
| Heparin | 26 (38.8) | 29 (46.8) |
| Albumin human | 27 (40.3) | 24 (38.7) |
| Metronidazole | 27 (40.3) | 24 (38.7) |
| Morphine | 28 (41.8) | 22 (35.5) |
| Nystatin | 27 (40.3) | 23 (37.1) |
| Acetylcysteine | 28 (41.8) | 21 (33.9) |
| Aciclovir | 29 (43.3) | 19 (30.6) |
- * Received by at least 40% of patients in either group.
Pharmacokinetics
On day 1, mean AUC0-24 of tacrolimus at equivalent doses was approximately 50% lower for tacrolimus qd than for tacrolimus bid (146 versus 264 ng·h/mL, respectively; Table 3 and Fig. 4). The AUC for tacrolimus qd on day 1 was outside the 90% confidence interval (80-125), and therefore was not equivalent to AUC with tacrolimus bid. However, AUC0-24 for tacrolimus qd was comparable with that of tacrolimus bid by day 14 (324 versus 287 ng·h/mL, respectively) and at week 6 (364 versus 301 ng·h/mL; respectively) although the mean daily doses of tacrolimus qd were higher after day 1 (Fig. 3A), ie, in order to achieve similar tacrolimus exposure, higher doses of tacrolimus qd were required. Furthermore, by day 4, trough levels were similar for both formulations (Fig. 3B). There was a strong correlation between AUC0-24 and C24 for both tacrolimus qd and tacrolimus bid (r = 0.92 and r = 0.76, respectively; Fig. 5).
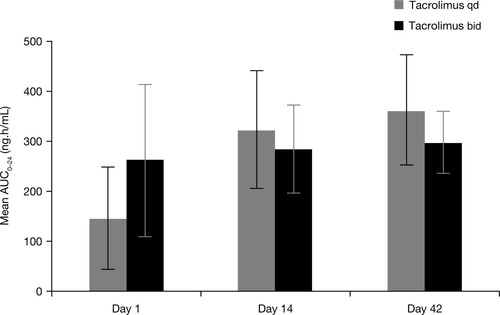
Mean AUC0-24 (SD) for tacrolimus (PK Evaluable Set).
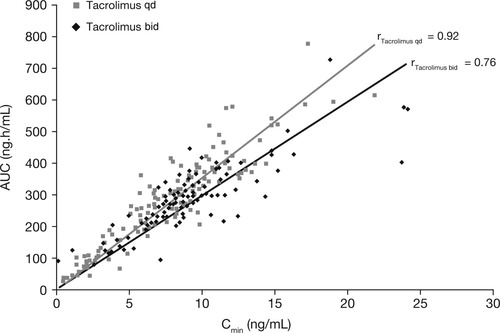
Correlation between AUC0-24 and Cmin for tacrolimus administered as tacrolimus qd and tacrolimus bid (PK Evaluable Set). Lines of best fit: tacrolimus qd: y = 34.401x; tacrolimus bid: y = 29.583x.
| PK Parameters | Tacrolimus qd (n = 45) | Tacrolimus bid (n = 32) |
|---|---|---|
| Day 1* | Mean (SD) | |
| Cmax (ng/mL) | 10.59 (6.26) | 19.75 (8.48) |
| C24 (ng/mL) | 4.21 (3.31) | 8.98 (5.90) |
| Tmax (hours)† | 5.0 (4.0) | 2.9 (1.8) |
| AUC0-24 (ng·h/mL) | 145.97 (103.03) | 263.82 (153.36) |
| ln(AUC0-24) ratio (90% confidence interval) tacrolimus qd:bid | 50.3% (39.0-65.0) | |
| Day 14‡ | Mean (SD) | |
| Cmax (ng/mL) | 25.65 (11.61) | 25.07 (12.13) |
| C24 (ng/mL) | 8.82 (3.18) | 8.53 (2.85) |
| Tmax (hours) | 2.8 (2.5) | 1.9 (1.5) |
| AUC0-24 (ng·h/mL) | 324.19 (119.07) | 286.99 (88.03) |
| ln(AUC0-24) ratio (90% confidence interval) tacrolimus qd:bid | 111.4% (97.6-127.3) | |
| Week 6§ | Mean (SD) | |
| Cmax (ng/mL) | 29.20 (10.28) | 28.52 (11.51) |
| C24 (ng/mL) | 9.96 (3.53) | 9.70 (3.21) |
| Tmax (hours) | 2.8 (1.7) | 1.8 (1.7) |
| AUC0-24 (ng·h/mL) | 364.28 (110.52) | 301.10 (60.76) |
| ln(AUC0-24) ratio (90% confidence interval) tacrolimus qd:bid | 117.9% (106.1-131.0) | |
- * Mean total daily dose on day 1: tacrolimus qd = 0.118 mg/kg; tacrolimus bid = 0.112 mg/kg.
- † Tacrolimus bid: Relative to preceding morning or evening dose.
- ‡ Mean total daily dose on day 14: tacrolimus qd = 0.221 mg/kg; tacrolimus bid = 0.176 mg/kg.
- § Mean total daily dose at week 6: tacrolimus qd = 0.209 mg/kg; tacrolimus bid = 0.165 mg/kg.
- Log-normalized AUC used for comparison between formulations.
Tmax occurred later for tacrolimus qd compared with tacrolimus bid (day 1: 5.0 versus 2.9 hours; day 14: 2.8 versus 1.9 hours; week 6: 2.8 versus 1.8 hours, respectively), reflecting the prolonged-release characteristics of the qd tacrolimus formulation.
Secondary Endpoints
Rejection and Survival Rates
The overall frequency of BPAR in the Full Analysis Set was comparable for tacrolimus qd and tacrolimus bid (26.9 and 27.4%, respectively). There were no marked differences in the classification or histological severity of the acute rejections reported.
One patient receiving tacrolimus qd (1.5%) and 3 patients receiving tacrolimus bid (4.8%) experienced graft loss during the study in the Full Analysis Set. In the tacrolimus qd group, this loss was as a result of death due to cardiac arrest (caused by pulmonary hypertension) whereas in the tacrolimus bid group, 2 graft losses were the result of retransplantation (graft dysfunction and graft ischemia) and 1 due to death caused by pneumonia. One patient in each group experienced graft loss after withdrawal from the study (due to graft dysfunction in the tacrolimus qd group and portal vein thrombosis in the tacrolimus bid group).
The estimated graft survival rates with tacrolimus qd and tacrolimus bid at week 6 were similar at 96.9 and 93.3%, respectively. There was no graft loss beyond week 1 for tacrolimus qd–treated patients and beyond week 3 for tacrolimus bid–treated patients.
Estimated patient survival rates at week 6 were similar for tacrolimus qd and tacrolimus bid (98.4 and 98.1%, respectively).
Adverse Events
AEs reported during the study were consistent with the well-described safety profile of tacrolimus bid.7 There were no marked differences in the incidence, nature, or severity of AEs between tacrolimus qd and tacrolimus bid (Fig. 6). The most frequently reported AEs (Medical Dictionary for Regulatory Activities high-level term) causally related to the study drug in the tacrolimus qd and tacrolimus bid groups were: renal failure and impairment (32.8% versus 29.0%), diabetes mellitus (17.9% versus 24.2%), vascular hypertensive disorders not elsewhere classified (14.5% versus 16.4%), and hyperglycemic conditions not elsewhere classified (9.0% versus 16.1%). Renal failure and impairment was the most frequently reported serious AE and was reported in 6.0% of tacrolimus qd-treated patients and 6.5% of tacrolimus bid–treated patients (P = not significant).
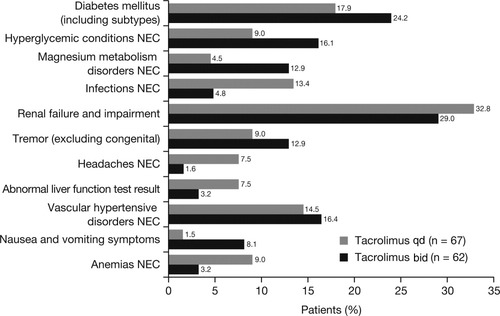
Most frequently reported AEs assessed by the investigator as causally related to study medication (Medical Dictionary for Regulatory Activities high-level term). Full Analysis Set. No differences between tacrolimus qd and tacrolimus bid were associated with P < 0.05 (Fisher's exact test). Renal failure was defined as any event reported by the investigator as: renal insufficiency, oliguria, acute renal failure, acute renal impairment not otherwise specified, anuria, and increase in serum creatinine.
Hepatic function was comparable between tacrolimus qd–treated and tacrolimus bid–treated patients throughout the study. At week 6, aspartate aminotransferase levels were 34.9 ± 53.6 and 37.7 ± 50.2 U/L, respectively, and alanine aminotransferase levels were 68.4 ± 138.8 and 77.8 ± 138.3 U/L, respectively. Renal function, as determined by serum creatinine levels and creatinine clearance, was comparable for tacrolimus qd and tacrolimus bid during the study (Table 4). At week 6, serum creatinine levels were 99.5 ± 30.7 and 99.2 ± 48.8 μmol/L and creatinine clearance rates were 83.3 ± 31.7 and 85.5 ± 25.7 mL/minute for tacrolimus qd and tacrolimus bid, respectively. There were no clinically significant findings for other laboratory variables, body weight, blood pressure or pulse rate.
| n | Tacrolimus qd (n = 67) | n | Tacrolimus bid (n = 62) | |
|---|---|---|---|---|
| Serum creatinine (μmol/L) Mean ± SD | ||||
| Day 0 | 67 | 78.9 ± 21.7 | 62 | 82.0 ± 26.6 |
| Day 1 | 65 | 93.0 ± 33.0 | 62 | 96.1 ± 32.9 |
| Day 14 | 58 | 98.2 ± 40.6 | 51 | 97.0 ± 46.0 |
| Week 4 | 53 | 98.9 ± 26.8 | 43 | 99.8 ± 51.8 |
| Week 6* | 51 | 99.5 ± 30.7 | 42 | 99.2 ± 48.8 |
| Creatinine clearance (mL/minute) Mean ± SD | ||||
| Day 0 | 67 | 111.7 ± 39.4 | 62 | 106.4 ± 38.6 |
| Day 1 | 65 | 100.3 ± 45.0 | 62 | 93.2 ± 36.2 |
| Day 14 | 58 | 90.2 ± 34.3 | 51 | 93.5 ± 38.1 |
| Week 4 | 53 | 84.2 ± 27.7 | 43 | 86.4 ± 28.4 |
| Week 6* | 51 | 83.3 ± 31.7 | 42 | 85.5 ± 25.7 |
- Full Analysis Set. Normal ranges: Serum creatinine < 133 μmol/L; Creatinine clearance 91 to 130 mL/minute.
- *Completers only.
- Comparisons between formulations at each time point did not reach statistical significance.
One-Year Follow-Up
Patients from the tacrolimus qd group had the option to enter long-term follow-up at the end of the study. Patients must have been fully informed and must have given written informed consent to demonstrate comprehension of the purpose and risks of the study to participate. Patients were excluded from the follow-up phase if they were pregnant, nursing or not using adequate contraception during the study. Of the 67 patients in the Full Analysis Set who had received at least 1 dose of tacrolimus qd, 47 (70.1%) patients chose to enter the long-term extension phase and continue treatment with tacrolimus qd.
At entry to follow-up the mean tacrolimus qd dose (SD) was 0.168 (0.105) mg/kg. At 1 year after entering the follow-up phase, mean tacrolimus qd dose (SD) was 0.096 (0.073) mg/kg. Safety and efficacy findings were consistent with the well-documented characteristics of tacrolimus bid1, 8, 9 with Kaplan-Meier estimated rates of both patient and graft survival of 95.7% at 1-year follow-up. There were 2 graft losses, which were both due to patient deaths (acute respiratory failure and abnormal hepatic function). Overall, the Kaplan-Meier estimated rate of patients free from BPAR at 1-year follow-up was 95.6%. At 1-year follow-up, mean (SD) serum creatinine and creatinine clearance rates were 101.0 μmol/L (20.5) and 86.3 mL/minute (27.1), respectively.
DISCUSSION
This is the first study to characterize the PK of tacrolimus in de novo liver transplant recipients. The primary objective of this study was to compare PK parameters of tacrolimus qd and bid following liver transplantation. Systemic exposure (AUC0-24) on day 1 was 50% lower for tacrolimus qd than for tacrolimus bid at equivalent doses. Following dose adjustments, the systemic exposure to tacrolimus as measured by whole-blood trough level was similar by day 4 for both formulations. In order to achieve similar tacrolimus exposure, higher doses of tacrolimus qd were required. The reasons for differences in the extent of absorption between the 2 tacrolimus formulations during the immediate posttransplant period are not yet fully understood. In order to achieve similar exposure to tacrolimus bid, the initial dose of tacrolimus qd would need to be higher. A higher starting dose of tacrolimus qd has been investigated in subsequent studies.
Because tacrolimus is a narrow therapeutic index medication, doses are optimized using whole-blood trough level monitoring as a surrogate marker of exposure (AUC). Thus it is critical to ensure that the relationship and correlation between AUC and trough levels is similar between any 2 formulations. This study has demonstrated a good correlation between whole-blood tacrolimus trough levels and AUC0-24, and a similar slope of the regression line, in de novo liver transplant patients, for both tacrolimus qd and tacrolimus bid. This indicates that, for drug-level monitoring, the same recommended target levels can be applied for both formulations to ensure similar exposure (AUC). This observation is also supported by data from a previous PK study in stable liver transplant patients converted from tacrolimus bid to tacrolimus qd.6
Although the study was not powered to show differences in efficacy and safety profiles, these were comparable for tacrolimus qd and bid during the 6-week study period, with patient survival rates over 98% and graft survival rates over 93% for both formulations. BPAR rates were also similar for tacrolimus qd and tacrolimus bid at 6 weeks. The overall safety profile of tacrolimus qd was comparable to that of tacrolimus bid in this 6-week study. Although some types of AE considered to be tacrolimus-related were higher in the tacrolimus qd arm (eg, infection, renal failure and impairment, headache, and abnormal liver function), other AEs were higher in the tacrolimus bid arm (eg, diabetes mellitus, hyperglycemia, tremor, nausea). Therefore, it is not possible to draw definite conclusions from the comparison between the 2 formulations from this 6-week study with relatively small patient numbers. The higher incidence of infection in the tacrolimus qd group was mainly a result of procedure-related infections (eg, wound and catheter-related infection); importantly, the overall incidence and pattern of infection, as well as the incidence of serious or dangerous infections, was similar between groups. The reasons for the greater incidence of headaches and anemia among tacrolimus qd recipients is unclear, particularly with the small sample size of the current study.
For tacrolimus qd patients who were entered into the long-term follow-up, efficacy and safety profiles were consistent with the well-described characteristics of tacrolimus bid after 1 year,1 with patient and graft survival rates in excess of 95% at 1-year follow-up. Renal function was similar between tacrolimus bid and qd formulations at the end of the 6-week study and remained stable with tacrolimus qd after 1 year of follow-up.
The strengths of the study design are that this study was adequately powered to compare PK parameters, and validated assay methods10 were used to determine tacrolimus concentrations. Notably, the PK Evaluable Set only included patients who completed all PK evaluations and had no other protocol violations. A potential limitation associated with the study may be that, due to the 6-week study duration and relatively low patient numbers, the study was not designed to show differences in efficacy and safety between tacrolimus qd and tacrolimus bid. These parameters will be investigated in further phase 3 trials that are currently underway.
The higher mean total daily doses with tacrolimus qd versus tacrolimus bid achieved similar exposure as measured by trough levels and as determined by the observed AUC at week 2 and week 6.
In conclusion, this study provides evidence that tacrolimus qd can be administered to de novo liver transplant recipients; furthermore, the equally good correlation and similar slope of the line for tacrolimus trough levels and AUC for both formulations suggests that targeting the same trough level should achieve similar total AUC over the 24-hour period for both tacrolimus qd and tacrolimus bid.
Acknowledgements
Tacrolimus Modified Release Liver Study Group: L. Fischer (Germany); P. Trunečka (Czech Republic); B. Gridelli (Italy); M. Colledan (Italy); A. Roy (Canada); D. D'Amico (Italy); A. Valdivieso (Spain); E. Varo (Spain); J. Langrehr (Germany); S. Lynch (Australia); D. Samuel (France); B.-G. Ericzon (Sweden); K. Boudjema (France); G. McCaughan (Australia); R. Margreiter (Austria); J. Tchervenko (Canada); C. Scudamore (Canada); C. Margarit (Spain); G.P. Pageaux (France); J. O'Grady (UK); L. Bäckman (Sweden); M. Meurisse (Belgium).
The design and results of this study have been presented previously in an abstract at the American Transplant Congress 2005: 6th Annual Joint Meeting of the American Society of Transplant Surgeons and the American Society of Transplantation; May 21-25, 2005; Seattle, WA, USA.
The authors thank Caroline Anthony, a professional medical writer with Acumed, for her assistance in drafting and revising the manuscript.