Early noninvasive measurement of the indocyanine green plasma disappearance rate accurately predicts early graft dysfunction and mortality after deceased donor liver transplantation
Abstract
Early diagnosis of graft dysfunction in liver transplantation is essential for taking appropriate action. Indocyanine green clearance is closely related to liver function and can be measured noninvasively by spectrophotometry. The objectives of this study were to prospectively analyze the relationship between the indocyanine green plasma disappearance rate (ICGPDR) and early graft function after liver transplantation and to evaluate the role of ICGPDR in the prediction of severe graft dysfunction (SGD). One hundred seventy-two liver transplants from deceased donors were analyzed. Ten patients had SGD: 6 were retransplanted, and 4 died while waiting for a new graft. The plasma disappearance rate was measured 1 hour (PDRr60) and within the first 24 hours (PDR1) after reperfusion, and it was significantly lower in the SGD group. PDRr60 and PDR1 were excellent predictors of SGD. A threshold PDRr60 value of 10.8%/minute and a PDR1 value of 10%/minute accurately predicted SGD with areas under the receiver operating curve of 0.94 (95% confidence interval, 0.89-0.97) and 0.96 (95% confidence interval, 0.92-0.98), respectively. In addition, survival was significantly lower in patients with PDRr60 values below 10.8%/minute (53%, 47%, and 47% versus 95%, 94%, and 90% at 3, 6, and 12 months, respectively) and with PDR1 values below 10%/minute (62%, 62%, and 62% versus 94%, 92%, and 88%). In conclusion, very early noninvasive measurement of ICGPDR can accurately predict early severe graft dysfunction and mortality after liver transplantation. Liver Transpl 15:1247–1253, 2009. © 2009 AASLD.
Severe graft dysfunction (SGD) is a serious and life-threatening condition following orthotopic liver transplantation (OLT) and the main reason for early retransplantation and mortality. Therefore, early diagnosis of initial graft nonfunction after transplantation is essential if appropriate action is to be taken.1
The routine diagnostic criteria used for the early evaluation of postoperative liver function are based on different nonspecific parameters, including aminotransferase and albumin levels, coagulation tests, persistence of metabolic acidosis and hypotension, renal failure, and neurological status.1-4 However, liver function tests are difficult to interpret in the postoperative period, and serial observations up to 72 hours are required; this makes it difficult to diagnose SGD early and accurately. Moreover, there is no universally accepted gold standard criteria for classifying the degree of graft function or diagnosing SGD.
Indocyanine green (ICG) is a water-soluble dye that is extracted by hepatic parenchymal cells and excreted almost entirely into the bile without significant extrahepatic elimination or enterohepatic recirculation. Therefore, the extraction rate of the dye is closely correlated with liver function.
Previous studies have shown that an ICG clearance rate of over 200 mL/minute at 24 hours after liver transplantation is associated with a favorable outcome.3 However, ICG clearance is not widely used in this context, probably because of the technical difficulties involved in the measurement of plasma ICG levels. A method for the estimation of the ICG plasma concentration by pulse spectrophotometry has been developed, allowing the noninvasive measurement of ICG clearance with the indocyanine green plasma disappearance rate (ICGPDR). This method has provided results similar to those obtained by serial blood sampling methods5 and is well correlated with the invasive aortic fiber optic method in patients undergoing OLT.6, 7 The predictive capability of noninvasive ICG clearance estimated by the plasma disappearance rate (PDR) in the prediction of prolonged jaundice in a small group of patients after living donor liver transplantation was described by Hori et al.8 However, prospective confirmation of these findings in a larger group of patients undergoing deceased donor liver transplantation is needed before clinical decisions can be based on ICG clearance.
The primary aim of our study was to prospectively determine the relationship between ICGPDR as an estimate of ICG clearance and early graft function after liver transplantation in a large series of patients. We also evaluated the role of ICGPDR in the prediction of SGD.
Abbreviations
AST, aspartate aminotransferase; AUC, area under the curve; CI, confidence interval; Cr, creatinine; HAT, hepatic artery thrombosis; ICG, indocyanine green; ICGPDR, indocyanine green plasma disappearance rate; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease; NPV, negative predictive value; OLT, orthotopic liver transplantation; PDR, plasma disappearance rate; PDR1, plasma disappearance rate measured within the first 24 hours after the surgical procedure is finished; PDRr60, plasma disappearance rate measured 60 minutes after graft reperfusion; PPV, positive predictive value; ROC, receiver operating characteristic; SGD, severe graft dysfunction.
PATIENTS AND METHODS
Characteristics of Patients and Operative Management
From February 2002 to February 2008, 172 adult patients undergoing OLT from deceased donors were consecutively included in the study. Demographic data and liver disease etiologies are shown in Table 1.
| Age (years) | 52 (9) |
| Gender (male/female) | 137/35 |
| Weight (kg) | 74 (10) |
| Height (cm) | 167 (8) |
| MELD score (points)* | 16.3 (5.6) |
| Etiology of liver disease [number (%)] | |
| Viral cirrhosis | 51 (29.6) |
| Hepatocellular carcinoma | 55 (31.9) |
| Alcoholic cirrhosis | 41 (23.8) |
| Fulminant hepatic failure | 5 (2.9) |
| Primary biliary cirrhosis | 3 (1.7) |
| Acute retransplantation | 6 (3.5) |
| Chronic rejection | 1 (0.6) |
| Sclerosing cholangitis | 4 (2.3) |
| Cryptogenic cirrhosis | 3 (1.7) |
| Amyloidotic neuropathy | 1 (0.6) |
| Hemochromatosis | 1 (0.6) |
| Cholangiocarcinoma | 1 (0.6) |
- Abbreviation: MELD, Model for End-Stage Liver Disease.
- * The MELD score does not take into account extra exception points.
The study was conducted according to the principles of the Declaration of Helsinki and with the approval of the local ethics committee. Written informed consent was obtained from each patient.
Anesthesia was induced with propofol (2 mg/kg) and fentanyl (2 μg/kg) and maintained with 1% to 2% end-tidal sevoflurane and fentanyl (1-3 μg/kg). Muscle relaxation was maintained with rocuronium. Mechanical ventilation was set to give an arterial oxygen pressure > 120 mm Hg and a carbon dioxide pressure of 35 to 45 mm Hg. The invasive arterial pressure, heart rate, electrocardiogram, pulse oximetry, end-tidal carbon dioxide pressure, esophageal temperature, pulmonary artery pressure, and continuous cardiac output were monitored routinely.
Crystalloids were infused and blood derivatives were transfused as required.
University of Wisconsin solution was used for liver preservation. All patients received ABO-compatible deceased donor livers. Recipient hepatectomy and liver replacement were performed by the standard technique with the piggyback method and without venovenous bypass.
ICG Test
A fresh water dilution of ICG monosodium salt with a concentration of 5 mg/mL was prepared. ICGPDR was obtained in all patients with a LiMON Monitor (Pulsion Medical Systems AG, Munich, Germany). This monitor measures ICG plasma concentrations noninvasively by pulse spectrophotometry with a finger-clip sensor that detects 4 near-infrared wavelengths. After a bolus intravenous injection of the dye (0.5 mg/kg; ICG Pulsion Medical Systems, AG, Munich, Germany) ICGPDR was calculated automatically by the time course of the blood ICG concentration (normal value, 18%-25%/minute).6 In less than 5% of measurements, the finger probe was improperly located, and the LiMON monitor did not provide any PDR value. In these cases, a repeat measurement was performed after a 30-minute washout period. Finally, a valid estimation of ICGPDR was obtained in all cases. PDR measurements were taken 60 minutes after graft reperfusion (PDRr60) once hepatic artery anastomosis was completed and within the first 24 hours after the surgical procedure was finished (PDR1) when hemodynamic stability was achieved [mean time between the end of surgery and PDR1 determination, 8.5 hours (range, 2-24; interquartile range, 5-11 hours). Importantly, 75% of the measurements were obtained within the first 11 hours after the end of surgery.
Postoperative Management
After surgery, patients were admitted to the intensive care unit. Graft function was evaluated with the aminotransferase level, coagulation tests, and bile production when available according to the Toronto Group criteria (Table 2) during the first 72 hours.2 This classification establishes 4 grades of function, grade I being excellent and grade IV being poor (SGD). Vascular complications, especially hepatic artery thrombosis (HAT), were examined routinely with Doppler ultrasonography on the first postoperative day, and this was followed by abdominal angiography when indicated. Blood, sputum, urine, peritoneal fluid, and abdominal wound discharge samples were cultured whenever infectious complications were suspected. Since the beginning of our liver transplant program, one of the investigators (J.M.P.), who was blinded to the ICGPDR results, evaluated the degree of hepatic function. When a T tube was not placed into the main bile duct, graft function was determined according to the remaining parameters of the Toronto classification.
| Degree of Graft Dysfunction | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| AST (U/L) | <1000 | >1000 | >2500 | >2500 |
| Bile production (mL/day) | >40 | >40 | <40 | 0 |
| Coagulopathy | Mild and improving | Mild and improving | Moderate | Severe |
- NOTE: This table was adapted from Greig et al.2 Mild coagulopathy is defined as INR < 2, moderate coagulopathy is defined as INR between 2 and 2.5, and severe coagulopathy is defined as INR > 2.5.
- Abbreviations: AST, aspartate aminotransferase; INR, international normalized ratio.
The study variables were the ICGPDR measured 60 minutes after graft reperfusion (PDRr60) and the ICGPDR measured within the first 24 hours after the surgical procedure was finished (PDR1).
Statistical Analysis
Unless otherwise stated, data are expressed as the mean (standard deviation). The Kolmogorov-Smirnov test was used to study the normality of the distribution of quantitative variables. Qualitative and quantitative variables were studied with the χ2 test and t test and analysis of variance, respectively. When significant differences were observed with the analysis of variance, further t tests were carried out between groups with a Bonferroni correction. In order to evaluate the influence of PDR values at different time points on patient survival, Kaplan-Meier curves were constructed and compared with the Breslow test. For this purpose, when there was retransplantation, only PDR values obtained at the time of the first liver transplant were analyzed.
In order to evaluate the predictive capacity for SGD of PDRr60 and PDR1 and the behavior of these markers with respect to classical liver function parameters [aspartate aminotransferase (AST), international normalized ratio (INR), and bilirubin on the first day], receiver operating characteristic (ROC) curves were plotted and compared.
The statistical software package SPSS, version 14.0 for Windows (SPSS, Inc., Chicago, IL), and Medcalc, version 9.4.1.0 (Medcalc Software, Mariakerke, Belgium), were used for analysis. Statistical significance was set as a 2-tailed P value of less than.05.
RESULTS
Ten patients (5.8%) were classified as having grade IV dysfunction after liver transplantation. Complete HAT diagnosed within the first 24 hours after transplantation was observed in 4 cases, whereas in the remaining 6 cases, no vascular cause for graft dysfunction was detected. All patients were listed for retransplantation, but only 6 of them received a new graft 24 to 96 hours after relisting. The remaining 4 patients died while waiting for a donor.
The different ICGPDR values and their relationship with the degree of graft function are shown in Fig. 1. Overall, there were significant differences in PDRr60, mainly because of low ICG elimination in group IV in comparison with the other groups. No differences were observed between the other groups. Moreover, these differences were maintained the first day after surgery, as assessed by PDR1.
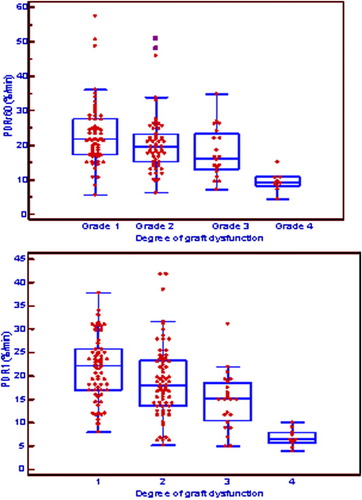
Relationship between PDRr60 and PDR1 (%/minute) and early graft function. Medians, 25th and 75th percentiles, and ranges are shown. There is a significant difference between function IV and the other function groups in both plasma disappearance rate values. Abbreviations: PDR1, plasma disappearance rate measured within the first 24 hours after the surgical procedure is finished; PDRr60, plasma disappearance rate measured 60 minutes after graft reperfusion.
There were no significant differences between donor and recipient age, cold ischemia time, Model for End-Stage Liver Disease score, and baseline serum creatinine between patients with SGD and patients without SGD (Table 3).
| Non-SGD | SGD | P Value | |
|---|---|---|---|
| Donor age | 47.87 (1.79) | 53.40 (6.99) | 0.36 |
| Baseline Cr | 1.04 (0.04) | 1.32 (0.24) | 0.30 |
| Recipient age | 52.28 (0.86) | 51.60 (2.45) | 0.81 |
| MELD score | 17.23 (0.67) | 22.329 (3.91) | 0.26 |
| Cold ischemia time | 8.99 (0.27) | 8.84 (1.11) | 0.88 |
- Abbreviations: Cr, creatinine; MELD, Model for End-Stage Liver Disease; SGD, severe graft dysfunction.
PDRr60 values were significantly greater in patients with SGD and HAT than in those without HAT [12.2 (2.5) versus 7.9 (1.9)%/minute; P = 0.02]. Only 1 patient with HAT and SGD had a relatively high PDRr60 value (15.1%/minute), which decreased to 5.6%/minute for PDR1. In this case, PDR1 determination was performed before the diagnosis of HAT with ultrasound. As expected, these differences were not observed in PDR1 values [7.8 (2.1) versus 6.0 (1.5)%/minute; P = 0.2].
PDRr60 and PDR1 were excellent predictors of grade IV function, as shown by areas under ROC curves of 0.94 [95% CI, confidence interval (CI), 0.89-0.97] and 0.96 (95% CI, 0.92-0.98) (Fig. 2), with no significant differences between the 2 curves (P = 0.31).
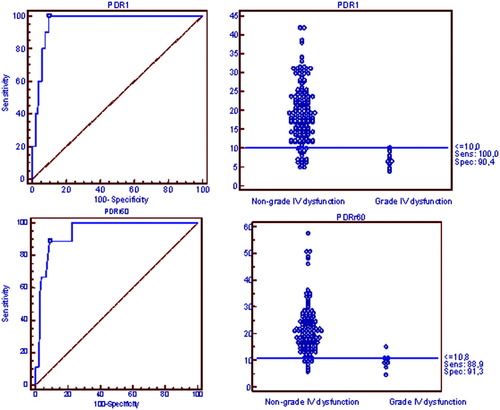
Receiver operating characteristic curves showing the predictive capacity of PDR for the development of grade IV function. Threshold points for PDRr60 and PDR1 are also shown. Abbreviations: PDR1, plasma disappearance rate measured within the first 24 hours after the surgical procedure is finished; PDRr60, plasma disappearance rate measured 60 minutes after graft reperfusion.
Interestingly, a threshold PDRr60 value of 10.8%/minute distinguished 2 different risk populations for the development of SGD with a sensitivity of 89% (95% CI, 51.7-98.2) and a specificity of 91.3% (95% CI, 85.3-95.4). Furthermore, the negative predictive value of this threshold was 99.2%, and this suggested that it would be highly unlikely to develop grade IV function in patients with a PDRr60 greater than 10.8%/minute. A threshold PDR1 value of 10%/minute also distinguished 2 different risk populations for the development of grade IV function with a sensitivity of 100% (95% CI, 69%-100%) and a specificity of 90.4% (95% CI, 84.7%-94.6%). As with PDRr60, the negative predictive value of this threshold was 100%. Interestingly, only 1 patient classified as group IV showed a PDRr60 > 10.8%/minute (15.1%/minute for a patient with postoperative development of HAT; all 10 patients in this group showed a PDR1 < 10%/minute).
In order to compare the diagnostic accuracy for SGD of both PDR determinations and classical parameters of graft dysfunction, ROC curves for INR, AST, and bilirubin serum levels on the first day after transplantation were obtained. Both PDR ROC curves showed significantly better areas under the curve than INR and bilirubin on day 1. In addition, the positive and negative predictive values of both PDR measurements were better than those of AST (Table 4).
| AUC | 95% CI | P Value | PPV | NPV | |
|---|---|---|---|---|---|
| Bilirubin | 0.599 | 0.502–0.690 | <0.001 versus PDRr60 <0.001 versus PDR1 | 13 (5.4–24.9) | 96 (88.9–99.1) |
| INR | 0.696 | 0.602–0.780 | 0.011 versus PDRr60 0.007 versus PDR1 | 22 (8.5–42.7) | 96 (90.4–98.9) |
| AST | 0.860 | 0.782–0.919 | 0.289 versus PDRr60 0.183 versus PDR1 | 23.1 (11.2–39.3) | 98.9 (94.1–99.8) |
| PDRr60 | 0.944 | 0.884–0.979 | 0.368 versus PDR1 | 40 (19.2–63.9) | 99.2 (95.7–99.9) |
| PDR1 | 0.967 | 0.915–0.991 | 40 (21.2–61.3) | 100 (97.4–100.0) |
- NOTE: The cutoff levels were as follows: 5 mg/dL for bilirubin, 4.87 for INR, 1690 UI/mL for AST, 10.8%/minute for PDRr60 (in the operating room), and 10%/minute for PDR1.
- Abbreviations: AST, aspartate aminotransferase; AUC, area under the curve; CI, confidence interval; INR, international normalized ratio; NPV, negative predictive value; PDR1, plasma disappearance rate measured within the first 24 hours after the surgical procedure is finished; PDRr60, plasma disappearance rate measured 60 minutes after graft reperfusion; PPV, positive predictive value.
The PDRr60 and PDR1 threshold levels reveal 2 different survival populations (Fig. 3). Thus, survival was significantly lower in those patients with PDRr60 values below 10.8%/minute (53%, 47%, and 47% versus 95%, 94%, and 90% at 3, 6, and 12 months, respectively; log rank, 27.2; P <.0001) and also with PDR1 values below 10%/minute (62%, 62%, and 62% versus 94%, 92%, and 88% at 3, 6, and 12 months, respectively; log rank, 13.2; P <.003).
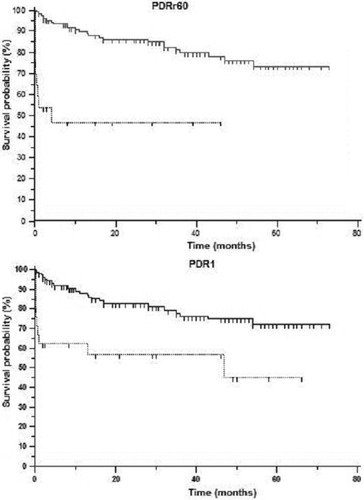
Survival probability after liver transplantation. Solid and dotted lines represent survival rates for the patients over or under the threshold points in PDRr60 and PDR1 (10.8%/minute and 10%/minute, respectively). Abbreviations: PDR1, plasma disappearance rate measured within the first 24 hours after the surgical procedure is finished; PDRr60, plasma disappearance rate measured 60 minutes after graft reperfusion.
DISCUSSION
Severe primary graft dysfunction is a life-threatening condition, and early diagnosis is essential. A simple and accurate method to identify poor graft function would allow timely intensive clinical or surgical management to bring about further improvements in the outcome of OLT. Although present therapeutic approaches designed to avoid retransplantation or to provide support to damaged liver function offer only small advantages (the use of prostaglandin E1 or N-acetyl-cystein prophylaxis),2, 9 it is conceivable that if future therapeutic interventions (ie, artificial liver support devices) are developed, early diagnosis of SGD may be useful to promptly apply such new therapies.
ICG is a water-soluble anionic compound that, after intravenous injection, binds mainly to albumin and plasma β-lipoproteins. It is taken up selectively by hepatocytes and is then excreted unchanged into bile by an adenosine triphosphate–dependent transport system. It is not metabolized and does not undergo enterohepatic recirculation. Therefore, ICG has been proposed as a means of assessing liver function in liver transplant recipients.1, 3, 8, 10 Classically, ICG concentrations are determined with spectrophotometry. Absorbance of plasma samples is read at different wavelengths, and this allows the ICG concentration and its hepatic clearance to be calculated. This procedure is time-consuming and expensive. Another method involves the use of a fiber optic aortic catheter inserted into the femoral artery. This method has been found to correlate well with graft function.11 Faybik et al.6 described a procedure to estimate the ICG concentration using pulse dye densitometry with a near-infrared 4-wavelength finger-clip sensor and central or peripheral venous access for injection of ICG. This procedure enables the measurement of ICG clearance with the PDR, which correlates well with classical measurements and with determinations obtained with fiber optic probes in critically ill patients, even in unstable liver recipients.
In this study, we show that ICGPDR measured as early as 1 hour after reperfusion or after a mean of 8 hours after OLT is strongly associated with initial graft function. These data suggest that a PDR value below the described threshold values should be considered an early indicator of poor graft outcome and may prompt more intensive care strategies and early therapeutic approaches. It is important to emphasize that PDR can be measured before unequivocal clinical expression of poor liver function. In addition, we have also shown that the predictive ability of PDR estimations is significantly better than that observed with classical parameters of liver dysfunction such as first-day bilirubin or INR values. In the case of AST, although the areas under the receiver operating curves were not significantly different, both PDR determinations showed better predictive values. Interestingly, classical clinical predictors of initial poor function such as donor and recipient age and cold ischemia time were similar between patients with SGD and patients without SGD. Therefore, PDR determinations seem to introduce relevant prognostic information that is not evident otherwise. This may be useful in clinical practice.
Furthermore, the high negative predictive value indicates that when ICGPDR values are over the specified threshold values, the probability of having SGD is very low, and this may be reassuring if there is initially torpid evolution.
Interestingly, we observed a marked similarity between the predictive ability of PDRr60 and PDR1 values. PDRr60 values were slightly higher than PDR1 values, probably because of the hyperperfusion status existing at this time.8, 12, 13
The results of the present study are similar to those reported by Hori et al.8 for the prediction of graft function after living donor liver transplantation, showing that low ICG clearance can predict poor outcome in graft recipients. However, the results of the previous study are not applicable to deceased donor liver transplantation because of the special characteristics of living donor liver transplantation, including the critical liver mass and the greater risk of vascular complications. Moreover, in Hori et al.'s report, graft dysfunction was evaluated with only the serum bilirubin level.
Interestingly, the ICGPDR value enabled us to distinguish between 2 different populations with very different survival rates; thus, patients with a PDRr60 value below 10.8%/minute and patients with a PDR1 below 10%/minute had significantly lower survival.
Our study has some limitations. First, ICG clearance depends not only on intrinsic liver function but also on liver perfusion; therefore, ICGPDR may not differentiate between severe vascular complications and primary nonfunction of the graft. Furthermore, very early ICGPDR may overestimate the degree of liver function in those patients who develop vascular complications later. In fact, we have shown that PDRr60 values are initially greater in patients with severe dysfunction associated with arterial thrombosis, although they decrease afterwards. Therefore, we suggest that both time frames for ICGPDR determination may be combined in the early diagnosis of SGD.
Unfortunately, the low number of events precludes the use of multivariate regression models. Therefore, the independent value of ICGPDR should be tested in a large population
Another important limitation is the lack of a generally accepted definition of SGD that can be used as a gold standard. In this study, we used the Toronto classification2 to evaluate early graft function because it includes the most important clinical criteria used to evaluate liver function (AST for cytolysis, INR for synthesis, and bile production for excretion). Other authors have proposed a similar classification using very similar parameters.14 However, not all types of biliary anastomoses allow the measurement of bile production. Several groups have attempted to devise a classification of primary graft dysfunction based on a combination of clinical and biochemical parameters from both the donor and the recipient or based on dynamic liver function tests, but this approach has not been adopted in routine clinical practice.15 Needle biopsy can also be used to assess graft condition, but this approach is clearly unpractical because of the high risk of bleeding and the heterogeneity of liver damage. In our study, the utility of the Toronto classification is supported by the fact that no patient classified as grade III or less finally needed immediate retransplantation and by the high observed mortality in the grade IV patients.
Another potential limitation of the study is the method of ICG measurement because a minimal amount of finger perfusion is necessary to be able to measure the variation in light absorption and thus estimate the ICG concentration. Therefore, in cases of severe hemodynamic instability or when high doses or vasoconstrictors are needed, the ICG concentration may not be estimated. In this study, we were able to obtain accurate estimations in all cases, although a second test had to be performed because of inappropriate positioning or movement of the finger probe in less than 5% of cases.
Finally, Bruegger et al.16 reported that the ICGPDR values measured during the anhepatic phase of 22 human OLT procedures differed from zero; therefore, a possible average inaccuracy of up to 1.55%/minute has to be taken into account. In our patients, all values were greater than 1.55, even in cases of SGD.
In summary, very early noninvasive measurement of ICG clearance estimated by ICGPDR can accurately predict early graft dysfunction and mortality after deceased donor liver transplantation. The use of this test may help design appropriate therapeutic approaches in this complicated setting.




