Vasopressin decreases portal vein pressure and flow in the native liver during liver transplantation
Abstract
Vasodilation due to impaired vascular tone is common in liver failure. Vasoconstrictor drugs are almost always required during the anhepatic phase of a liver transplant to maintain blood pressure unless venovenous bypass is employed. Arginine-vasopressin can be used as a vasoconstrictor instead of or in addition to norepinephrine for this purpose, but the effect of vasopressin on the portal vein pressure and flow in this setting is unknown. Portal vein pressure, portal vein blood flow, hemodynamic variables, and plasma vasopressin levels were measured in 16 patients during liver transplantation after ligation of the hepatic artery before and after a vasopressin infusion of 3.8 ± 1.1 units/hour. Measurements were performed on the native liver prior to caval clamping. After vasopressin infusion, the portal vein pressure decreased significantly from 24.0 ± 6.5 to 21.5 ± 7.4 mm Hg [mean ± standard deviation (SD), P = 0.006]. The portal vein blood flow also decreased (from 1.01 ± 0.53 to 0.76 ± 0.53 L/minute, mean ± SD, P < 0.0001), as did the portal vein blood flow to cardiac output ratio (from 0.14 ± 0.06 to 0.10 ± 0.07, mean ± SD, P < 0.0001). In conclusion, vasopressin significantly decreased portal vein pressure and flow of the native liver without decreasing cardiac output or intestinal perfusion in patients undergoing liver transplantations. Liver Transpl 14:1664–1670, 2008. © 2008 AASLD.
Patients in hepatic failure who require a liver transplant often exhibit profound vasodilatation and are able to maintain adequate perfusion pressure only by increasing cardiac output.1 Without the use of portocaval bypass or preservation of the recipient vena cava (the piggyback technique), cardiac preload decreases significantly upon clamping of the inferior vena cava, and decreased cardiac output and arterial blood pressure follow.
Sympathomimetic drugs such as norepinephrine and epinephrine are used to maintain an adequate arterial blood pressure during the anhepatic phase. We have observed that a low-dose infusion of arginine-vasopressin (AVP), either alone or in combination with norepinephrine, is also potentially effective for this purpose, but we did not know if vasopressin had a beneficial effect on portal blood flow and pressure in patients undergoing liver transplantations. Vasopressin is a potent vasoconstrictor in vasodilatory septic shock,2-4 and vasopressin analogues such as ornipressin and terlipressin have been used successfully to treat hepatorenal syndrome.5, 6 Vasopressin causes splanchnic vasoconstriction, but it was not known if there is a direct effect of vasopressin on portal blood flow and pressure that may be beneficial in preventing overperfusion of the transplanted liver. Previous studies that evaluated portal hemodynamics either measured portal flow but not pressure or used animal models that are not necessarily reflective of a clinical situation.
We report a unique method to simultaneously measure human portal vein blood flow and pressure in the native cirrhotic liver before and after vasopressin infusion.
Abbreviations
ABG, arterial blood gas; AVP, arginine-vasopressin; CO, cardiac output; CVP, central venous pressure; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; FFP, fresh frozen plasma used intraoperatively; g-a, gastric/arterial; LOS, length of stay; LRLT, living related liver transplantation; MAP, mean arterial pressure; MELD, Model for End-Stage Liver Disease; MPAP, mean pulmonary artery pressure; NAFLD, nonalcoholic fatty liver disease; OLT, orthotopic liver transplantation; PRBC, packed red blood cells used intraoperatively; PV, portal vein; PVF/CO ratio, portal vein flow to cardiac output ratio; SBP, systolic blood pressure; SD, standard deviation; SPAP, systolic pulmonary artery pressure; SVR, systemic vascular resistance.
PATIENTS AND METHODS
Patients
The Institutional Review Board of Columbia University approved this study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as well as New York State and US federal guidelines. Adult patients undergoing orthotopic liver transplantation (cadaveric or living related) at Columbia University Medical Center were eligible for inclusion in this study. Exclusion criteria included inability to give informed consent and hemodynamic instability requiring vasopressor drugs prior to clamping of the vena cava.
Surgical Technique
Orthotopic liver transplantation was undertaken via a bilateral subcostal incision with midline extension. The liver was mobilized by the division of its ligamentous attachments. Exposure to the portal vein was facilitated by division of the peritoneum of the hepatoduodenal ligament. The cystic duct and artery were identified and ligated, and the gallbladder was retracted superiolaterally. The right and left hepatic arteries were dissected, and the common bile duct was divided at the bifurcation of the right and left hepatic ducts. The portal vein was then dissected and cleaned from the bifurcation proximally to the level of the pancreatic neck prior to application of the flow probe and insertion of the pressure transducer needle.
Measurements
After induction of anesthesia and prior to surgery, an 18F gastric tonometry tube (Datex-Ohmeda, Madison, WI) was inserted into the stomach to measure gastric pCO2. Prior to any further measurements, the hepatic artery had to be ligated in order to gain access to the portal vein and allow the application of the flow probe and the cannula to measure portal vein pressure. All measurements were done on the native, diseased liver shortly before caval cross-clamping and the anhepatic phase. If the patient was hemodynamically unstable (eg, required vasopressor administration) or implantation of the liver transplant graft was considered urgent, the patient was excluded from the study, and no measurements were taken.
A pulmonary artery catheter (with continuous cardiac output measurement; Edwards Lifesciences, Irvine, CA) was inserted per routine prior to surgery. At baseline and after vasopressin infusion, hemodynamic parameters (systolic blood pressure, diastolic blood pressure, mean arterial pressure, pulmonary artery pressure, and central venous pressure) were measured, and 5 mL of arterial blood was drawn for an arterial blood gas and vasopressin level analysis.
Measurement of the Portal Vein Pressure
After ligation of the hepatic artery and surgical exposure of the portal vein, a 21-gauge needle was inserted into the portal vein just distal to the bifurcation between the left and right portal veins, an area that was resected during the hepatectomy shortly afterwards. The needle was connected to a pressure transducer and remained in this position for the entire duration of the study (Fig. 1).
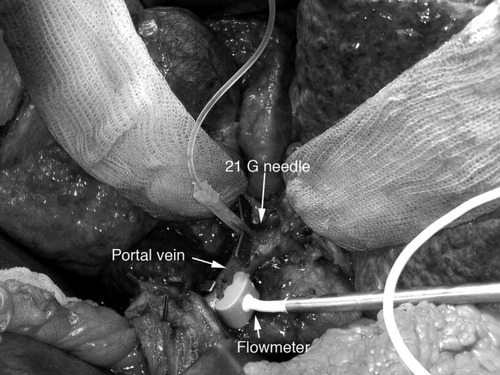
Experimental setup for the in vivo measurement of the portal vein blood flow and pressure (consent for publication was obtained from the patient).
Measurement of the Portal Vein Flow
A vascular flow probe (10 or 12 mm) and an HT-107 flowmeter (both from Transonic Systems, Inc., Ithaca, NY) were used to measure portal vein blood flow. Immediately prior to the measurements, the flow probe was calibrated by submersion in sterile water and zeroing of the flowmeter. The flow probe was then attached to the portal vein and the field was submerged in sterile water. The portal vein flow was measured twice to calculate the average portal vein flow (Fig. 1).
Vasopressin Infusion
After the baseline measurements, vasopressin (8-arginine-vasopressin, Monarch Pharmaceuticals, Inc., Bristol, TN) was administered at 3 units/hour and adjusted from 3 to 6 units/hour to maintain a mean arterial pressure above 60 mm Hg. The vasopressin plasma half-life is 1 to 2 minutes,7 and steady-state conditions were achieved at the time of the postvasopressin hemodynamic measurements.
Vasopressin Levels (Enzyme-Linked Immunosorbent Assay)
Arterial blood (5 mL) was drawn at each time point and spun at 1000g for 15 minutes at 4°C, and the plasma was frozen at −70°C. Vasopressin levels were determined with a commercially available enzyme-linked immunosorbent assay kit (Assaydesign, Ann Arbor, MI) according to the manufacturer's instructions. The limit of detection for this assay is 0.024 pg/mL.8
Statistics
Values are presented as mean ± standard deviation. Comparisons of paired variables were made either with a paired t test for variables with normal distribution or a Wilcoxon matched-pairs test for variables without Gaussian distribution. Gaussian distribution was determined with the Kolmogorov-Smirnov test. P values were 2-tailed, and P < 0.05 was considered significant. For the purpose of a pretrial power analysis, we assumed that vasopressin would decrease portal blood flow by 25% (mean portal blood flow before = 1 L/minute, mean portal blood flow after = 0.75 L/minute, standard deviation of the difference = 0.2 mL/L). We concluded that we would require 12 subjects to achieve 80% power with α = 0.05.
SAS 9.1 (SAS, Inc., Cary, NC), SPSS 11.0.4 (SPSS, Inc., Chicago, IL), and Graphpad Prism 4.0 (San Diego, CA) software was used for the statistical analysis.
RESULTS
Demographics
After informed consent, 18 patients were enrolled from June 20, 2005 to May 18, 2006 in this study. Sixteen patients successfully completed all measurements; 2 patients were excluded because they required vasoconstrictors prior to the first measurement. The average Model of End-Stage Liver Disease score was 16.4 ± 8.0 (range, 4.5-34.5). Twelve patients (80%) required liver transplantation for hepatitis C (in 2 patients in combination with hepatocellular carcinoma); 4 patients (25%) underwent living related liver transplantation (Table 1).
| All (n = 16) | LRLT (n = 4) | Cadaveric OLT (n = 12) | |
|---|---|---|---|
| Preoperative | |||
| Female, n (%) | 4 (25%) | 2 (50%) | 2 (16.7%) |
| Age (years), mean ± SD | 59.8 ± 7.4 | 54.1 ± 6.6 | 62.7 ± 6.9 |
| MELD score, mean ± SD | 16.4 ± 8.0 | 13.4 ± 4.4 | 17.4 ± 8.8 |
| Ascites, n (%) | 8 (50%) | 1 (25%) | 7 (58%) |
| Indication for OLT | |||
| Hepatitis C, n (%) | 12 (75%) | 3 (75%) | 9 (75%) |
| ETOH, n (%) | 2 (12.5%) | 1 (25%) | 1 (8.3%) |
| Graft failure, n (%) | 1 (6.25%) | 0 | 1 (8.3%) |
| NAFLD, n (%) | 1 (6.25%) | 0 | 1 (8.3%) |
| Reoperation, n (%) | 1 (6.25%) | 0 | 1 (8.3%) |
| PRBC (units), mean ± SD | 5.6 ± 5.3 | 4.3 ± 3.2 | 5.0 ± 5.0 |
| FFP (units), mean ± SD | 9.8 ± 6.43 | 8.0 ± 6.9 | 10.3 ± 6.6 |
| Hospital LOS (days), mean ± SD | 27 ± 26.6 | 17.5 ± 4.7 | 32 ± 30.9 |
- Abbreviations: FFP, fresh frozen plasma used intraoperatively; LOS, length of stay; LRLT, living related liver transplantation; MELD, Model for End-Stage Liver Disease; NAFLD, nonalcoholic fatty liver disease; OLT, orthotopic liver transplantation; PRBC, packed red blood cells used intraoperatively; SD, standard deviation.
Vasopressin Infusion
AVP (3.8 ± 1.1 units/hour; range, 3-6 units/hour) was infused through a central venous catheter over 16.6 ± 11.1 minutes (range, 5-52 minutes) before the second measurement commenced.
Portal Vein Pressure
The portal vein pressure was 24 ± 6.54 mm Hg at baseline and decreased significantly to 21.5 ± 7.37 mm Hg with AVP infusion (P = 0.0056)—a reduction of 11.6% ± 12.4%. The portal vein pressure decreased in all but 2 patients. In 1 patient, the portal vein pressure remained unchanged (25 mm Hg), and in 1 patient, the portal vein pressure increased from 24 to 26 mm Hg (Table 2 and Fig. 2).
| Before | After | P Value | |
|---|---|---|---|
| Time between measurements, minutes | 16.6 ± 11.1 | ||
| Vasopressin infusion, units/hour | 3.8 ± 1.1 | ||
| PV pressure | 24 ± 6.54 | 21.5 ± 7.37 | 0.0056 |
| PV flow, L/minute | 1.01 ± 0.53 | 0.76 ± 0.46 | <0.0001 |
| PVF/CO ratio | 0.14 ± 0.06 | 0.10 ± 0.07 | <0.0001 |
| Plasma vasopressin level, pg/mL | 8.3 ± 10.5 | 46.8 ± 25.3 | <0.0001 |
| SBP, mm Hg | 115.5 ± 15.1 | 124.1 ± 17.9 | 0.039 |
| DBP, mm Hg | 63.1 ± 8.4 | 66.1± 7.8 | 0.154 |
| MAP, mm Hg | 80.6 ± 8.9 | 85.7 ± 9.9 | 0.073 |
| SPAP, mm Hg | 27.4 ± 6.7 | 29.1 ± 6.9 | 0.129 |
| DPAP, mm Hg | 14.9 ± 4.7 | 15.5 ± 5.5 | 0.529 |
| MPAP, mm Hg | 19.0 ± 4.9 | 20.0 ± 5.5 | 0.307 |
| CVP, mm Hg | 10.8 ± 3.3 | 12.5 ± 7.7 | 0.233 |
| CO, L/minute | 7.341 ± 1.985 | 7.368 ± 1.690 | 0.411 |
| SVR, dyn second/cm5 | 837 ± 260 | 813 ± 224 | 0.386 |
| ABG analysis | |||
| pH | 7.424 ± 0.076 | 7.424 ± 0.067 | 0.254 |
| pCO2, mm Hg | 33.9 ± 3.2 | 33.2 ± 4.0 | 0.842 |
| HCO3, mmol/L | 22.7 ± 3.2 | 43.9 ± 74.4 | 0.354 |
| Gastric pCO2, mm Hg | 39.1 ± 13.2 | 40.7 ± 15.3 | 0.135 |
| g-a pCO2 gradient, mm Hg | 6.9 ± 12.1 | 3.9 ± 7.2 | 0.842 |
- NOTE: To convert millimeters of mercury into kilopascals, divide by 7.5006.
- Abbreviations: ABG, arterial blood gas; CO, cardiac output; CVP, central venous pressure; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; g-a, gastric/arterial; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; PV, portal vein; PVF/CO ratio, portal vein flow to cardiac output ratio; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; SVR, systemic vascular resistance.
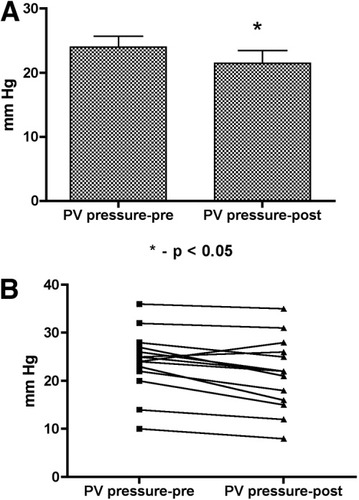
Changes in the portal vein (PV) pressure before and after vasopressin infusion: (A) mean/standard error of the mean and (B) individual values.
Portal Vein Flow
The portal vein flow decreased significantly with the AVP infusion. The portal vein flow was 1.01 ± 0.53 L/minute at baseline and decreased to 0.76 ± 0.46 L/minute (P < 0.0001)—a 26.7 ± 14.8% reduction (Table 2 and Fig. 3). The portal vein flow decreased in all patients, ranging from −5% to −54%. The ratio of portal vein blood flow to cardiac output also decreased significantly from 0.14 ± 0.06 to 0.10 ± 0.07 (P < 0.001; Table 2 and Fig. 4).
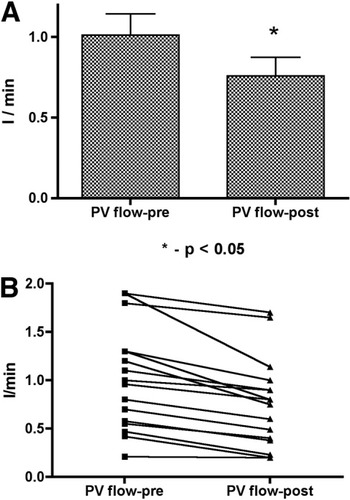
Changes in the portal vein (PV) blood flow before and after vasopressin infusion: (A) mean/standard error of the mean and (B) individual values.
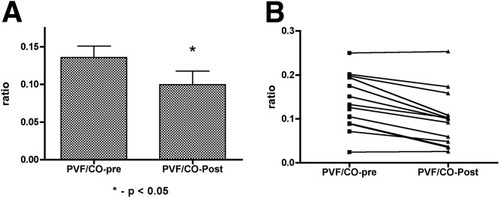
Changes in the portal vein (PV)/cardiac output (CO) ratio before and after vasopressin infusion: (A) mean/standard error of the mean and (B) individual values.
Hemodynamic Variables
The mean and diastolic blood pressures, central venous pressure, pulmonary artery pressure, and cardiac output did not change significantly during the infusion of AVP. The systolic blood pressure trended higher (115.5 ± 15.1 to 124.1 ± 17.9 mm Hg; P = 0.039). The mean arterial pressure decreased in 5 patients (between −2% and −19%) and increased in 11 patients (between 0.5% and 23%). There was no change in the arterial pH, arterial carbon dioxide partial pressure, or arterial bicarbonate concentration (Table 2).
Gastric Tonometry
The gastric pCO2 remained unchanged during the AVP infusion. The gradient between arterial and gastric pCO2, a measure of splanchnic hypoperfusion,9 did not change significantly either (Table 2).
Plasma Vasopressin Levels
Prior to the AVP infusion, plasma vasopressin levels were 8.3 ± 10.5 pg/mL, approximately 2 to 4 times the reported normal value.10-12 Eight patients had no detectable baseline plasma vasopressin levels. Vasopressin levels prior to infusion correlated with the baseline systemic vascular resistance (Pearson r = 0.651, P = 0.016). After the AVP infusion, plasma vasopressin levels increased to 46.8 ± 25.3 pg/mL, which is approximately the level expected after a vasopressin infusion of 0.02 units/minute and 4 times higher than levels observed in cardiogenic shock.12, 13 Vasopressin levels after AVP infusion correlated with the second portal vein measurement (Pearson's r = 0.613, P = 0.014) and weakly with the percent change of the portal vein flow (Pearson's r = 0.544, P = 0.036) but not with the absolute portal vein pressure or changes in the portal vein pressure.
DISCUSSION
This study was able to demonstrate that a low-dose infusion of vasopressin during liver transplantation decreases portal vein blood flow and pressure in the native diseased liver without a significant short-term reduction of cardiac output, changes in hemodynamics, or signs of splanchnic hypoperfusion.
A reduction of portal vein blood flow and portal vein pressure may be beneficial in the early phase of a liver transplant: a reduction of portal vein pressure will limit the engorgement of the implanted liver and enhance oxygen-rich hepatic artery blood flow.14, 15 Hepatic artery flow and portal vein flow are interrelated, and decreased portal vein flow causes an increase in hepatic artery flow mediated by an increase of adenosine in the vicinity of hepatic arterioles. This hepatic arterial buffer response preserves a constant total hepatic blood flow with changes in portal blood flow.16-18 Because hepatic arterial tone is regulated through local adenosine washout by intrahepatic portal venules, denervation of the liver during the transplant is not going to abate the hepatic arterial buffer response.
Decreased portal vein blood flow may also reduce the metabolic work of the newly transplanted liver.19 A portal vein pressure over 20 mm Hg has been associated with rapid graft hypertrophy and poor outcome after living related liver transplants.20, 21 Increased portal vein flow is associated with accelerated volume gain of living related liver grafts and more rapid improvement of coagulation parameters, but the effect of decreased portal vein flow in the early phase after liver transplantation requires further studies.
Vasopressin is a potent vasopressor in vasodilatory septic shock, whereas it has almost no effect on arterial pressure in healthy volunteers. Septic shock requiring catecholamine vasoconstrictors is associated with a plasma vasopressin deficiency (relative to acute endotoxemic shock) and enhanced peripheral vascular vasopressin sensitivity.3, 4 In hepatic cirrhosis, a vasodilatory state can be observed, and vasopressin and vasopressin analogs (ornipressin or terlipressin) have been employed in the treatment of hepatorenal syndrome.5, 22, 23
A vasopressin plasma level of 8 pg/mL, observed in our patients, is elevated with respect to the hormone's water metabolism but low with respect to its vasoconstrictor effect. This study was designed to detect not vasopressin deficiency but the correlation of baseline vasopressin levels with baseline systemic vascular resistance points toward this.
During liver transplantation at the time of caval cross-clamping, cardiac output can often not be maintained without venovenous bypass, and vasoconstrictor agents are required to maintain arterial pressure. In our experience since 1999, vasopressin instead of or in addition to norepinephrine sustains adequate arterial blood pressure, and venovenous bypass is very rarely required (<1% of liver transplants).
Vasopressin, a splanchnic vasoconstrictor, may have an advantage over catecholamines as it decreases splanchnic hyperemia, which aggravates portal vein pressure and ascites formation with hepatic cirrhosis.24, 25 We found that the reduction of portal vein pressure and portal vein flow with vasopressin was due to splanchnic vasoconstriction and not due to a reduction of mean arterial pressure, central venous pressure, or cardiac output.
We did not detect any sign of splanchnic hypoperfusion such as metabolic acidosis, increased gastric pCO2, or arterial-gastric pCO2 gradient, and thus vasopressin appears to maintain adequate gastric mucosal perfusion at least for the very short duration of the study. Interestingly, portal vein pressure decreased less than expected in light of the reduction of portal vein flow, and this indicates that vasopressin may have a direct vasoconstrictor effect on the portal vein and thereby increase portal vein resistance.
Limitations
This study is limited by the fact that we could not measure the effect of vasopressin on hepatic artery flow: a simultaneous measurement of hepatic artery flow and portal vein flow would have required a proximal dissection of the hepatic artery beyond the requirements for the surgery, and cannulation of the hepatic artery even with a small needle carries the risk for dissection. We did not want to expose the subjects to these substantial risks and therefore measured portal vein flow and pressure after ligation of the hepatic artery. Krejci et al.26 recently reported a similar decrease of portal vein flow with vasopressin in healthy and septic pigs that was associated with a sustained increase in hepatic artery flow. We can only assume that there is a similar increase of hepatic artery flow with decreased portal vein pressure and portal vein flow in human cirrhotic and transplanted livers but cannot exclude that vasopressin has a direct vasoconstrictor effect on the hepatic artery and could therefore diminish the hepatic arterial buffer response.
We also did not compare vasopressin with other vasoconstrictor drugs such as norepinephrine, and we cannot conclude that it is superior to norepinephrine. Further studies will also need to ascertain at what time after liver transplantation a reduction of portal vein flow is disadvantageous and impedes graft function. The novel experimental design used here for the in vivo measurement of portal vein pressure and portal vein flow will, however, allow further studies of the effect of drugs on hepatic perfusion.




