Protection from phototoxic injury during surgery and endoscopy in erythropoietic protoporphyria
Abstract
Erythropoietic protoporphyria is an inherited condition characterized by pronounced solar photosensitivity and in a minority of patients severe liver disease that necessitates liver transplantation for survival. Phototoxic injury to abdominal organs and skin has been reported in several cases of liver transplantation surgery, including a few transplants in which protective light filters were used. This study discusses the optimal characteristics of light filters used during liver transplantation surgery. An experimental model is used to evaluate the relative protection of different filters, and the results are compared with theoretical calculations regarding the risk for phototoxic injury from light sources in health-care procedures. Whether protective measures are warranted in other illuminated procedures besides liver transplantation has been discussed often but never studied. This study elucidates the risk for phototoxic injury in endoscopy, laparoscopy, and non–liver transplant surgery. A theoretical model and epidemiological data are considered. Our findings indicate that endoscopy, laparoscopy, and surgical procedures other than liver transplantation are safe in the noncholestatic protoporphyria patient and that general recommendations for using filters in these situations are not warranted. Among the tested filters, a flexible yellow filter omitting wavelengths below 470 nm is recommended for liver transplant surgery. This filter has been readily accepted by surgeons and offers a good balance between protection and altered visual color perception. The experimental model, using hemolysis of protoporphyrin-loaded erythrocytes as a measure of phototoxicity, has substantiated theoretical findings on relative filter protection. Liver Transpl 14:1340–1346, 2008. © 2008 AASLD.
Erythropoietic protoporphyria (EPP) is an inherited disorder of heme synthesis caused by a mutated ferrochelatase gene resulting in overproduction and accumulation of protoporphyrin.1 Protoporphyrin is a photoactive compound. The main phenotypic characteristic of EPP is painful sensitivity to intense light, predominantly in the blue-violet region around 400 nm. Protoporphyrin, which also has hepatotoxic properties, is eliminated exclusively via the liver. Some EPP patients develop cholestatic liver failure, in most cases when the disease has already progressed to cirrhosis. Liver transplantation is an established life-saving treatment in this setting. More than 40 liver transplants have been performed for EPP worldwide,2 with phototoxic injury to abdominal organs and skin reported in several cases.1, 3-6 This has prompted recommendations to use protective filters to exclude the most harmful wavelengths from surgical luminaires. Although uncomplicated surgery without filters has been reported, phototoxic damage during liver transplantation surgery using filtered light has occurred as well.3 This raises questions about what the optimal characteristics of filters are and which surgical situations necessitate shielding with light filters in EPP. It is not known whether liver transplantation surgery is the only procedure that calls for protective measures or if protection is required as well in other procedures, such as laparoscopy and endoscopy. Consequently, recommendations for filter use vary among centers.
This study aims to answer 3 main questions: (1) what are the optimal characteristics of a filter for surgical lights, (2) can the relative protection of different filters be evaluated in an experimental model, and (3) what is the risk for phototoxic tissue injury in laparoscopy, endoscopy, and surgical procedures other than liver transplantation?
In attempting to answer these questions, 6 variables are reviewed and considered to develop a theoretical framework for the problem. These are (1) the spectral irradiance of light sources, (2) spectral filter transmission, (3) the absorption spectrum of protoporphyrin, (4) light exposure in different procedures, (5) visibility and working conditions for surgeons under filtered light, and (6) epidemiological data on procedures that a national cohort of EPP patients has undergone.
No in vivo method or model is available with which to evaluate light-induced photochemical reactions in EPP patients. On the basis of previous experimental work,7, 8 we have used an erythrocyte model to evaluate relative protective filter effects.
Abbreviations
EPP, erythropoietic protoporphyria; ERCP, endoscopic retrograde cholangiopancreatography; OR, operating room.
PATIENTS AND METHODS
Determinations of the Spectral Irradiance of Luminaires
A Spectrascan PR705 spectrophotometer was used to determine spectral irradiance from standard halogen operating room (OR) luminaires (Angineu, 300 W and 120 and 80 kLux), a xenon head light (Luxtec LX300 light source with a Luxtec UltraLite headlight), a laparoscope (Karl Storz Xenon 300), and endoscopes (Olympus CLV-160 300-W xenon light source, 160A L/I colonoscope, and GIF-160 gastroscope). To resemble clinical practice, measurements were performed at 100 cm for the main OR lights, at 40 cm for the head light, at 5 cm for the laparoscope, and at 1 cm for the endoscopes. All light sources were turned to maximal output and focus.
Mathematical Processing
Weighted spectral irradiance (Fig. 1C) is the product of irradiance and normalized protoporphyrin absorption at each wavelength. The biologically active irradiance in Fig. 1D represents the product of the sum of the weighted spectral irradiance for the 3 main OR luminaires (the head light and 2 main lights) and normalized filter transmittance at each wavelength.

Measured and calculated characteristics of the light sources, filters, protoporphyrin absorption spectrum, and biological light effects. (A) Spectral irradiance for all light sources in OR, laparoscopy, and endoscopy. (B) Normalized absorption spectrum of PPIX and transmittance spectra for the 4 studied filters. (C) The added irradiance from the 3 principal surgical luminaires (the headlight and 2 main lights) is compared to laparoscopic and endoscopic irradiance after being weighted with the protoporphyrin absorption spectrum in panel B. (D) Theoretical biological effects of irradiation. The added OR irradiance was weighted with the protoporphyrin absorption spectrum (“All OR light” in panel C) and further weighted with the filter transmittance spectra in panel B. Numbers in parentheses represent the areas under corresponding curves. Abbreviations: OR, operating room; PPIX, protoporphyrin IX.
Filter Transmission
The filters, CLS-200-X and TA-81 from Madico, Inc., Supergel #22 Deep Amber from Rosco, Inc., and 61011 from Reflectiv SA (Cretil, France), were obtained from Ganta Trading (Oslo, Norway). Filter transmittance was measured spectrophotometrically (Shimadzu UV2101PC, Shimadzu Corp.).
Protoporphyrin Absorption Spectrum
Protoporphyrin IX (Frontier Scientific, Logan, UT) was dissolved in dimethyl sulfoxide, and the absorption spectrum was scanned from 300 to 700 nm on a spectrophotometer (Shimadzu UV2101PC, Shimadzu Corp.).
Surgeon Survey
Surgical luminaires were equipped with 3 different filters (Deep Amber, TA-81, and 61011) during a liver transplantation pause. Four surgeons present were asked to assess visibility and working conditions. CLS-200-X was not evaluated because it is a clear film that induces minimal color distortion and has previously been used in several liver transplants.3, 9 Furthermore, an Olympus CLV-U40 endoscopy light source was equipped with a glass filter with spectral transmission almost identical to that of 61011. Two gastroenterologists were asked to assess visibility during gastroscopy and colonoscopy, and 2 surgeons evaluated visibility and working conditions in the same manner during laparoscopic cholecystectomy.
Patient Survey
Fifty EPP patients in Sweden were asked to report surgical procedures and endoscopies that they had undergone, whether they had experienced any objective or subjective complications or discomfort related to light, and whether they had been protected from light during the procedure in any way. Reported information was confirmed or completed when medical records were readily available. Information on 3 patients who expired during the last decade from EPP liver failure was included, but liver transplantation data were excluded.
Experimental Erythrocyte Model for Evaluating the Protective Effect of Different Filters
Heparin anticoagulated blood (10 mL) was drawn from healthy volunteers. The erythrocytes were washed 3 times and diluted to a concentration of 8.0 × 1010cells/L in a buffer (154 mM NaCl), 10 mM N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid, and 7 mM glucose (pH 7.4). The red cells were made light-sensitive by the addition of protoporphyrin IX (Frontier Scientific) at a concentration of 50 μM dissolved in dimethyl sulfoxide (Merck, Darmastad, Germany). Cellular loading of protoporphyrin was carried out by 20 repetitive additions of 40-μL aliquots of the protoporphyrin solution to 100 mL of diluted erythrocytes under magnetic stirring and incubation for 15 minutes at 22°C. This gave an erythrocyte protoporphyrin concentration of 56 μmol/L (3156 μg/dL). Irradiation of the protoporphyrin-enriched erythrocytes was performed in a cuvette measuring 7 cm × 7 cm with a light path of 3 mm. The light source was an OR luminaire (5×50W halogen, Hereaeus, Germany) with an irradiance of 212 W m−2 measured at the place of the cuvette (100-cm distance from the light source). The irradiance was measured with a Ramses ACC hyperspectral irradiance sensor equipped with a cosine collector (TriOS GmbH, Oldenburg, Germany). The suspension of protoporphyrin-loaded erythrocytes was placed in the cuvette and was exposed to unfiltered or filtered light for different durations and with the placement of different filters (CLS-200-X, 61011, and TA-81) between the light source and the cuvette. The cuvette temperature was monitored. The temperature never rose more than 1°C. After irradiation, the erythrocyte suspension was left for 75 minutes at 22°C in the dark and was then centrifuged (1300g, 10 minutes). The degree of cell damage was measured as the amount of hemoglobin released from the erythrocytes to the medium. The concentration of hemoglobin in the supernatant was measured spectrophotometrically (Shimadzu UV2101PC, Shimadzu Corp.) at a wavelength of 540 nm. The small amount of spontaneous hemolysis observed in parallel samples that were kept in the dark for the same duration was subtracted from the observed total light-induced hemolysis in each sample.
RESULTS
Measurements and Calculations
Spectral irradiance from luminaires is demonstrated in Fig. 1A. There is a notable difference in output between OR luminaires and laparoscopy on the one hand and endoscopy and background OR light on the other hand. The protoporphyrin absorption spectrum in Fig. 1B explains the ability of different wavelengths to excite protoporphyrin to cause photochemical tissue injury. To get a better understanding of the biological effect of light in clinical situations, data from Fig. 1A were mathematically weighted against the normalized absorption spectrum of protoporphyrin in Fig. 1B to obtain the curves in Fig. 1C, which illustrate weighted spectral irradiation from the 3 main OR lights combined, laparoscopy, and endoscopy. Viewing filter transmittance spectra together with the protoporphyrin absorption spectrum in Fig. 1B facilitates an understanding of the extent to which filters reduce or block wavelengths that excite protoporphyrin. Figure 1D illustrates a theoretical understanding of the differences in biological effects between OR light without filters and with the 3 studied filters. The combined output from the 3 main OR luminaires is displayed after weighting for protoporphyrin absorption and further weighting for filter transmissions. The calculated areas under the curves represent theoretical measures of the phototoxic effect of different light qualities.
Surgeon Survey
Surgeons found the Deep Amber red filter unacceptable because of pronounced distortion of color perception. This filter was omitted from further study. The orange filter (TA-81) caused a moderate influence on color perception but caused no significant restrictions on working conditions and was readily accepted for use. The yellow filter (61011) caused minimal visual distortion. The only negative comment from the user surveys was that the difference in color nuance between the cystic artery and the bile duct seen in laparoscopic cholecystectomy was less prominent. It was noted but not considered an obstacle. Altogether, the surgeons did not consider visibility or working conditions significantly impaired when they worked under the yellow (61011) or orange (TA-81) filter.
Patient Survey
Information on surgical procedures and endoscopies was available from 48 patients in the Swedish EPP cohort, 32 of whom had undergone procedures. The reported 60 surgical and 38 endoscopic procedures are presented in Table 1. A yellow filter with a transmission closely resembling that of filter 61011 was used for protection in 1 laparoscopic cholecystectomy, 2 gastroscopies, and 1 colonoscopy. Protective covering of the surrounding skin was used in 1 case of open arm-fracture surgery. A iodophor-impregnated drape, further discussed later, was used in 1 case of thoracic surgery. All other procedures were performed according to the standard procedure with unfiltered light. For none of the procedures were objective or subjective complications that could be related to light reported.
| Open abdominal | Cerebral hematoma | 1 | ENT | 7 | |
| Bowel resection | 2 | Orthopedic | Urogenital | ||
| Cholecystectomy | 8 | Ankle | 4 | Testis | 3 |
| Hysterectomy | 2 | Arm | 3* | Prostate | 1 |
| Appendectomy | 4 | Arthroscopy | 2 | Cystoscopy | 1 |
| Caesarean section | 3 | Vertebral | 1 | Laparoscopy | 3† |
| Duodenal ulcer | 1 | Hip | 1 | Endoscopy | |
| Renal | 2 | Varia | Gastroscopy | 20†§ | |
| Open thoracic | Hernia | 7 | Colonoscopy | 14† | |
| Lobectomy | 1‡ | Varices | 3 | ERCP | 4§ |
- NOTE: Data on 60 surgical procedures in 32 individuals and 38 endoscopies in 10 individuals were found. Protective measures were used in 6 cases. The mean of the average habitual erythrocyte protoporphyrin concentrations was 28 μmol/L (3265 μg/dL); they ranged from 5 (282 μg/dL) to 122 μmol/L (6869 μg/dL). Three endoscopies were performed during protoporphyric liver failure. No evidence of subjective or objective phototoxic damage was found in any of the procedures.
- Abbreviation: ERCP, endoscopic retrograde cholangiopancreatography; ENT, ear, nose and throat.
- * The surrounding skin was covered.
- † A protective yellow filter was used (1 laparoscopy, 2 gastroscopies, and 1 colonoscopy).
- ‡ The surrounding skin was covered by an iodophor-impregnated drape.
- § Patients were in cholestatic erythropoietic protoporphyria liver failure (2 endoscopic retrograde cholangiographies and 1 gastroscopy without a filter).
Experimental Model for Assessing the Protection of Different Filters
Results from the experimental model with hemolysis as a function of exposure for filtered and unfiltered light of different durations are displayed in Fig. 2. Through the shielding of the erythrocytes with filters, the irradiation time to comparable degrees of hemolysis was prolonged. A brown, transparent drape impregnated with iodophor (Ioban2 from 3M) provided protection from phototoxic injury to a higher degree than CLS-200-X (data not shown).
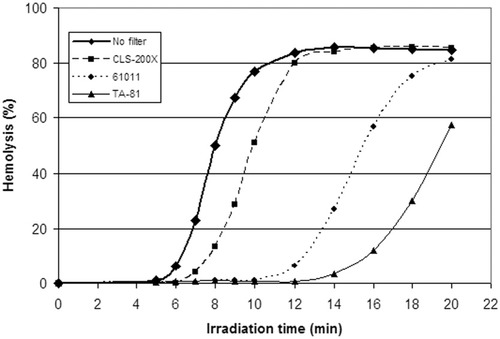
Results from the experimental erythrocyte model designed to examine cell damage as a function of exposure for different doses of unfiltered and filtered operating room light and to assess the relative protection of different filters. When the cuvettes containing protoporphyrin-loaded erythrocytes were shielded with a clear (CLS-200-X), yellow (61011), or orange (TA-81) filter, the irradiation time causing equivalent amounts of phototoxic cell damage, measured as hemolysis, was prolonged by 25%, 100%, and 150%, respectively.
DISCUSSION
The use of protective light filters in liver transplantation for EPP is a standard recommended practice. That some patients have suffered tissue damage despite the use of filtered light is disturbing and constitutes one reason for this study. Another reason is the often discussed, but never studied, uncertainty of whether filters should also be used in surgical procedures other than liver transplantation. Recommendations vary between different publications and centers.
The risk of phototoxic damage to EPP patients from illumination depends on at least 3 variables: light quality, duration of irradiation, and patient variables. Figure 1C illustrates the first variable, light quality. It explains the difference in biological effects between 3 distinct clinical situations: OR surgery, laparoscopy, and endoscopy. OR illumination is clearly more intense than laparoscopic illumination and dwarfs the light intensity in endoscopy. When we consider the second variable, duration of irradiation, differences are even more pronounced. Liver transplantation surgery typically involves 6 to 10 hours of intense abdominal illumination, whereas most laparoscopic procedures last less than 1 hour. In endoscopy, each segment of the intestinal or gastric mucosa is illuminated for seconds, except in a few therapeutic procedures including endoscopic retrograde cholangiopancreatography, for which the duration of duodenal irradiation may approach an hour.
In this context, the open thoracic procedure in Table 1, a 3-hour lobectomy, is of greatest interest. This case [erythrocyte protoporphyrin concentration of 36.2 μmol/L (2038 μg/dL)] was discussed with the anesthesiologists, surgeons, and ward nurses, who found no signs of phototoxic tissue damage or unexpected postoperative symptoms. The light quality is identical to that in liver transplantation surgery, and the light dose (duration) is 30%-50% of that in liver transplantation surgery, but the procedures differ greatly when we consider the third aspect of phototoxicity risk, the individual patient factor. A typical EPP liver transplant recipient is in cholestatic liver failure. Because of reduced hepatic capacity to process and excrete protoporphyrin, levels in erythrocytes, plasma, and presumably perivascular tissues are sharply increased,10 causing pronounced photosensitivity. In 2 of our cases, this meant reduced tolerance even for the background ceiling light in the pretransplant phase. As 1 of these 2 cases underwent liver transplantation, the only luminaire used was the headlight turned to minimum output.1 Nevertheless, abdominal phototoxic damage complicated by intestinal ulceration, bleeding, and ultimately multiorgan failure followed.
Considering the differences in these 3 variables (light intensity, duration, and protoporphyrin concentration) between liver transplant surgery, other OR surgery, laparoscopy, and endoscopy, we do not find it surprising that no evidence of phototoxic tissue damage was found in any of the procedures reported among Swedish EPP patients (Table 1). In addition, biopsies taken after 50 minutes of duodenal illumination in one of the endoscopic retrograde cholangiopancreatography cases demonstrated no histological abnormality when examined by light microscopy.
It is reasonable to conclude that the risk for phototoxic injury from endoscopy and surgery other than liver transplantation is low and that protective measures are not needed. On the basis of these data, a risk for injury cannot, however, safely be dismissed when prolonged OR surgery is being performed in a cholestatic EPP patient. Protective filters should be considered in that situation.
Among 42 reported cases of EPP liver transplantation worldwide, 8 cases of phototoxic injury have been described,1, 3-6 of which 2 were protected by filters.3 Documentation is incomplete for the majority of cases. Incise drapes with antimicrobial activity, such as the iodophor-impregnated drape mentioned previously, are used in many centers in an effort to reduce the incidence of surgical site infections. It is not known to what extent similar drapes have been used in EPP liver transplant surgery. If used, they may have protected patients from skin burns but not from intra-abdominal injuries. Because the exact causes of postoperative complications are often not obvious, speculation about a higher true incidence of phototoxic injuries, not recognized as such, is possible.
The easiest and probably most effective protective measure for liver transplant surgery is to use a filter that blocks the most harmful wavelengths. Several considerations arise when the optimal filter is being selected. Protective capacity is primary, but good color rendering and practical applicability are also very important. The 3 filters selected for further examination in this study were chosen for several reasons. All are thin, flexible films that are preglued, easily applied, heat-resistant, and commercially available. Differences in color (clear, yellow, and orange) due to a stepwise increase in color absorption from the low end of the visible spectrum (blocks violet, blue, and green, respectively) warrant a didactic value (Fig. 1B). CLS-200-X is a clear film that has been used and described in 13 previous cases.3, 9 TA-81 has been used in 1 previous case11 and, with respect to transmission, resembles a thick, inflexible filter described in 2 cases12 as well as a “welding curtain” used in 1 case.13 61011 is a yellow film that has previously never been described.
Methods for direct in vivo quantification of phototoxicity are not available. We used an experimental in vitro model (Fig. 2) to demonstrate phototoxic effects of actual OR light on protoporphyrin-enriched cells and to compare the relative protection of different filters. Erythrocytes were loaded with protoporphyrin in amounts seen in clinical EPP and were exposed to different doses of unfiltered and filtered OR light. Photochemically induced membrane damage8 measured as hemolysis then served as a marker for phototoxicity. Animals or cell cultures photosensitized by endogenously produced protoporphyrin after stimulation with 5-aminolaevulinic acid have been used for similar purposes.14 These models resemble our model but differ from clinical EPP in protoporphyrin distribution and are hampered by costs and laboriousness.
The validity of our experimental model may be challenged. In the clinical situation, phototoxic injury occurs after hours of irradiation, whereas hemolysis occurs within minutes in the experimental model. The erythrocyte concentration was selected for pragmatic reasons. It allowed practical irradiation times. Few studies on protoporphyrin tissue concentration in EPP are available. In one study, the concentration in the epidermis was estimated to be the equivalent of 0.15 μM,15 and in another, where the patient died from EPP liver disease, a skin protoporphyrin level of 4.36 μg/g of wet weight,16 equivalent to approximately 8 μM, was found. In our preliminary studies, the relative filter protection was the same when studied with erythrocyte protoporphyrin concentrations of 20 and 56 μM. This suggests that relative filter protection is independent of the cellular protoporphyrin concentration.
The risk of phototoxic injury during surgery is related to the tissue protoporphyrin concentration. The circulating levels in EPP liver transplantation depend on the severity of liver failure and on the preoperative treatment.17 Protoporphyrin tissue levels might increase during surgery as light-induced release from erythrocytes8, 18 allows pericapillary tissue uptake, but these dynamics have never been studied in vivo.
Results from the experimental model in Fig. 2 may be used to calculate the relative protection of different filters. By comparing irradiation times needed to induce major cell damage, for example, 20% hemolysis, we found that the clear CLS-200-X filter prolonged the irradiation time by 25% in comparison with unfiltered light, 61011 prolonged the irradiation time by 100%, and TA-81 prolonged the irradiation time by 150%. This indicates that the 61011 filter protects 4 times better and the TA-81 filter protects 6 times better than CLS-200-X.
The clear CLS-200-X filter just partially blocks the wavelengths mainly responsible for protoporphyrin excitation (Fig. 1B). This may explain why phototoxic injury has been documented despite its use and why it is not optimal for liver transplant surgery in EPP. Both 61011 and TA-81 offer acceptable color rendering and working conditions for surgeons, whereas 61011 induces less distortion of color perception. In our view, the yellow 61011 filter offers the best balance between protection and visual distortion.
The areas under the respective curves in Fig. 1D represent calculated biologically active irradiation, whereas the irradiation times needed to induce equivalent hemolysis (20%) in Fig. 2 illustrate actual biological effects of irradiance in an experimental model. Results from the 2 models are roughly proportional. Both the theoretical and experimental models appear to be valid tools for estimating the relative protection of different filters.
Collectively, our findings suggest that endoscopy, laparoscopy, and surgical procedures other than liver transplantation are safe in the noncholestatic EPP patient. General recommendations for protective filters during other procedures in health care are not warranted. Among the tested protective filters, 61011 is the most optimal for use during liver transplantation surgery in EPP patients. The experimental model is a useful tool in the study of protection from phototoxic injury. Experimental results substantiate theoretical findings and vice versa.
Acknowledgements
We thank Dr. Guy McCoy for his critical reading of this article.




