Risk factors for recurrence of autoimmune hepatitis after liver transplantation†‡
See Editorial on Page 1169
This study was presented in part at the 59th Annual Meeting of the American Association for the Study of Liver Diseases in San Francisco, CA on November 4, 2008.
Abstract
Autoimmune hepatitis has been reported to recur after liver transplantation. The aim of our study was to evaluate the risk factors associated with recurrence of autoimmune hepatitis. Forty-six patients that underwent liver transplantation because of end-stage liver disease secondary to autoimmune hepatitis were studied. Recurrence of autoimmune hepatitis was diagnosed in 11 of the 46 (24%) patients, and the overall 5-year probability of recurrence was 18%. By univariate Cox analysis, the features before liver transplantation associated with a higher risk of recurrence were concomitant autoimmune disease [hazard ratio (HR), 3.74; 95% confidence interval (CI), 1.05–13.36; P = 0.04], high aspartate aminotransferase (HR, 1.09; 95% CI, 1.03–1.14; P = 0.002), high alanine aminotransferase (HR, 1.09; 95% CI, 1.03–1.20; P = 0.003), and high immunoglobulin G (IgG; HR, 1.25; 95% CI, 1.11–1.41; P = 0.0003). Moreover, patients with recurrence had a higher frequency of moderate to severe inflammatory activity (HR, 5.3; 95% CI, 1.55–18.79; P = 0.008) and plasma cell infiltration in the liver explant (HR, 5.8; 95% CI, 1.52–22.43; P = 0.01). In the multivariate Cox analysis, only the presence of moderate to severe inflammation (HR, 6.9; 95% CI, 1.76–26.96; P = 0.006) and high IgG levels before liver transplantation (HR, 7.5; 95% CI, 1.45–38.45; P = 0.02) were independently associated with the risk of autoimmune hepatitis recurrence. In conclusion, patients with concomitant autoimmune disease, high aspartate aminotransferase, alanine aminotransferase, and IgG before the transplant, or moderate to severe inflammatory activity or plasma cell infiltration in the liver explant have a higher risk of recurrent disease. These findings suggest that recurrence of autoimmune hepatitis may reflect incomplete suppression of disease activity prior to liver transplantation. Liver Transpl 15:1254–1261, 2009. © 2009 AASLD.
Autoimmune hepatitis is a chronic inflammatory disease of the liver of unknown etiology. In general, the diagnosis is based on histological abnormalities, characteristic clinical and biochemical findings, and abnormal levels of serum globulins, including autoantibodies.1, 2
Autoimmune hepatitis is an infrequent indication for liver transplantation (LT), accounting for approximately 5% of cases in most centers.3 It is associated with good outcomes after LT, reaching survival rates at 1 and 5 years of approximately 90% and 80%, respectively.1, 4-6
Even though LT is consider the best therapeutic option for patients with end-stage liver disease secondary to autoimmune hepatitis, this disease can recur after LT with a reported incidence ranging from 15% to 40%.6-8
Risk factors associated with recurrence of autoimmune hepatitis after LT have not been completely elucidated. Nor is it known whether patients who develop recurrence have a worse prognosis, such as a higher frequency of graft loss, liver failure, or inferior survival.
In this retrospective analysis of prospectively acquired data, we evaluate the risk factors associated with recurrence of autoimmune hepatitis after LT. We also compare the outcomes of patients with and without recurrent disease to determine whether recurrent autoimmune hepatitis constitutes a negative predictor factor.
Abbreviations
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; AZA, azathioprine; CI, confidence interval; CMV, cytomegalovirus; CNI, calcineurin inhibitor; EBV, Epstein-Barr virus; HLA, human leukocyte antigen; HR, hazard ratio; Ig, immunoglobulin; INR, international normalized ratio; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; PDN, prednisone; ULN, upper limit of normal.
PATIENTS AND METHODS
Study Population
Forty-six patients with autoimmune hepatitis who underwent LT secondary to end-stage liver disease were included in the study. Patients with overlapping syndromes (diagnosis criteria for autoimmune hepatitis plus primary biliary cirrhosis or primary sclerosing cholangitis) or concomitant chronic liver disease were excluded. All patients satisfied the new simplified scoring criteria for a probable or definitive autoimmune hepatitis diagnosis (mean score, 7 ± 0.1 points; range, 6–8 points; median, 7 points).9 The mean age of the study population at LT was 42 ± 2 years (range, 13–65 years; median, 44 years), and 34 patients (74%) were women. Our investigation was approved by the institutional review board of the University of Alberta Hospital.
Clinical and Laboratory Assessments
Data recovered from the medical charts included the gender, age at diagnosis and LT, time between the diagnosis of autoimmune hepatitis and LT, concomitant autoimmune diseases (eg, autoimmune thyroiditis, pernicious anemia, celiac disease, and immune thrombocytopenic purpura), presence of ascites or variceal bleeding before LT, liver biochemistries, international normalized ratio, and immunoglobulin G (IgG) levels just before LT, Model for End-Stage Liver Disease score, treatment before LT, post-LT viral infections, rejection episodes, ABO compatibility, and immunosuppressive regimens.
Cytotoxicity and Cytotoxicity Cross-Match
Each patient's sensitization was reported as the percentage value of the panel of reactive antibodies, which was determined by complement-dependent cytotoxicity, enzyme-linked immunosorbent assay, or flow cytometry. Briefly, the complement-dependent cytotoxicity assay estimates the panel of reactive antibodies by adding recipient serum to microtiter plates that have a pool of lymphocytes with defined human leukocyte antigens (HLAs). In general, PRA can then be determined on the basis of the number of cytotoxic reactions that are observed. Enzyme-linked immunosorbent assay uses microtest trays containing known HLA antigens to which recipient serum is added. Finally, flow cytometry measures the fluorescence after recipient serum has been added to a defined set of HLA antigen flow beads, and a positive test is determined by the mean channel shift in intensity.10 We considered a positive cytotoxic screen when the PRA value was greater than 5%.
The final crossmatch from fresh serum was done between patients and donors by flow cytometry. This assay can define a positive T or B cell reaction and determine whether the antibody present is an IgG or an IgM.10 A positive cytotoxic cross-match test was considered with a grading equal to or greater than 30%.
Histological Assessments
Liver tissue examinations of the explants were analyzed in all patients. An evaluation for necroinflammatory activity (grade) and extent of fibrosis (stage) in the explants was performed on the basis of the Batts and Ludwig criteria.11
For analysis, the findings of the liver explants were divided into no to mild inflammatory activity and moderate to severe inflammatory activity. Briefly, mild activity was considered when we had to search to find any inflammatory foci in the biopsy; moderate activity was considered when the majority of portal areas had inflammation, but it extended around less than 50% of the circumference of most portal areas; and severe activity was considered when the majority of portal areas had interface hepatitis extending around more than 50% of the circumference.12 Plasma cell infiltration was defined as the presence of plasma cells in the hepatic parenchyma, portal tracts, or both.
An examination of liver tissue after LT was performed at least once for all patients in the evaluation of graft dysfunction. The mean number of biopsies performed during the follow-up after LT was 3 ± 0.1 (total, 128; median, 3; range, 1–5)
Donor and Graft Characteristics
Evaluated characteristics included living related or cadaveric transplants, age, gender, biochemistry parameters, cardiac or respiratory arrest, lowest systolic and diastolic pressures recorded, lowest urine output recorded, use of vasopressors, cold and warm ischemia times, and surgery time.
Recurrent Autoimmune Hepatitis and Other Endpoints
Recurrent autoimmune hepatitis was defined as the presence of portal and periportal lymphoplasmacytic infiltrates in liver tissue in patients with sustained elevations of aminotransferases levels (more than 2-fold in at least 2 separate determinations) and in the absence of other causes of allograft dysfunction. Survival was considered from the date of LT to the date of last follow-up (censored) or date of death (uncensored).
Statistical Analyses
Cumulative probabilities of autoimmune hepatitis recurrence after LT and survival were calculated with the Kaplan-Meier method, and they were compared with the log-rank (Mantel-Cox) test.13, 14 Patients who did not develop autoimmune hepatitis recurrence and died and those who were lost during follow-up were censored at the time of death or at the time of their last visit. Prognostic factors for autoimmune hepatitis recurrence were analyzed by Cox regression univariate analysis.15 Those variables with a P value less than 0.1 in the univariate analysis were included in the Cox regression multivariate analysis. Data are presented as the mean ± standard error of the mean in the tables and text.
RESULTS
Frequency and Probability of Autoimmune Hepatitis Recurrence
Autoimmune hepatitis recurrence was diagnosed in 11 of the 46 (24%) patients after LT. The mean follow-up after LT of the 46 patients was 79 ± 10 months (range, 6–287 months; median, 51 months). Our transplant program started in 1989; however, we followed a patient that underwent transplantation in Montreal in 1984. The interval to the development of recurrence in the 11 patients was 48 ± 16 months (range, 9–144 months; median, 30 months). The probability of recurrent autoimmune hepatitis was 18% at 5 years and 32% at 10 years (Fig. 1), and the overall incidence of recurrence manifested as graft dysfunction after LT was 3.6 cases per 100 patient years [95% confidence interval (CI), 2.46–30.92 cases per 100 patient years; patient years, n = 304].
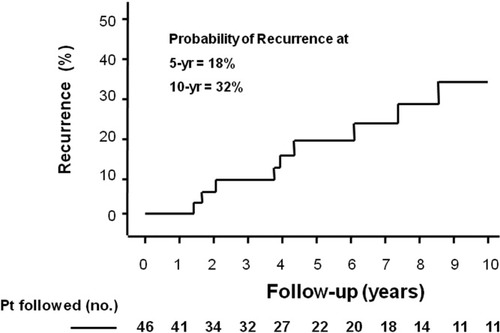
Cumulative probability of autoimmune hepatitis recurrence after liver transplantation. The overall 5-year probability of recurrent disease was 18% in all patients, and the overall 10-year probability of recurrent disease was 32%, but fewer patients were followed. The ordinate has been shortened to 50% to better depict the plot line.
Features Before LT in Patients with Disease Recurrence
By univariate Cox analysis, the following variables were associated with a higher risk of autoimmune hepatitis recurrence: concomitant autoimmune disease [hazard ratio (HR), 3.74; 95% CI, 1.05–13.36; P = 0.04], high serum aspartate aminotransferase (AST; HR, 1.09; 95% CI, 1.03–1.14; P = 0.002), high serum alanine aminotransferase (ALT; HR, 1.09; 95% CI, 1.03–1.20; P = 0.003), and high IgG levels (HR, 1.25; 95% CI, 1.11–1.41; P = 0.0003).
Also, patients with autoimmune hepatitis recurrence had a higher frequency of moderate to severe inflammatory activity (HR, 5.3; 95% CI, 1.55–18.79; P = 0.008) and plasma cell infiltration in the liver explant (HR, 5.8; 95% CI, 1.52–22.43; P = 0.01) in comparison with patients that did not develop recurrent disease (Table 1).
| Features Before LT | Recurrence (n = 11) | No Recurrence (n = 35) | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| Age at diagnosis of AIH | 32 ± 6 | 36 ± 3 | 0.99 | 0.94–1.03 | 0.6 |
| Age at LT (years) | 37 ± 6 | 43 ± 3 | 0.98 | 0.95–1.03 | 0.3 |
| Men:women | 3: 8 | 9: 26 | 1.67 | 0.43–6.46 | 0.5 |
| Indications for LT* | |||||
| Acute liver failure | 0 | 3 (9) | — | — | — |
| Chronic liver failure | 11 (100) | 32 (91) | |||
| Concomitant autoimmune disease | 4 (36) | 5 (14) | 3.74 | 1.05–13.36 | 0.04 |
| Time between AIH diagnosis and LT (months) | 57 ± 14 | 64 ± 13 | 0.99 | 0.98–1.09 | 0.8 |
| Ascites before LT | 8 (73) | 22 (63) | 0.90 | 0.23–3.5 | 0.9 |
| Variceal bleeding before LT | 5 (45) | 9 (26) | 0.43 | 0.12–1.49 | 0.2 |
| PDN alone before LT | 7 (64) | 23 (66) | 1.3 | 0.36–4.58 | 0.7 |
| PDN and AZA before LT | 3 (27) | 10 (29) | 1.04 | 0.27–4.1 | 0.9 |
| ALT (nL; <50 U/L) | 145 ± 53 | 47 ± 5 | 1.09 | 1.03–1.20 | 0.003 |
| ALT > 3-fold ULN | 3 (27) | 2 (6) | 8.49 | 2.09–34.48 | 0.02 |
| AST (nL; <38 U/L) | 175 ± 53 | 59 ± 5 | 1.09 | 1.03–1.14 | 0.002 |
| AST > 3-fold ULN | 6 (55) | 6 (17) | 11.98 | 3.33–43.12 | 0.0001 |
| AP (nL; 30–130 U/L) | 78 ± 20 | 80 ± 13 | 1.0 | 0.99–1.04 | 0.9 |
| Bilirubin (<20 μmol/L) | 46 ± 14 | 60 ± 14 | 1.0 | 0.99–1.01 | 0.6 |
| INR | 1.61 | 1.58 | 1.9 | 0.46–7.62 | 0.4 |
| IgG (nL; 6.9–16.2 g/L) | 24.2 ± 2.4 | 14.5 ± 2.4 | 1.25 | 1.11–1.41 | 0.0003 |
| IgG > 1-fold ULN | 7 (64) | 9 (26) | 3.91 | 1.14–13.40 | 0.03 |
| MELD score | 19 ± 2 | 20 ± 1 | 1.0 | 0.93–1.08 | 1.0 |
| Positive cytotoxic screen | 3 (27) | 10 (29) | 0.98 | 0.24–3.97 | 0.9 |
| Positive cross-match | 1 (9) | 7 (20) | 2.0 | 0.25–16.23 | 0.5 |
| Moderate to severe inflammatory activity† | 7 (64) | 10 (29) | 5.3 | 1.55–18.79 | 0.008 |
| Plasma cells† | 5 (45) | 9 (26) | 5.8 | 1.52–22.43 | 0.01 |
- NOTE: Numbers in parentheses are percentages.
- Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; AZA, azathioprine; CI, confidence interval; HR, hazard ratio; IgG, immunoglobulin G; INR, international normalized ratio; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; PDN, prednisone; ULN, upper limit of normal.
- * A Cox analysis could not be performed because acute liver failure was not present in patients with AIH recurrence.
- † In the liver explants. Bold numbers reflect significantly different from each other.
No association was found with other features such as the age at diagnosis of autoimmune hepatitis, age at LT, gender, indication for LT (acute or chronic liver failure), time between the diagnosis of autoimmune hepatitis and LT, complications of chronic liver disease before LT (eg, ascites and variceal bleeding), treatment before LT (prednisone alone or prednisone and azathioprine), bilirubin, alkaline phosphatase, international normalized ratio, Model for End-Stage Liver Disease score, or cytotoxic screen tests (Table 1).
Features After LT in Patients with Disease Recurrence
No association was found with other features such as the type of bile duct anastomosis, type of immunosuppression, long-term use of prednisone, rejection episodes, ABO mismatch, or cytomegalovirus or Epstein-Barr virus mismatch (Table 2).
| Features After LT | Recurrence (n = 11) | No Recurrence (n = 35) | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| Bile duct anastomosis | |||||
| End to end | 7 (64) | 25 (71) | 0.34 | 0.89–1.33 | 0.1 |
| Roux-en-Y | 4 (36) | 10 (29) | |||
| CNI immunosuppression | |||||
| Tacrolimus | 7 (64) | 23 (66) | 0.4 | 0.09–1.74 | 0.2 |
| Cyclosporine | 4 (36) | 12 (34) | |||
| Prednisone after LT | 10 (91) | 29 (83) | 0.39 | 0.05–3.12 | 0.4 |
| Long-term prednisone† | 6 (55) | 14 (40) | 1.2 | 0.37–4.06 | 0.7 |
| Mycophenolate mofetil | 9 (82) | 25 (71) | 1.2 | 0.70–15.9 | 0.1 |
| Sirolimus | 2 (18) | 8 (23) | 1.7 | 0.36–7.76 | 0.5 |
| Dacluzimab | 1 (9) | 5 (14) | 0.78 | 0.09–6.4 | 0.8 |
| Acute rejection episodes | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.95 | 0.48–1.86 | 0.9 |
| ABO mismatch | 0 | 0 | — | — | — |
| CMV mismatch | 2 (18) | 3 (9) | 0.24 | 0.05–1.21 | 0.09 |
| EBV mismatch‡ | 0 | 2 (6) | — | — | — |
- NOTE: Numbers in parentheses are percentages.
- Abbreviations: AIH, autoimmune hepatitis; CI, confidence interval; CMV, cytomegalovirus; CNI, calcineurin inhibitor; EBV, Epstein-Barr virus; HR, hazard ratio; LT, liver transplantation.
- † More than 1 year after LT.
- ‡ A Cox analysis could not be performed because EBV mismatch was not present in patients with AIH recurrence.
Donor Features in Patients with Disease Recurrence
No association was found with several donor characteristics, such as age, gender, cold-warm ischemia time, surgical time, lowest blood pressure recorded, lowest urine output recorded, use of vasopressors, biochemical parameters, and a higher risk of autoimmune hepatitis recurrence (Table 3).
| Donor Features | Recurrence (n = 11) | No Recurrence (n = 35) | HR | 95% CI | P Value |
|---|---|---|---|---|---|
| Living-related transplant | 1 (9) | 2 (6) | 0.26 | 0.29–2.32 | 0.2 |
| Donor age (years) | 27 ± 3 | 39 ± 3 | 0.96 | 0.92–1.02 | 0.2 |
| Men:women | 6: 5 | 19: 16 | 1.25 | 0.38–4.15 | 0.7 |
| Surgery time (hours:minutes) | 9:17 ± 1:07 | 7:15 ± 0:38 | 1.01 | 0.96–1.06 | 0.7 |
| Cold ischemia time (hours:minutes) | 7:32 ± 1:03 | 8:29 ± 1:09 | 0.94 | 0.79–1.12 | 0.4 |
| Warm ischemia time (minutes) | 47 ± 3 | 50 ± 4 | 0.99 | 0.94–1.06 | 0.9 |
| Lowest pH level (mol/L)* | 7.37 ± 0.2 | 7.36 ± 0.1 | 0.97 | 0.94–1.06 | 0.1 |
| Maximum lactate (mmol/L) | 4 ± 1.4 | 3 ± 0.7 | 1.02 | 0.76–1.36 | 0.9 |
| Lowest PO2 (mm Hg) | 116 ± 23 | 124 ± 12 | 0.99 | 0.98–1.05 | 0.5 |
| Maximum bilirubin (<20 μmol/L) | 14 ± 4 | 15 ± 3 | 1.0 | 0.98–1.04 | 0.9 |
| Maximum ALT (nL; <50 U/L) | 36 ± 8 | 48 ± 10 | 0.98 | 0.94–1.02 | 0.2 |
| Maximum AST (nL; <38 U/L) | 95 ± 28 | 74 ± 12 | 1.0 | 0.98–1.02 | 0.9 |
| Maximum AP (nL; 30–130 U/L) | 74 ± 11 | 89 ± 10 | 0.99 | 0.97–1.01 | 0.4 |
| Cardiac or respiratory arrest | 3 (27) | 9 (26) | 0.97 | 0.24–3.88 | 0.9 |
| Lowest systolic pressure (mm/Hg) | 94 ± 6 | 95 ± 3 | 1.0 | 0.95–1.06 | 0.9 |
| Lowest diastolic pressure (mm/Hg) | 60 ± 5 | 57 ± 3 | 1.04 | 0.98–1.04 | 0.2 |
| Lowest urine output (mL/hour) | 61 ± 24 | 84 ± 15 | 0.99 | 0.98–1.01 | 0.6 |
| Use of vasopressors† | 6 (55) | 23 (66) | 1.19 | 0.35–3.98 | 0.8 |
- NOTE: Numbers in parentheses are percentages.
- Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; CI, confidence interval; HR, hazard ratio.
- * Negative logarithm of the hydrogen ion concentration ([H+]).
- † Dopamine, norepinefrine, or vasopressin.
Features Associated with Disease Recurrence in Multivariate Analysis
In the multivariate Cox regression analysis, only the presence of moderate to severe inflammation in the explant (HR, 6.9; 95% CI, 1.76–26.96; P = 0.006) and high IgG levels before LT (HR, 7.5; 95% CI, 1.45–38.45; P = 0.0) were independently associated with the risk of autoimmune hepatitis recurrence (Figs. 2 and 3).
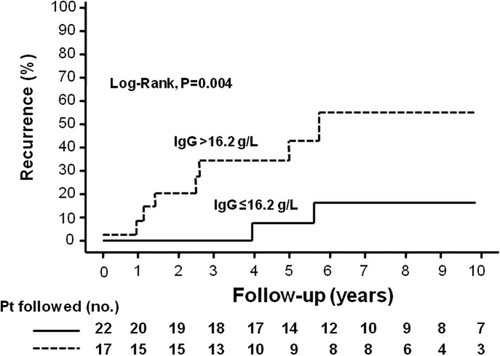
Cumulative probability of autoimmune hepatitis recurrence after liver transplantation according to immunoglobulin G (IgG) levels: (- - -) IgG > 16.2 g/L and (—) IgG ≤ 16.2 g/L. The 5-year probability of recurrence was 31% and 7%, respectively (P = 0.004, log-rank test). The 10-year probability of recurrence was 52% and 14% in these same groups, but fewer patients were followed.
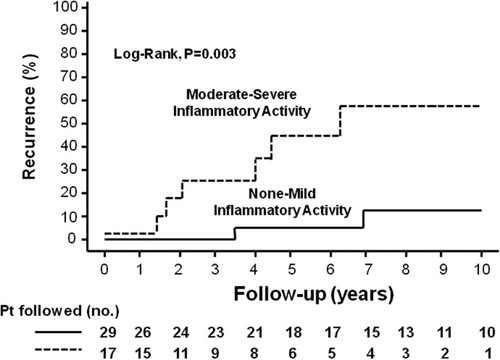
Cumulative probability of autoimmune hepatitis recurrence after liver transplantation according to inflammatory activity in liver explants: (- - -) moderate to severe inflammatory activity and (—) no to mild inflammatory activity. The 5-year probability of recurrence was 45% and 5%, respectively (P = 0.003, log-rank test). The 10-year probability of recurrence was 59% and 11% in these same groups, but fewer patients were followed.
Patient Survival Associated with Recurrent Disease
The overall 5- and 10-year probability of survival was 81% and 77%, respectively. The 5-year probability of survival was 82% and 76% in patients without and with autoimmune hepatitis recurrence, respectively (log-rank test; P = 0.9). Moreover, the 10-year probability of survival was 77% and 76% in these same groups, but fewer patients were followed (Fig. 4).
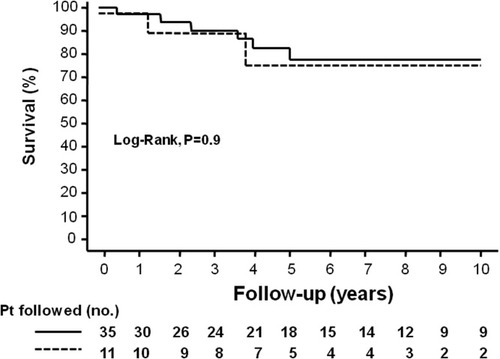
Kaplan-Meier curve indicating the survival of patients (—) with and (- - -) without autoimmune hepatitis recurrence. The 5-year probability of survival was 82% and 76%, respectively (P = 0.9, log-rank test). The 10-year probability of survival was 78% and 76% in these same groups, but fewer patients were followed.
DISCUSSION
Our study indicates that the incidence of recurrent autoimmune hepatitis manifested as graft dysfunction is 18% and 32% at 5 and 10 years, respectively, with an overall incidence of recurrence of 3.6 cases per 100 patient years. The presence of concomitant autoimmune disease, abnormal serum levels of AST, ALT, and IgG before LT, and the presence of moderate to severe inflammatory activity or plasma cell infiltration in the liver explants have a predictive value for disease recurrence. The risk for autoimmune hepatitis recurrence increases by 1- to 12-fold in comparison with patients who do not present any of these clinical, biochemical, and histological features (Tables 1 and 2).
Similarly to previous studies, we found that the incidence of rejection and the treatment of acute cellular rejection are not significantly different between patients with and without autoimmune hepatitis recurrence.6 Moreover, we did not find differences in the frequency of pretransplant administration of prednisone either alone or in combination with azathioprine, the use of different immunosuppressive regimens, including the long-term use of prednisone after LT, and the risk of autoimmune hepatitis recurrence (Table 2).
The optimal choice of immunosuppression after LT in this subgroup of patients is still controversial; however, as reported by Gautam et al.,16 we did not find differences in recurrence rates between recipients of tacrolimus and recipients of cyclosporine. Nor did we find differences for other immunosuppression regimens (ie, sirolimus, mycophenolate mofetil, and dacluzimab); however, these were used in few patients in this study. In addition, we did not demonstrate a protective effect for the long-term administration of prednisone on autoimmune hepatitis recurrence.
We did not find any association between the risk of autoimmune hepatitis recurrence and the donor variables that were evaluated (Table 3). These observations suggest that the propensity to recurrence is limited only to recipient factors, which may be related to a hyperactive immune system or the persistence of pathogenic mechanisms that allow ongoing injury to the new graft.
Recurrence of autoimmune hepatitis after LT has been reported by several transplant centers17-26 with recurrence rates ranging from 17% to 42% at 5 years.4 Recently, a systematic review involving 13 original articles reported a weighted recurrence rate of 22% after a median interval of 26.4 months.16
Our finding of high serum levels of IgG in conjunction with moderate or severe activity in the explants of patients with autoimmune hepatitis recurrence suggests that recurrence may be related to an increased inflammatory state mediated by plasma cells and incompletely suppressed by corticosteroid therapy before LT.27-29
Moreover, the results of the present study extend those from an earlier study with fewer patients.30 In that study, patients with high-grade inflammation in the native liver had a higher risk of autoimmune hepatitis recurrence; however, the authors did not analyze associations of biochemical parameters before LT and the risk of recurrent disease.
Interestingly, similar findings in patients with autoimmune hepatitis without LT have been reported previously: patients who were treated until the serum AST and IgG levels normalized had a lower frequency of relapse after corticosteroid withdrawal despite comparable histological findings.31 This suggests that relapse in native livers and recurrence after LT may share pathogenic mechanisms.
One limitation of our study is that we were not able to determine the HLA antigens in our patients with autoimmune hepatitis. Typically, patients with HLA-DR3 are younger at disease onset, they respond less well to corticosteroid therapy, and they have worse outcomes (a higher frequency of hepatic-related death or LT requirement). Alternatively, patients with HLA-DR4 are more commonly women, have a higher frequency of concurrent immune diseases, and respond better to corticosteroid treatment.32 In patients with autoimmune hepatitis recurrence, some studies have found an increased incidence of HLA-DR3,21, 33 whereas others have not been able to find such associations.4, 22 For example, 1 study found that the risk of autoimmune hepatitis recurrence was higher in HLA-DR3–positive recipients of HLA-DR3–negative grafts, and recurrences were not seen in recipients of HLA-DR3–positive grafts. Furthermore, they found that HLA-B8 status did not affect disease recurrence, and neither HLA-B8 nor the DR3 status of the graft or recipient had an effect on the observed frequency of rejection.34 However, HLA mismatch has not been associated with recurrence in several subsequent studies.7, 19, 21, 35
Another limitation of our study is that liver biopsies were not protocolized but instead were performed only in the context of graft dysfunction evaluation; therefore, it is possible that the incidence of autoimmune hepatitis recurrence has been underestimated. However, all the patients evaluated in this study had at least 1 liver biopsy during their follow-up, the frequency of autoimmune hepatitis recurrence in the absence of abnormal biochemistries is unusual, and the significance of clinically and biochemically silent recurrence is unknown.36
The fact that elevated aminotransferase and IgG levels constitute markers associated with a high risk of disease recurrence is important because unlike histological findings assessed in liver explants, these markers can be obtained in the preoperative stage and therefore can be used to predict recurrence prior to transplantation. Our current findings raise the possibility that the frequency of recurrence could be reduced if patients are treated before LT with the aim of normalizing or further reducing raised aminotransferase and IgG levels; however, the applicability of this strategy should be evaluated prospectively.
Finally, as reported in other series,6 we did not find differences in survival at 5 and 10 years after LT between patients with and without autoimmune hepatitis recurrence. This could be related to the low number of patients with recurrence because during a period of almost 20 years, only 46 patients underwent LT secondary to end-stage liver disease due to autoimmune hepatitis in our center.
In summary, patients who undergo LT secondary to end-stage autoimmune hepatitis with concomitant autoimmune disease who have high pretransplant serum levels of AST, ALT, or IgG before transplantation or who have moderate or severe inflammatory activity or plasma cell infiltration in the liver explants have a significantly higher risk of recurrent disease. Our results suggest that autoimmune hepatitis that is difficult to control is more likely to recur after LT; however, survival outcome is good even with recurrence. These findings may have important implications in posttransplantation immunosuppressive strategies.
Acknowledgements
The authors thank Glenda Meeberg (Data Coordinator, Liver Transplant Program, Capital Health) for her statistical support.




