Improved rat steatotic and nonsteatotic liver preservation by the addition of epidermal growth factor and insulin-like growth factor-I to University of Wisconsin solution
Abstract
This study examined the effects of epidermal growth factor (EGF) and insulin-like growth factor-I (IGF-I) supplementation to University of Wisconsin solution (UW) in steatotic and nonsteatotic livers during cold storage. Hepatic injury and function were evaluated in livers preserved for 24 hours at 4°C in UW and in UW with EGF and IGF-I (separately or in combination) and then perfused ex vivo for 2 hours at 37°C. AKT was inhibited pharmacologically. In addition, hepatic injury and survival were evaluated in recipients who underwent transplantation with steatotic and nonsteatotic livers preserved for 6 hours in UW and UW with EGF and IGF-I (separately or in combination). The results, based on isolated perfused liver, indicated that the addition of EGF and IGF-I (separately or in combination) to UW reduced hepatic injury and improved function in both liver types. A combination of EGF and IGF-I resulted in hepatic injury and function parameters in both liver types similar to those obtained by EGF and IGF-I separately. EGF increased IGF-I, and both additives up-regulated AKT in both liver types. This was associated with glycogen synthase kinase-3β (GSK3β) inhibition in nonsteatotic livers and PPARγ overexpression in steatotic livers. When AKT was inhibited, the effects of EGF and IGF-I on GSK3β, PPARγ, hepatic injury and function disappeared. The benefits of EGF and IGF-I as additives in UW solution were also clearly seen in the liver transplantation model, because the presence of EGF and IGF-I (separately or in combination) in UW solution reduced hepatic injury and improved survival in recipients who underwent transplantation with steatotic and nonsteatotic liver grafts. In conclusion, EGF and IGF-I may constitute new additives to UW solution in steatotic and nonsteatotic liver preservation, whereas a combination of both seems unnecessary. Liver Transpl 16:1098–1111, 2010. © 2010 AASLD.
In human liver transplantation, a long ischemic period is a predicting factor for posttransplantation graft dysfunction. As such, some transplantation groups hesitate to transplant liver grafts preserved for more than 10 hours. However, in shorter ischemic periods, liver transplantation may also result in primary organ dysfunction.1 If this happens in healthy livers, the risks of dysfunction or nonfunction of the graft are still greater in the presence of steatosis.2, 3 For this reason, many steatotic livers are discarded for transplantation, exacerbating the critical shortage of donor livers.4, 5 The main aim of organ preservation, therefore, is to strive to prolong steatotic liver graft tolerance.1, 6
The most widely used preservation solutions, including University of Wisconsin (UW) solution, deprive the donor organ of essential trophic factors. Trophic factor deprivation is associated with deleterious effects such as apoptosis, cell-cycle arrest, and cell death.7-9 A recent study indicated that the treatment with insulin-like growth factor-I (IGF-I) and epidermal growth factor (EGF) protected steatotic and nonsteatotic livers against warm ischemia/reperfusion (I/R) injury.10 The interaction between EGF and IGF-I is a field of increasing interest, with various results reported in warm hepatic ischemia and isolated hepatocytes.10, 11 The main purpose of the present study was to evaluate the effect of EGF and IGF-I as additives to UW solution in steatotic and nonsteatotic liver preservation and to clarify the relationship between EGF and IGF-I under these conditions. The second purpose was to evaluate the involvement of a serine-threonine protein kinase, AKT, in the effects of EGF and IGF-I on steatotic and nonsteatotic liver preservation. This approach was based on: (1) data reported in the heart and brain indicating that EGF and IGF-I activate AKT12-14; and (2) numerous reports indicating that AKT promotes cell survival in different models of hepatic ischemia.15-17 Finally, the third purpose of this study was to evaluate whether AKT regulated glycogen synthase kinase-3β (GSK3β) and peroxisome proliferator-activated receptor-γ (PPARγ) in both liver types. This approach was based on: (1) data reported in the literature indicating that, in the liver, AKT's substrates include GSK3β, which is an important regulator of cell survival18, 19; and (2) previous studies revealing the key role of PPARγ in steatotic livers under warm ischemia conditions10, 20 and its up-regulation by AKT.15, 21 Our findings could lead to the design of new additives to UW solution for preserving steatotic and nonsteatotic liver grafts during cold ischemia.
Abbreviations:
AKTinh, AKT inhibitor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSP, sulfobromophthalein; EGF, epidermal growth factor; GSK3β, glycogen synthase kinase-3β; IGF-I, insulin-like growth factor-I; I/R, ischemia/reperfusion; Ln, lean; mRNA, messenger RNA; Ob, obese; pAKT, phosphorylated AKT; PCR, polymerase chain reaction; pGSK3β, phosphorylated GSK3β; PPARγ, peroxisome proliferator-activated receptor-γ; TR, orthotopic liver transplantation; UW, University of Wisconsin.
MATERIALS AND METHODS
Experimental Animals
In the studies based on isolated perfused liver model, homozygous (obese, Ob) and heterozygous (lean, Ln) Zucker rats (Iffa-Credo, L'Abresle, France) aged 16-18 weeks were used.22 Ob rats showed severe macrovesicular and microvesicular fatty infiltration in hepatocytes (between 60% and 70% steatosis), whereas Ln rats showed no evidence of steatosis. In the studies based on a liver transplantation model, homozygous (Ob) and heterozygous (Ln) Zucker rats (Iffa-Credo) aged 10-11 weeks were used.23 Ob rats showed moderate macrovesicular and microvesicular fatty infiltration into hepatocytes (between 40% and 60% steatosis), whereas Ln rats showed no evidence of steatosis. All procedures were performed under isofluorane inhalation anesthesia.22, 23 This study respected European Union regulations (Directive 86/609 EEC) for animal experiments. Animals were randomly distributed into groups as described below.
Experimental Design
Isolated Perfused Liver Model.
- 1
Control 1 (Cont 1): Livers from 16 Zucker rats (8 Ln and 8 Ob) were flushed via the portal vein without ischemic preservation.24
- 2
Control 2 (Cont 2): Livers from 16 Zucker rats (8 Ln and 8 Ob) were flushed via the portal vein without ischemic preservation and perfused ex vivo (see below).24
- 3
UW: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW solution.24
- 4
UW+EGF: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW solution with the addition of recombinant human epidermal growth factor at a concentration of 10 μg/L.25, 26
- 5
UW+IGF-I: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW with the addition of recombinant human IGF-I at a concentration of 10 μg/L.25, 26
- 6
UW+EGF+IGF-I: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW solution with the addition of EGF at a concentration of 10 μg/L and IGF-I at a concentration of 10 μg/L.25, 26
- 7
UW+EGF+AKTinh: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW solution with the addition of EGF at a concentration of 10 μg/L and LY294002, an inhibitor of AKT (AKTinh), at a concentration of 1.5 mg/L.27
- 8
UW+IGF-I+AKTinh: Livers from 16 Zucker rats (8 Ln and 8 Ob) were preserved for 24 hours in UW solution with the addition of IGF-I at a concentration of 10 μg/L and LY294002, an inhibitor of AKT, at a concentration of 1.5 mg/L.27
At the end of the protocol, aliquots of the effluent flushed from groups 1, 3, 4, 5, and 6 were sampled for measurements of aminotransferases. In addition, to account for the period of rewarming during surgical implantation in vivo,24, 28 livers from groups 2, 3, 4, 5, 6, 7, and 8 were warmed to 22°C for 30 minutes prior to reperfusion. Livers were then connected via the portal vein to a recirculating perfusion system for 120 minutes at 37°C.24, 29 Time 0 was the point at which the portal catheter was satisfactorily connected to the circuit. As previously reported,24, 29 during the first 15 minutes of perfusion (initial equilibration period), the flow was steadily increased until stabilization of the portal pressure was achieved at 12 mm Hg (Pression Monitor BP-1; Pression Instruments, Sarasota, FL). The flow was controlled by a peristaltic pump (Minipuls 3; Gilson, France).24, 29 The reperfusion liquid consisted of a cell culture medium (William's medium E; BioWhittaker, Barcelona, Spain) with a Krebs-Heinseleit–like electrolyte composition enriched with 5% albumin as oncotic supply. The buffer was continuously ventilated with 95% O2 and 5% CO2 gas mixture. The buffer was subsequently passed through a heat exchanger (37°C) and a bubble trap prior to entering the liver.24, 28 During 120 minutes of normothermic reperfusion, the effluent fluid was collected at 30-minute intervals to measure aminotransferases. Bile output, hepatic clearance (expressed as percentage of sulfobromophthalein [BSP] in bile samples), cleaved caspase-3 (a critical downstream element in the apoptotic cascade),30 cleaved caspase-9 (involved in mitochondrial apoptotic cascade),31 caspase-12 (involved in endoplasmic reticulum apoptotic cascade),32 EGF, IGF-I, AKT, GSK3β, and PPARγ were all evaluated after 120 minutes of reperfusion. Histology and TUNEL (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling) assay were also carried out after 120 minutes of reperfusion.
Liver Transplantation Model.
- 1
Sham (n = 12 rats): Ln and Ob animals (6 in each group) were subjected to transversal laparotomy, and silk ligatures were applied in the right suprarenal vein, diaphragmatic vein, and hepatic artery.
- 2
TRUW (n = 24 rats). This group was divided into two subgroups: (1) (n = 12 rats, 6 donor rats and 6 recipient rats, 6 transplantations). Steatotic livers from Ob Zucker rats (n = 6 rats) were flushed and preserved for 6 hours in UW solution.23 Standard orthotopic liver transplantation (TR) in Ln Zucker rats (n = 6 rats) was performed according to the Kamada cuff technique, without hepatic artery reconstruction33; (2) (n = 12 rats, 6 donor rats and 6 recipient rats, 6 transplantations). Nonsteatotic livers from Ln Zucker rats (n = 6 rats) were flushed and preserved for 6 hours in UW solution.23 Standard orthotopic liver transplantation in Ln Zucker rats (n = 6 rats) was performed according to the Kamada cuff technique, without hepatic artery reconstruction.33
- 3
TRUW+EGF (n = 24 rats): Same as group 2, but steatotic and nonsteatotic livers were flushed and preserved for 6 hours in UW solution with the addition of EGF at a concentration of 10 μg/L.25, 26
- 4
TRUW+IGF-I (n = 24 rats): Same as group 2, but steatotic and nonsteatotic livers were flushed and preserved for 6 hours in UW solution with the addition of IGF-I at a concentration of 10 μg/L.25, 26
- 5
TRUW+EGF+IGF-I (n = 24 rats): Same as group 2, but steatotic and nonsteatotic livers were flushed and preserved for 6 hours in UW solution with the addition of EGF at a concentration of 10 μg/L and IGF-I at a concentration of 10 μg/L.25, 26
Plasma and liver samples were collected 4 hours after transplantation. A cold ischemic period of 6 hours is long enough to induce liver damage after transplantation in both liver grafts and to allow high survival at 4 hours after transplantation.23, 34 Therefore, under these experimental conditions, we evaluated the effect of EGF and IGF-I as additive in UW solution on I/R injury associated with transplantation by measuring the biochemical and histological parameters of hepatic injury. To evaluate the effect of EGF and IGF-I as additive to UW solution on survival in recipients who underwent transplantation with steatotic or nonsteatotic liver grafts, the following experimental groups were added: TRUW Ln, recipients who underwent transplantation with nonsteatotic liver grafts preserved for 6 hours in UW solution; TRUW Ob, recipients who underwent transplantation with steatotic liver grafts preserved for 6 hours in UW solution; TRUW+EGF Ln, recipients who underwent transplantation with nonsteatotic liver grafts preserved for 6 hours in UW solution with the addition of EGF at a concentration of 10 μg/L25, 26; TRUW+EGF Ob, recipients who underwent transplantation with steatotic liver grafts preserved for 6 hours in UW solution with the addition of EGF at a concentration of 10 μg/L25, 26; TRUW+IGF-I Ln, recipients who underwent transplantation with nonsteatotic liver grafts preserved for 6 hours in UW solution with the addition of IGF-I at a concentration of 10 μg/L25, 26; and TRUW+IGF-I Ob, recipients who underwent transplantation with steatotic liver grafts preserved for 6 hours in UW solution with the addition of IGF-I at a concentration of 10 μg/L.25, 26 For each experimental group mentioned above, there were 20 rats (10 donor rats and 10 recipient rats) and 10 transplantations. As previously reported,23 recipient survival was monitored for 14 days.
Preliminary studies from our group demonstrated that the concentrations of EGF (10 μg/L) and IGF-I (10 μg/L) were the most effective in protecting the two livers against cold ischemia damage.
Biochemical Determinations
Aminotransferase Assay.
Hepatic injury was evaluated according to aminotransferase levels using commercial kit from Boehringer Mannheim (Munich, Germany).
Bile Output.
Liver function was assessed by measuring bile production.24, 29 Bile was collected through the cannulated bile duct, and output is reported as microliters per gram of liver.
Hepatic Clearance.
Similar to bile output, hepatic clearance was considered another parameter of hepatic function.24 Thirty minutes after the onset of perfusion (t30), 1 mg of BSP was added to the perfusate. The concentration of BSP in bile samples (t120) was measured at 580 nm with an ultraviolet-visible spectrometer. Bile BSP excretion was expressed as a percentage of perfusate content (t120 bile/t30 perfusate × 100).24
Reverse Transcription and Quantitative Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (PCR) analysis was performed by the premade Assays-on-Demand TaqMan probes (Rn00563336_m1 for egf, Rn00710306_m1 for igf1, and Rn00667869_m1 for β-actin; Applied Biosystems, Foster City, CA). The TaqMan gene expression assay was performed according to the manufacturer protocol (Applied Biosystems).
Western Blotting.
Western blotting was performed as described elsewhere10 using the following antibodies: EGF and IGF-I (Santa Cruz Biotechnology, Santa Cruz, CA), total and phosphorylated AKT, cleaved caspase-3, cleaved caspase-9 and caspase-12 (Cell Signaling Technology, Beverly, MA), total and phosphorylated PPARγ (Abcam, UK), total and phosphorylated GSK3β (BD Transduction Laboratories, San Jose, CA) and β-actin (Sigma Chemical, St. Louis, MO). The bands were viewed by means of an enhanced chemiluminescence kit (Bio-Rad Laboratories, Hercules, CA). The values were obtained by densitometric scanning and the Quantity One software program. The scanning values for EGF, IGF-I, cleaved caspase-3, cleaved caspase-9, and caspase-12 were divided by the scanning values for β-actin, and those for phosphorylated AKT, PPARγ, and GSK3β by total AKT, PPARγ and GSK3β, respectively.
Histology and TUNEL Assay
Liver tissues were fixed in 10% neutral buffered formalin and embedded in Paraplast, and 5-μm sections were stained with hematoxylin and eosin according to standard procedures. In an experimental model of isolated perfused liver, the extent of cell damage was graded semiquantitatively from 0 (no damage) to 3 (severe damage with disintegration of hepatic cords), as described elsewhere.29 In an experimental model of liver transplantation, sections stained with hematoxylin and eosin were evaluated by a point-counting method on an ordinal scale as follows: grade 0, minimal or no evidence of injury; grade 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; grade 2, moderate-to-severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and grade 3, severe necrosis with disintegration of hepatic cords, hemorrhage, and neutrophil infiltration.23 Steatosis in liver was evaluated by oil red-O staining on frozen specimens. Briefly, 5-μm cryosections were fixed in 60% isopropanol and stained with 0.3% oil red-O in 60% isopropanol and subsequently washed with 60% isopropanol. Sections were counterstained with hematoxylin, in line with standard procedures. For TUNEL assay, DNA fragmentation was determined using a TUNEL assay in deparaffinized liver samples as described elsewhere. TUNEL-positive nuclei were counted.35
Statistics
Data are expressed as means ± standard error and were compared statistically by variance analysis, followed by the Student-Newman-Keuls test. P < 0.05 was considered significant.
RESULTS
Effect of EGF and IGF-I as Additives in UW Solution in Isolated Perfused Liver
Steatotic livers preserved in UW solution (UW group) showed higher aminotransferase levels than nonsteatotic livers after cold ischemia and after reperfusion (Fig. 1, A and B, respectively). This confirms the increased sensitivity of this type of liver to cold ischemia and reperfusion. Figure 1B and the other figures that correspond to hepatic injury during reperfusion (for example, Fig. 7) only show the values of aminotransferases at 120 minutes of reperfusion, but a similar pattern was observed at 30, 60, and 90 minutes of reperfusion (data not shown). The addition of EGF (10 μg/L) or IGF-I (10 μg/L) to UW solution (UW+EGF and UW+IGF-I groups, respectively) reduced hepatic injury in both liver types (Fig. 1A,B). Lower concentrations of either EGF or IGF-I did not protect against hepatic injury, whereas higher concentrations of either EGF or IGF-I resulted in hepatic injury parameters higher than those observed for the UW group (data not shown). We also evaluated whether, the combination of EGF and IGF-I, at the effective concentrations (10 μg/L), would increase the benefits obtained when both drugs were added separately to UW solutions. The results indicated that the combined addition of EGF and IGF-I to UW solution (UW+EGF+IGF-I group) produced similar results in terms of hepatic injury to those obtained when EGF and IGF-I were added separately. The results of damage score (Fig. 1B) followed a pattern similar to that described for aminotransferases. The histological study of nonsteatotic liver of the UW group showed moderate cell swelling disintegration of hepatic architecture (Fig. 2A), whereas in the UW+EGF (Fig. 2B) and UW+IGF-I (Fig. 2C) groups, hepatocyte integrity was maintained. UW+EGF+AKTinh (Fig. 2D) and UW+IGF-I+AKTinh (Fig. 2E) resulted in histological lesions in nonsteatotic livers similar to those of observed in the UW group. The histological study of steatotic livers of the UW group showed severe cell swelling disintegration of hepatic architecture (Fig. 3A), whereas in the UW+EGF (Fig. 3B) and UW+IGF-I (Fig. 3C) groups, hepatocyte integrity was maintained. UW+EGF+AKTinh (Fig. 3D) and UW+IGF-I+AKTinh (Fig. 3E) resulted in histological lesions in steatotic livers similar to those of observed in the UW group.
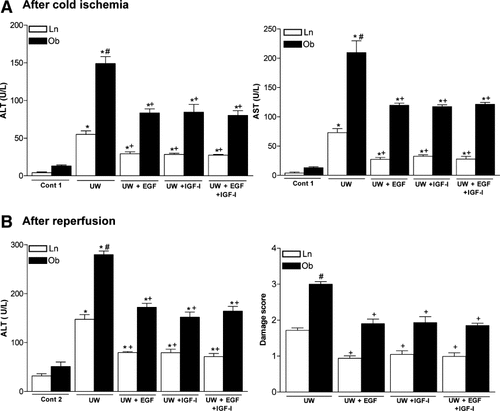
(A) Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in flushing effluent after 24 hours of cold ischemia. (B) ALT levels in perfusate and damage score in livers after 120 minutes of reperfusion (isolated perfused liver model). *P < 0.05 versus Cont, +P < 0.05 versus UW. Aminotransferase levels and damage score are significantly higher in steatotic livers of the UW group than in the nonsteatotic livers of the UW group (#P < 0.05). There were no significant differences in hepatic injury parameters between the UW+EGF, UW+IGF-I, and UW+EGF+IGF-I groups.
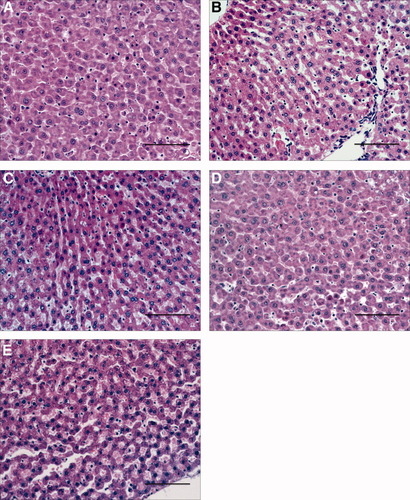
Histological change in nonsteatotic livers after 120 minutes of reperfusion (isolated liver perfused model). (A) Nonsteatotic livers preserved in UW. Moderate cell swelling and disintegration of hepatic architecture is evident. (B,C) Nonsteatotic livers preserved in (B) UW+EGF and (C) UW+IGF-I. Hepatic morphology was better preserved compared to that recorded in nonsteatotic livers preserved in UW solution. (D,E) Nonsteatotic livers preserved in (D) UW+EGF+AKTinh and (E) UW+IGF-I+AKTinh. Histological lesions are similar to that recorded in nonsteatotic livers preserved in UW. (Hematoxylin and eosin stain; bar = 100 μm.)
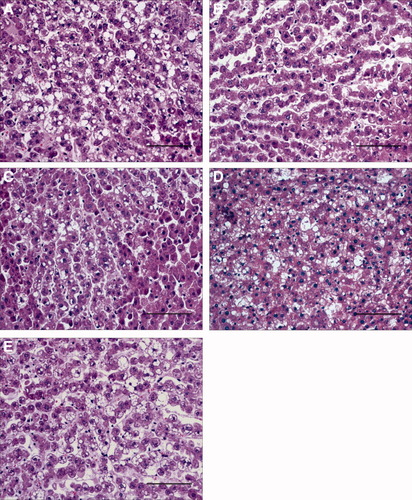
Histological change in steatotic livers after 120 minutes of reperfusion (isolated liver perfused model). (A) Steatotic livers preserved in UW. Severe cell swelling and disintegration of hepatic architecture is evident. (B,C) Steatotic livers preserved in (B) UW+EGF and (C) UW+IGF-I. Hepatic morphology was better preserved compared to that recorded in steatotic livers preserved in UW solution. (D,E) Steatotic livers preserved in (D) UW+EGF+AKTinh and (E) UW+IGF-I+AKTinh. Histological lesions similar to that recorded in steatotic livers preserved in UW are evident. (Hematoxylin and eosin stain; bar 100 μm.)
On the question of apoptotic cell death, nonsteatotic livers preserved in UW solution (UW group) showed a higher percentage of TUNEL-positive cells and caspase-3, caspase-9, and caspase-12 levels than the Cont 2 group did (Fig. 4). The addition of EGF or IGF-I to UW solution (UW+EGF and UW+IGF-I groups) reduced cell TUNEL staining and caspase-3, caspase-9, and caspase-12 levels in nonsteatotic livers. The combined addition of EGF and IGF-I to UW solution (UW+EGF+IGF-I group) produced similar results in term of apoptosis in nonsteatotic livers to those obtained when EGF and IGF-I were added separately. The percentage of TUNEL-positive cells and caspase-3, caspase-9, and caspase-12 levels in steatotic livers was at baseline in all groups (Fig. 4), which is in line with previous studies indicating that apoptosis is predominant in nonsteatotic livers.30
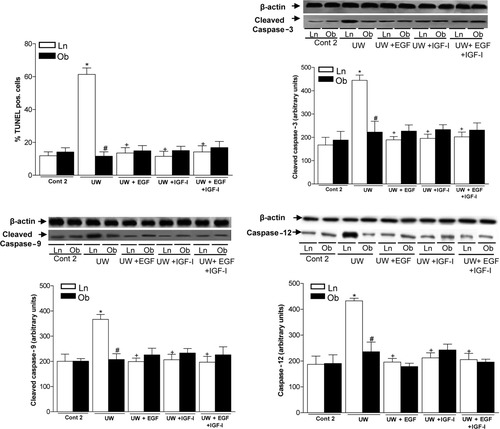
TUNEL-positive cells, cleaved caspase-3, cleaved caspase-9, and caspase-12 in livers after 120 minutes of reperfusion (isolated liver perfused model). For cleaved caspase-3, cleaved caspase-9, and caspase-12 protein levels in liver, representative western blot is shown at the top and densitometric analysis is shown at the bottom. *P < 0.05 versus Cont, +P < 0.05 versus UW. TUNEL-positive cells, cleaved caspase-3, cleaved caspase-9, and caspase-12 levels are significantly lower in steatotic livers of the UW group than in the nonsteatotic livers of the UW group (#P < 0.05). There were no significant differences in hepatic injury parameters between the UW+EGF, UW+IGF-I, and UW+EGF+IGF-I groups.
Liver function was assessed by measuring bile production and BSP clearance. Steatotic livers preserved in UW solution (UW group) showed lower bile output and percentage of BSP in bile than did nonsteatotic livers (Fig. 5). The addition of EGF or IGF-I to UW solution (UW+EGF and UW+IGF-I groups) significantly improved both liver function parameters in the two liver types. In line with the parameters of hepatic damage, the combined addition of EGF and IGF-I to UW solution (UW+EGF+IGF-I group) produced similar results in terms of liver function to those obtained when both EGF and IGF-I were added separately to UW (Fig. 5).
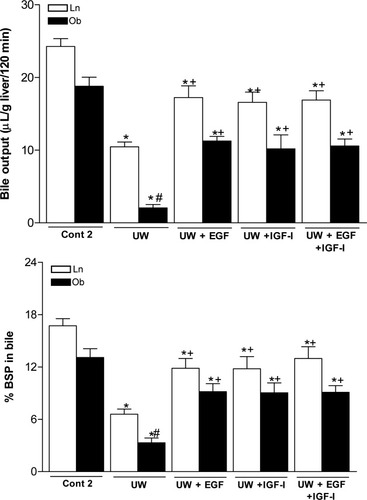
Bile output and percentage of BSP in bile of steatotic and nonsteatotic livers after 120 minutes reperfusion (isolated liver perfused model). *P < 0.05 versus Cont, +P < 0.05 versus UW. Bile output and percentage of BSP in bile are significantly lower in steatotic livers of the UW group than in the nonsteatotic livers of the UW group (#P < 0.05). There were no significant differences in liver function parameters between the UW+EGF, UW+IGF-I, and UW+EGF+IGF-I groups.
The results given here show a relationship between EGF and IGF-I in both liver types. As shown in Fig. 6A, IGF-I addition to UW solution (UW+IGF-I group) did not alter hepatic EGF, because the egf mRNA and EGF protein levels observed in both liver types were similar to those obtained for the UW group. However, in both liver types, EGF addition to UW solution (UW+EGF group) increased hepatic igf1 mRNA expression and IGF-I protein levels over those obtained for the UW group (Fig. 6B). Thus, EGF increased IGF-I in both liver types.
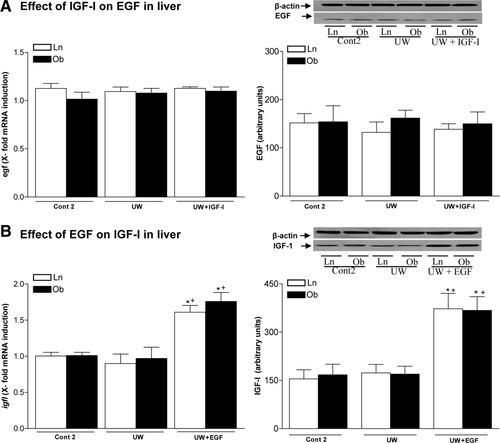
(A) Effect of IGF-I on egf mRNA expression and EGF protein levels in steatotic and nonsteatotic livers after 120 minutes of reperfusion. (B) Effect of EGF on igf1 mRNA expression and IGF-I protein levels in steatotic and nonsteatotic livers after 120 minutes reperfusion (isolated liver perfused model). For egf and igf1 mRNA expression in liver, PCR fluorescent signals for EGF and IGF-I were standardized to PCR fluorescent signals obtained from an endogenous reference (β-actin). Comparative and relative quantifications of egf and igf1 gene products normalized to β-actin and control (Cont 2 group) were calculated by the 2−ΔΔCT method. For EGF and IGF-I protein levels in liver, representative western blot is shown at the top and densitometric analysis is shown at the bottom. *P < 0.05 versus Cont, +P < 0.05 versus UW.
Next, we tested the hypothesis that IGF-I exerts its action in both liver types through AKT. IGF-I addition to UW (UW+IGF-I group) increased phosphorylated AKT in both liver types over the values for the UW group (Fig. 7A). Total AKT was unchanged in all groups. The relevance of the changes in AKT induced by IGF-I in both liver types was also investigated. As mentioned before, in our conditions, both necrosis and apoptosis occurs in nonsteatotic livers preserved in UW solution, whereas necrosis was the predominant cell death mechanism in the presence of steatosis. In nonsteatotic livers, UW+IGF-I reduced aminotransferases, damage score, percentage of TUNEL-positive cells, and caspase-3, caspase-9, and caspase-12 levels compared with the UW group whereas UW+IGF-I+AKTinh resulted in necrosis and apoptosis parameters of the same order as those of UW group (Fig. 7B). In steatotic livers, UW+IGF-I reduced aminotransferases and damage score values compared with the UW group; whereas UW+IGF-I+AKTinh resulted in hepatic damage parameters of the same order as those of the UW group (Fig. 7B). Thus, IGF-I increased AKT overexpression, which in turn protected both liver types. Because EGF may exert its protective role by increasing IGF-I expression, we also confirmed that AKT inhibition removed the beneficial effects of EGF. In fact, UW+EGF increased phosphorylated AKT (pAKT) in both liver types compared with the results of the UW group (Fig. 7A). AKT inhibition removed the beneficial effects of EGF on necrosis and apoptosis in nonsteatotic livers and on necrosis in steatotic livers (Fig. 7B).
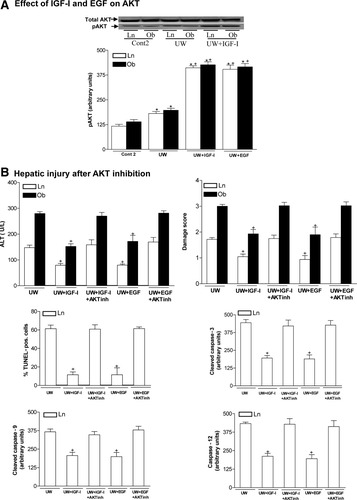
(A) Effect of IGF-I and EGF on AKT in steatotic and nonsteatotic livers after 120 minutes of reperfusion. Representative western blot at the top and densitometric analysis at the bottom. (B) Effect of AKT on hepatic injury (ALT, damage score, TUNEL-positive cells, cleaved caspase-3, cleaved caspase-9, and caspase-12) (isolated liver perfused model). *P < 0.05 versus Cont, +P < 0.05 versus UW.
We next evaluated whether AKT's substrates might include GSK3β and PPARγ. In nonsteatotic livers, UW+IGF-I and UW+EGF inactivated GSK3β (increased phosphorylated GSK3β) compared with the UW group (Fig. 8). The inhibition of AKT in nonsteatotic livers preserved in UW with IGF-I or EGF (UW+IGF-I+AKTinh and UW+EGF+AKTinh groups) resulted in phosphorylated GSK3β levels similar to those of the UW group. No differences with the Cont 2 group in phosphorylated GSK3β levels were found in steatotic livers of any group (Fig. 8). Regarding phosphorylated PPARγ, UW+IGF-I and UW+EGF increased phosphorylated PPARγ levels only in steatotic livers. The inhibition of AKT in steatotic livers preserved in UW with IGF-I or EGF (UW+IGF-I+AKTinh and UW+EGF+AKTinh) resulted in phosphorylated PPARγ levels similar to those of the UW group. No differences with the Cont 2 group in phosphorylated PPARγ levels were found in nonsteatotic livers of any group (Fig. 8). The protein levels of total GSK3β and PPARγ were unchanged in all groups.
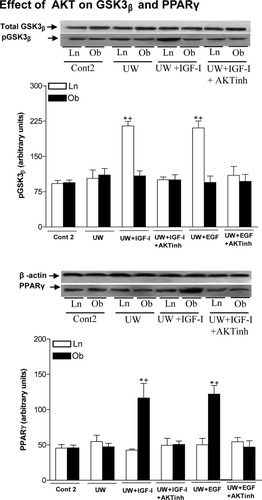
Effect of AKT on GSK3β and PPARγ in steatotic and nonsteatotic livers after 120 minutes of reperfusion (isolated liver perfused model). For GSK3β and PPARγ protein levels in liver, representative western blot is shown at the top and densitometric analysis is shown at the bottom. *P < 0.05 versus Cont, +P < 0.05 versus UW.
Effect on Liver Transplantation of EGF and IGF-I as Additives to UW Solution
Steatotic livers preserved in UW solution (TRUW group) showed higher aminotransferase levels (AST and ALT) than nonsteatotic livers did after transplantation (Fig. 9A). This confirms the increased sensitivity of this type of liver to hepatic I/R injury associated with transplantation. The addition of EGF (10 μg/L) or IGF-I (10 μg/L) to UW solution (TRUW+EGF and TRUW+IGF-I groups) reduced hepatic injury in both liver types after transplantation (Fig. 9A). We also evaluated whether the combination of EGF and IGF-I increased the benefits obtained when both drugs were added separately to UW solutions. The results indicated that the combined addition of EGF and IGF-I to UW solution (TRUW+EGF+IGF-I group) produced similar results in terms of hepatic injury to those obtained when both EGF and IGF-I were added separately to UW. The histological results (histological pictures not shown and damage score values in Fig. 9A) were consistent with the biochemical parameters of hepatic injury. The histological study of nonsteatotic liver of the TRUW group showed multifocal areas of moderate coagulative necrosis and neutrophil infiltration, randomly distributed throughout the parenchyma. TRUW+EGF, TRUW+IGF-I, and TRUW+EGF+IGF-I reduced the extent and number of necrotic areas in nonsteatotic livers, because patchy areas of incipient hepatocyte necrosis and scattered areas of coagulative hepatocyte necrosis were observed. This was consistent with damage score results. For example, in nonsteatotic liver grafts, damage score values were: 1.87 ± 0.12 and 0.92 ± 0.16, in TRUW and TRUW+IGF-I groups, respectively (Fig. 9A). The histological study of the steatotic livers of the UW group showed extensive and confluent areas of severe coagulative necrosis with neutrophil infiltration. The TRUW+EGF, TRUW+IGF-I, and TRUW+EGF+IGF-I groups showed reduced extent and number of necrotic areas in steatotic livers because multifocal areas of moderate coagulative necrosis with neutrophil infiltration were observed. For example, in steatotic liver grafts, damage score values were: 2.88 ± 0.08 and 1.80 ± 0.11 in TRUW and TRUW+IGF-I, respectively (Fig. 9A). Recipients who underwent transplantation with nonsteatotic grafts preserved in UW (TRUW Ln group) had an 80% survival rate (8 of 10) at 14 days (Fig. 9B). Survival in recipients who underwent transplantation with nonsteatotic grafts preserved in UW with addition of IGF-I or EGF (TRUW+EGF Ln and TRUW+IGF-I Ln groups) was 100% (10 of 10). Recipients who underwent transplantation with steatotic grafts preserved in UW (TRUW Ob group) showed 30% survival (3 of 10) at 14 days, with most of the deaths occurring within 2 days (Fig. 9B). The use of IGF-I or EGF as additives to UW solution (TRUW+EGF Ln and TRUW+IGF-I Ln groups) reduced lethality in recipients who underwent transplantation with steatotic grafts, and resulted in a 70% survival rate (7 of 10) at 14 days (Fig. 9B).
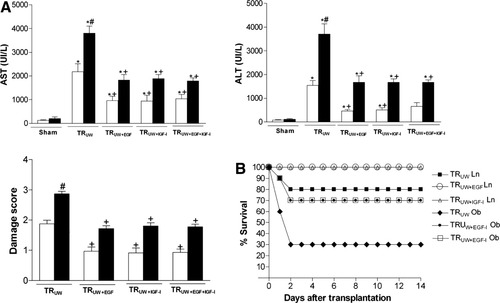
(A) Aminotransferase (AST and ALT) levels and damage score in nonsteatotic and steatotic liver grafts at 4 hours after transplantation. (B) Survival of recipients who underwent transplantation with nonsteatotic or steatotic liver grafts at 14 days after transplantation (liver transplantation model). *P < 0.05 versus Cont, +P < 0.05 versus UW. Aminotransferase levels and damage score are significantly higher in steatotic liver grafts of the TRUW group than in the nonsteatotic liver grafts of the TRUW group (#P < 0.05). There were no significant differences in hepatic injury parameters between the TRUW+EGF, TRUW+IGF-I, and TRUW+EGF+IGF-I groups.
DISCUSSION
The results of the present study, based on isolated perfused liver and liver transplantation, indicate that the addition of EGF (10 μg/kg in both liver types) and IGF-I (10 μg/kg in both liver types) to UW solution improved the capacity of this standard preservation solution in steatotic and nonsteatotic livers subjected to a prolonged cold ischemic period of 24 hours. It also reduced hepatic injury and increased survival in recipients who underwent transplantation with steatotic or nonsteatotic liver grafts preserved for 6 hours. This is of particular relevance to clinical practice, because numerous pharmacological strategies that are effective in nonsteatotic livers may not be useful in the presence of steatosis. Lower and higher doses of EGF and IGF-I than that reported here (10 μg/L) did not protect against hepatic I/R injury. It must be emphasized that, to be effective, additives to UW need to reach the liver at a suitable concentration, which could explain, at least partially, why low concentrations of EGF and IGF-I provided no protective effects. Possible explanations for the failure of concentrations of EGF and IGF-I that were higher than that reported here (10 μg/L) could be related to factors intrinsic to the drugs themselves (ie, toxic side-effects, lack of specificity, and so forth).
There is a relationship between EGF and IGF-I, whereby EGF production by IGF-I has been reported in warm hepatic ischemia.10 In addition, EGF stimulates IGF-I synthesis in isolated hepatocytes.11, 36, 37 Moreover, the effects of EGF are additive to IGF-I or synergetic with it in epithelial cells.38 Our results suggest that EGF and IGF-I had a similar degree of effectiveness on hepatic injury and liver function. In addition, it should be noted that the combination of EGF and IGF-I in UW preservation solution resulted in similar findings to those obtained when both additives were added separately to UW. Moreover, as explained below, EGF and IGF-I exerted their cytoprotective effects by similar mechanisms (AKT-GSK3β in nonsteatotic livers and AKT-PPARγ in steatotic livers). These results could be explained by the fact that EGF exerted its beneficial effects by means of IGF-I overexpression. Thus, IGF-I seems to be the most appropriate therapeutic strategy because its effects are more direct than EGF.
Previous results in warm hepatic ischemia reported that I/R impaired IGF-I synthesis in steatotic livers.10 However, this was not the case under cold hepatic ischemia conditions, because the results reported here indicated hepatic igf1 mRNA and IGF-I protein levels that were similar in both liver types. As occurred with IGF-I, hepatic egf mRNA and EGF protein levels were similar in both liver types under cold ischemia conditions. Thus, IGF-I production by EGF showed a similar increase in both mRNA and protein levels in both liver grafts. These results lead to the view that the effect of I/R on IGF-I synthesis in steatotic liver seems to depend on the type of ischemia.
It has been reported that growth factors such as IGF-I can activate AKT in heart and brain.12, 13 In our study, EGF (which increased IGF-I) and IGF-I up-regulated AKT, which in turn protected both liver types. Once activated, AKT activates downstream transcription factors that trigger survival signaling, which protects livers against I/R injury.15-17 In heart and brain, AKT inactivates GSK3β by phosphorylation, a mechanism by which neurons and myocytes become resistant to cell death stimuli.39-42 PPARγ up-regulation induced by AKT has been previously described in stellate cells and heart.15, 21 The key role of PPARγ overexpression in attenuating warm I/R injury in steatotic livers has been previously demonstrated by our group.10, 20 The precise mechanism by which activation of AKT protected both liver types was not investigated in our study. However, the possibility that AKT could promote graft cytoprotection through interaction with some of the downstream effectors, including GSK3β in nonsteatotic livers and PPARγ in the presence of steatosis should not be ruled out. Indeed, our results indicated that changes in AKT activity were reflected in differences in GSK3β and PPARγ. We find that AKT overexpression induced by EGF and IGF-I was associated with an increase of pGSK3β (leading to its inactivation) in nonsteatotic livers and with PPARγ overexpression in the presence of steatosis. Furthermore, AKT inhibition abolished the effects of EGF and IGF-I on GSK3β and PPARγ. Thus, AKT protected both liver types, possibly by different mechanisms, inactivating GSK3β in nonsteatotic livers and inducing PPARγ overexpression in steatotic livers. This is in line with reports indicating that the multiple intracellular signaling induced by AKT depends on cell type.15, 43, 44 Taking these observations into account and the structural and functional differences between hepatocytes with or without fatty infiltration,45-47 it is not surprising that our results show a differential effect of AKT on GSK3β and PPARγ, depending on the type of liver.
In conclusion, pharmacological strategies based on EGF and IGF-I as additives to UW solution protected against hepatic injury and ameliorated liver function in steatotic and nonsteatotic livers preserved in UW solution, although they do not need to be added in combination. Moreover, IGF-I addition to UW could be a more appropriate clinical therapy than EGF because its effects are more direct. EGF (which increases IGF-I) and IGF-I up-regulated AKT, thus protecting both liver types against cold ischemia injury. GSK3β in nonsteatotic livers and PPARγ in steatotic livers act downstream of AKT. In terms of clinical application, our findings may open up new possibilities for therapeutic intervention.
Acknowledgements
We are grateful to Robin Rycroft at the Language Advisory Service of the University of Barcelona for revising the English text.




