Percutaneous transhepatic treatment of hepaticojejunal anastomotic biliary strictures after living donor liver transplantation
Abstract
Endoscopic treatment has largely replaced surgery as the initial treatment for biliary strictures following living donor liver transplantation; however, this treatment is nearly impossible in patients who have previously undergone hepaticojejunostomy (HJ). We therefore retrospectively evaluated the efficacy of percutaneous transhepatic treatment in patients who developed HJ strictures following living donor liver transplantation. Percutaneous transhepatic biliary drainage and subsequent balloon dilation of biliary strictures were performed on 83 patients. Serial exchanges of drainage tubes with larger diameters up to 14 Fr were performed at 4-week intervals. Drainage tubes were removed if follow-up cholangiography revealed fluent passage of the contrast medium without recurrence of symptoms or changes in the biochemical findings. The clinical outcome, tube independence rate, and patency rate following drainage tube removal were retrospectively evaluated. Except for 2 patients who had failed negotiation of biliary strictures, clinical success was achieved in all 81 patients following percutaneous transhepatic treatment, and the drainage tubes were removed from 76 (93.8%) of these 81 patients. Tubes were removed 11.2 ± 7.4 months after initial percutaneous transhepatic biliary drainage. The recurrence rate at a mean of 36.0 ± 26.2 months following drainage tube removal was 15.8%. One- and three-year primary patency rates were 95.3% ± 2.7% and 80.9% ± 5.2%, respectively. In conclusion, percutaneous transhepatic treatment is an effective alternative treatment for HJ strictures following living donor liver transplantation. However, further research will be required in order to minimize the duration of treatment and the stricture recurrence rate following tube removal. Liver Transpl 14:1323–1332, 2008. © 2008 AASLD.
Developments in surgical techniques and immunosuppressive drugs have contributed to improved outcomes for living donor liver transplantation (LDLT). However, anastomotic biliary stricture remains an important cause of morbidity following LDLT. Endoscopic treatment has largely replaced surgery as the initial treatment of biliary strictures because of the lower morbidity and mortality of the former.1-6 However, endoscopic treatment is difficult in patients who have previously undergone hepaticojejunostomy (HJ). In such situations, percutaneous transhepatic treatment, including percutaneous transhepatic biliary drainage (PTBD), balloon dilation, and stent placement, may be a good alternative.
We retrospectively evaluated the efficacy of PTBD and balloon dilation followed by indwelling drainage tube placement in patients who developed HJ biliary strictures following LDLT.
Abbreviations
CI, confidence interval; HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; HJ, hepaticojejunostomy; LDLT, living donor liver transplantation; N/A, not available; OPD, outpatient department; PTBD, percutaneous transhepatic biliary drainage.
PATIENTS AND METHODS
Patient Population
Between January 1997 and June 2006, 92 (7.76%) of the 1201 patients who underwent LDLT at our institution also underwent percutaneous transhepatic treatment to manage HJ biliary strictures. None of our patients underwent surgical or endoscopic treatment as the initial therapeutic method for HJ biliary strictures. Among them, 9 patients were excluded because of either patient death (n = 8) unrelated to PTBD or retransplantation (n = 1). Demographic data for the remaining 83 patients are summarized in Table 1. The initial diagnosis of biliary stricture was based on combinations of clinical symptoms, biochemical data, and the results of imaging with various modalities, including ultrasonography, computed tomography, and diisopropyl iminodiacetic acid scanning. The diagnosis was confirmed by percutaneous transhepatic cholangiography. On the basis of the configuration of the cholangiographic findings, biliary strictures were classified as simple or complex anastomotic strictures, with the complex anastomotic type including bifurcated or trifurcated biliary strictures.
| Item | Number of Patients |
|---|---|
| Males versus females | 65:18 |
| Age | 21–64 years (mean, 48 ± 9) |
| Underlying diseases | |
| Liver cirrhosis (HBV) | 38 (45.8%) |
| Liver cirrhosis (HBV) and HCC | 29 (34.9%) |
| Liver cirrhosis (others)* | 16 (19.3%) |
| Graft lobe | |
| Single lobe versus dual lobes | 49:34 |
| Interval between LDLT and PTBD | 0.3–55.2 months (mean, 9.0 ± 9.2) |
| ≤6 months | 42 (50.6%) |
| >6 months | 41 (49.4%) |
| Symptoms and signs | |
| Cholangitis | 75 (90.4%) |
| Jaundice | 40 (48.2%) |
| Abnormal liver enzymes† | 23 (27.7%) |
| Itching | 9 (10.8%) |
| Type of biliary strictures | |
| Simple anastomotic | 53 (63.9%) |
| Complex anastomotic | 30 (36.1%) |
| Hepatic arterial stenosis | 1 (1.2%) |
- Abbreviations: HBV, hepatitis B virus infection; HCC, hepatocellular carcinoma; LDLT, living donor liver transplantation; PTBD, percutaneous transhepatic biliary drainage.
- * Including alcoholic liver cirrhosis (n = 4), primary biliary cirrhosis (n = 4), fulminant hepatitis (n = 3), liver cirrhosis of unknown cause (n = 2), cavernous hemangioma (n = 1), Wilson's disease (n = 1), and cholangiocarcinoma (n = 1).
- † Elevation of serum aspartate aminotransferase and alanine aminotransferase to >2 times their normal ranges.
Procedure Details
We obtained the approval of our hospital's institutional review board to conduct a retrospective review of the patients' medical and imaging records. Written informed consent for treating biliary strictures was obtained from each patient or his or her legal guardian.
Our treatment protocol is summarized in Fig. 1. PTBD and subsequent balloon dilation of biliary strictures were performed under conscious sedation using intravenous pethidine hydrochloride (Demerol, Keukdong Pharmaceuticals, Seoul, Korea) and local anesthesia using intramuscular lidocaine (Jeil Pharmaceuticals, Taegu, Korea). The intrahepatic bile duct was punctured with a 21-gauge Chiba needle (Cook, Bloomington, IN) under fluoroscopic or ultrasonographic guidance. The needle was exchanged for a 5-Fr coaxial dilator and a 6- to 7-Fr sheath (Terumo, Tokyo, Japan) over a 0.018-inch (Cook) or 0.035-inch angled hydrophilic (Terumo) guide wire. The 0.035-inch guide wire and a 5-Fr cobra or Kumpe catheter (Cook) were used to traverse a stricture. An internal-external 8.5- or 10.2-Fr drainage tube (Cook) was placed across the stricture following balloon dilation using a 6- to 10-mm-diameter balloon catheter (Boston Scientific, Galway, Ireland). The balloon catheter was inflated for 1 minute until the disappearance of the hourglass deformity created by the stricture. In patients who had complex-type biliary strictures, cannulation of other strictures and subsequent balloon dilations were attempted through the initial PTBD route. If this was not possible, a second PTBD and subsequent balloon dilation were performed. Patients were then discharged when their symptoms and biochemical data improved.
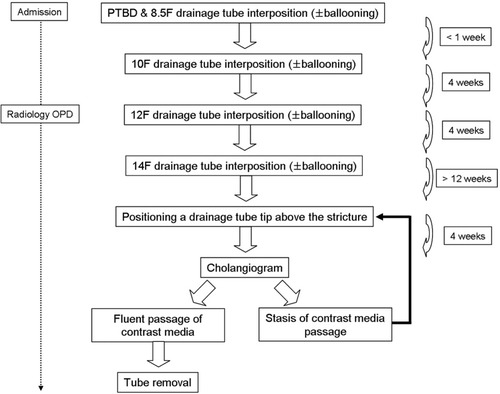
Schematic diagram of our treatment protocol. Abbreviations: OPD, outpatient department; PTBD, percutaneous transhepatic biliary drainage.
Serial exchanges of the internal drainage tubes for tubes of a larger diameter up to 14 Fr were performed at 4-week intervals on an outpatient basis. At that time, the drainage tube was cut 3 to 5 cm from the skin entry site, with the tip clamped to minimize discomfort from the drainage tube. Following at least 12 weeks of 14-Fr internal drainage tube interposition, the tube was positioned above the stricture, and the tip was clamped for 4 weeks in order to assess the presence of elastic restenosis of the treated HJ anastomosis.
Drainage tubes were removed when follow-up cholangiography revealed fluent passage of the contrast medium without recurrence of patient symptoms or changes in biochemical data. “Fluent passage” was defined subjectively as easy visualization of the small bowel beyond the stricture under mild-pressure manual infusion of the contrast medium, absence of bile duct dilation in comparison with a previous cholangiogram under higher pressure manual infusion of the contrast medium, and nearly total passage of the contrast medium into the small bowel in 10 minutes. However, if there was evidence of restenosis, the drainage tube was repositioned across the stricture for an additional 12 weeks or more following repeat balloon dilation.
Follow-Up
Serum aspartate aminotransferase, alanine aminotransferase, bilirubin, and alkaline phosphatase were measured prior to PTBD on the dates of drainage tube exchanges and on the dates of drainage tube removal. Following drainage tube removal, patient symptoms and biochemical data were routinely assessed every 8 to 12 weeks at the liver transplant outpatient clinic.
Analysis of Outcomes
“Technical success of balloon dilation” was defined as successful balloon dilation followed by drainage tube interposition across the biliary strictures. “Clinical success” was defined as the disappearance of patient symptoms and normalization of biochemical data or their decrease to less than 1.5 times the normal levels. “Primary patency” was defined as the time interval from the drainage tube removal until recurrent symptoms or the last follow-up. Complications were classified as major and minor according to the guidelines of the Society of Interventional Radiology Standards of Practice Committee.7 Major complications were defined as those necessitating major therapy, those necessitating an unplanned increase in the level of care or prolonged hospitalization (>48 hours), and those resulting in permanent adverse sequelae or death. Minor complications were defined as those requiring no therapy or nominal therapy, including overnight admission for observation only.
Statistical Analysis
The primary and secondary patency of clinical success was analyzed with the Kaplan-Meier method. Multivariate Cox proportional hazards modeling was used to assess factors influencing drainage tube removal and recurrence following drainage tube removal, including age, sex, type of grafted lobes, interval between LDLT and PTBD, type of biliary stricture, number of PTBDs, and duration of indwelling tube placement. Variables were selected in a stepwise forward selection manner, with entry and retention set with a P value of 0.05 considered to indicate a significant difference. The risk of any variable was expressed as a hazard ratio with a corresponding 95% confidence interval. All analyses were performed with SPSS software, version 12.0 (SPSS, Chicago, IL).
RESULTS
Percutaneous transhepatic cholangiography revealed simple anastomotic strictures in 53 (63.9%) patients and complex anastomotic strictures in 30 (36.1%) patients (Figs. 2 and 3). Thirteen of the 30 patients with complex anastomotic strictures could be treated with 1 PTBD; however, 16 required 2 PTBDs and 1 needed 3 PTBDs in order to treat the complex anastomotic strictures. In all, 101 PTBDs were performed in 83 patients. Procedural complications during treatment are listed in Table 2.
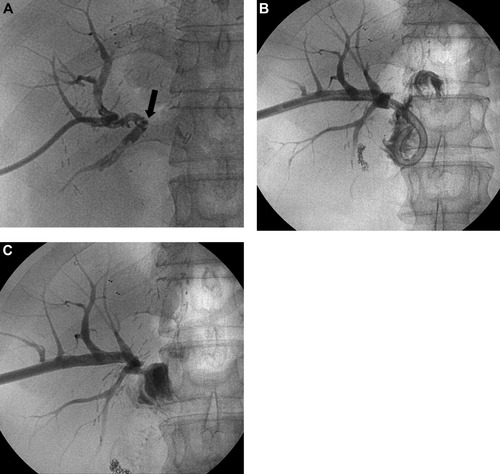
A case of a simple anastomotic biliary stricture. (A) Cholangiogram shows occlusion of the hepaticojejunostomy site (arrow). (B) Follow-up cholangiogram 4 months after 14-Fr internal drainage tube placement shows fluent passage of the contrast medium. (C) Cholangiogram 4 weeks after the positioning of the tip of a 14-Fr drainage tube above the stricture shows fluent passage of the contrast medium.
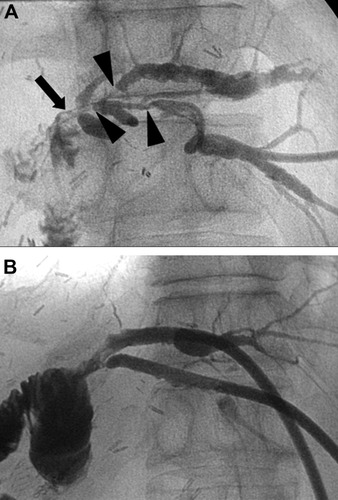
A case of complex anastomotic biliary strictures. (A) Percutaneous transhepatic cholangiogram shows strictures at the hepaticojejunostomy site (arrow) and orifices of the proximal intrahepatic ducts (arrowheads). (B) Cholangiogram 4 weeks after the positioning of the tips of 14-Fr drainage tubes above the stricture shows fluent passage of the contrast medium.
| Complication | Number of Patients | Incidence | Management |
|---|---|---|---|
| Major (n = 15; 18.1%) | |||
| Cholangitis | 12 | 14.5% | Antibiotics and drainage tube exchange |
| Hemobilia | 2 | 2.4% | Transarterial embolization |
| Drainage tube incorporation into the jejunum | 1 | 1.2% | Removal with a snare wire |
| Minor (n = 3; 3.6%) | |||
| Drainage tube extraction | 3 | 3.6% | Reinsertion along a previous percutaneous tract |
| Total | 18 | 21.7% |
Negotiation of biliary strictures and subsequent balloon dilation were technically successful in 81 (97.6%) of 83 patients after a mean of 1.6 ± 1.0 (range, 1-6) cannulation attempts. Removal of bile duct stones was also performed in 19 (22.9%) patients. However, cannulation of biliary strictures failed in 2 (2.4%) patients despite 8 and 9 cannulation attempts, respectively; 1 patient underwent subsequent surgical revision, and the other patient still maintained external drainage. Both of these patients are currently healthy and have normal liver function.
Except for 2 patients who had failed negotiation of their biliary strictures, clinical success was achieved in all 81 patients following PTBD, with drainage tubes removed from 76 (93.8%) of these 81 patients (Figs. 2 and 3). Tubes were removed 11.2 ± 7.4 (range, 2.8-35.2) months after the initial PTBD. Multivariate Cox regression analysis showed that the interval between LDLT and PTBD was the only independent factor associated with the period of drainage tube maintenance (Table 3); that is, patients with longer than a 6-month interval between LDLT and PTBD required a shorter duration of drainage tube placement.
| Variable | Number of Patients (%) | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|---|
| Type of biliary stricture | ||||
| Simple type | 53 (63.9) | 1 | 0.946 | |
| Complex type | 30 (36.1) | 0.978 | 0.522–1.833 | |
| Age | ||||
| ≤50 years | 45 (54.2) | 1 | 0.156 | |
| >50 years | 38 (45.8) | 0.701 | 0.429–1.145 | |
| Sex | ||||
| Female | 18 (21.7) | 1 | 0.530 | |
| Male | 65 (78.3) | 1.196 | 0.683–2.094 | |
| Number of PTBDs | ||||
| 1 | 66 (79.5) | 1 | 0.572 | |
| ≥2 | 17 (20.5) | 1.257 | 0.568–2.781 | |
| Graft lobe | ||||
| Single lobe | 48 (57.8) | 1 | 0.923 | |
| Dual lobes | 35 (42.2) | 1.026 | 0.608–1.732 | |
| Interval between LDLT and PTBD | ||||
| >6 months | 42 (50.6) | 1 | <0.001 | |
| ≤6 months | 41 (49.4) | 0.338 | 0.206–0.555 |
- Abbreviations: CI, confidence interval; LDLT, living donor liver transplantation; PTBD, percutaneous transhepatic biliary drainage.
Twelve (15.8%) of the 76 patients experienced recurrent symptoms at a mean of 18.2 ± 14.7 (range, 5.2-61.3) months after tube removal. Multivariate Cox regression analysis showed that there were no independent factors associated with recurrence, although patients with less than a 6-month interval between LDLT and PTBD were likely to have recurrence (Table 4). All of the patients with recurrence underwent repeat PTBD and balloon dilation to treat recurrent biliary strictures, and clinical success was achieved in 11 patients. One patient died of ductopenic rejection 2 months after repeat PTBD. Drainage tubes were removed from 9 of the 11 patients 13.3 ± 7.4 (range, 3-23) months after the repeat PTBD; the 2 remaining patients are still undergoing our treatment protocol, having maintained internal drainage tubes for 3 and 6 months, respectively. Eight of the 9 patients were still healthy without recurrence for a median follow-up of 12.5 (range, 6.5-99.8) months; however, 1 patient underwent a third session of PTBD 50 months after the second PTBD because of recurrence.
| Variable | Number of Patients (%) | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| Type of biliary stricture | ||||
| Simple type | 48 (63.2) | 1 | 0.763 | |
| Complex type | 28 (36.8) | 1.278 | 0.259–6.298 | |
| Age | ||||
| ≤50 years | 40 (52.6) | 1 | 0.600 | |
| >50 years | 36 (47.4) | 1.399 | 0.399–4.909 | |
| Sex | ||||
| Female | 17 (22.4) | 1 | 0.688 | |
| Male | 59 (77.6) | 1.344 | 0.317–5.692 | |
| Number of PTBDs | ||||
| 1 | 61 (80.3) | 1 | 0.281 | |
| ≥2 | 15 (19.7) | 0.333 | 0.045–2.460 | |
| Graft lobe | ||||
| Single lobe | 43 (56.8) | 1 | 0.605 | |
| Dual lobes | 33 (43.2) | 0.679 | 0.156–2.946 | |
| Interval between LDLT and PTBD | ||||
| >6 months | 38 (50.0) | 1 | 0.058 | |
| ≤6 months | 38 (50.0) | 3.434 | 0.958–12.306 | |
| Treatment duration | ||||
| ≤271 days | 42 (55.3) | 1 | 0.061 | |
| >271 days | 34 (44.7) | 5.941 | 0.921–38.310 |
- Abbreviations: CI, confidence interval; LDLT, living donor liver transplantation; PTBD, percutaneous transhepatic biliary drainage.
During the mean follow-up period of 36.0 ± 26.2 (range, 2.0-112.3) months in the 76 patients, the 1-, 2-, and 3-year primary and secondary patency rates were 95.3% ± 2.7% and 100%, 80.9% ± 5.2% and 100%, and 80.9% ± 5.2% and 100%, respectively (Fig. 4).
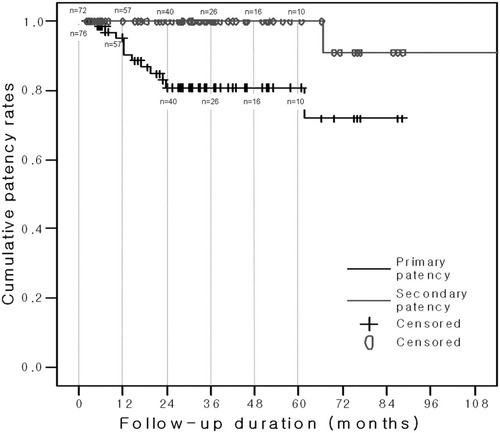
Kaplan-Meier survival curve demonstrating the degree of patency following drainage tube removal in 76 patients.
However, drainage tubes could not be removed from 5 (6.2%) of the 81 patients because of residual strictures requiring further indwelling drainage tube placement. Three of these patients had simple anastomotic strictures, and the remaining 2 had complex anastomotic strictures. In these patients, cholangiograms performed 4 weeks after drainage tube positioning above the strictures showed significant restenosis or occlusion (Fig. 5). In addition, they showed significant elevations in liver enzymes or complained of recurrent symptoms at that time. Because of the risk of early recurrence, these patients underwent repeat drainage tube placement across strictures following repeat balloon dilation. They have maintained these internal drainage tubes for 38.0 ± 24.0 (range, 15.4-77.0) months.
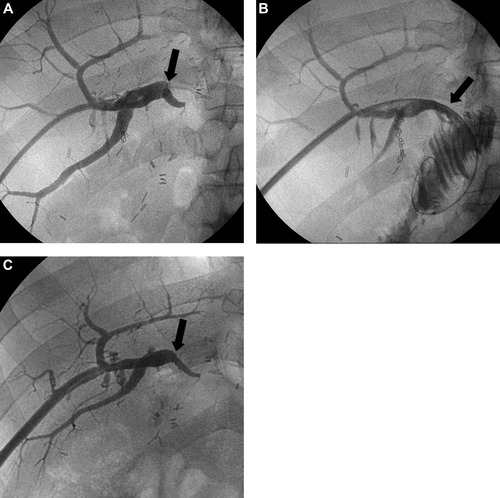
A case of early reocclusion occurring 4 weeks after the positioning of the tips of 14-Fr drainage tubes above the stricture. (A) Percutaneous transhepatic cholangiogram shows complete occlusion at the hepaticojejunostomy site (arrow). (B) Cholangiogram obtained immediately after 14-Fr internal drainage tube removal following 3 months of internal drainage shows fluent passage of the contrast medium through the hepaticojejunostomy site (arrow). (C) However, a cholangiogram 4 weeks after the positioning of the tip of a 14-Fr tube above the stricture shows reocclusion at the hepaticojejunostomy site (arrow).
DISCUSSION
We found that the clinical success and tube independence rates of percutaneous transhepatic treatment of HJ anastomotic biliary strictures after LDLT were 100% (81/81) and 93.8% (76/81), respectively. These clinical success and tube independence rates were superior to previously reported data (51%-100% clinical success rate and 94.9% tube independence rate) in cadaveric liver transplant recipients (Table 5).8-13 In addition, our observed recurrence rate, which was 15.8% at a mean follow-up of 36.0 ± 26.2 months, was superior to the 27%-44% recurrence rates (at a mean follow-up of 5 months to 5.8 years) reported in other studies.8-11, 13
| Reference | Number of Patients | Catheter Diameter | Balloon Size | Treatment Duration | Initial Success Rate | Follow-Up Duration | Recurrence Rate | 1-Year Primary Patency Rate |
|---|---|---|---|---|---|---|---|---|
| Righi et al.8 | 51 | 8–10 Fr | 6–18 mm | 3–90 days (mean, 22) | 100% | 1–41 months (mean, 9.7) | 33% | N/A |
| Roumilhac et al.9 | 14 | N/A | 8–12 mm | N/A | 93% | 6–48 months (median, 26) | 36% | 71% |
| Sung et al.10 | 76 | N/A | 6–10 mm | >4 weeks* | 51% | 6 months to 10 years (mean, 5.8 years) | 21% | 97% |
| Saad et al.11 | 38 | 8–12 Fr | 6–14 mm | 0–6 weeks | 85% | 0.5–38 months (mean, 5) | 44% | 45% |
| Choo et al.12 | 12 | 14–18 Fr | 6–12 mm | 30–90 days (mean, 60) | 92% | 8–40 months (mean, 19) | 17% | 82% |
| Zajko et al.13 | 56 | 8.5–14 Fr | 4–15 mm | 5–8 weeks | 86% | 6–120 months (mean, 40) | 27% | 73% |
| Current study | 83 | 14 Fr | 6–10 mm | 3–35 months (mean, 11) | 100% | 2–112 months (mean, 36) | 16% | 95% |
- Abbreviation: N/A, not available.
- * Three balloon dilation sessions were usually performed within a 4- to 8-week interval between procedures.
Our treatment protocol differed from those reported elsewhere. We hypothesized that the effect of balloon dilation might be temporary and that immunosuppressive therapy following LDLT may delay the healing of balloon-dilated biliary strictures. We therefore placed a relatively large diameter (14-Fr) indwelling tube for a long period of time, that is, at least 12 weeks. In comparison, Righi et al.8 placed 8- to 10-Fr drainage tubes for 3-90 days and reported a 33% recurrence rate at a mean follow-up of 9.7 months (Table 5). Saad et al.11 reported a 44% recurrence rate at a mean follow-up of 5 months after the placement of 8- to 12-Fr drainage tubes for 0-6 weeks. However, Choo et al.12 reported a relatively low recurrence rate (17%) at a mean follow-up of 19 months after the placement of 14- to 18-Fr drainage tubes for 30-90 (mean, 60) days.
Although there is no consensus regarding the best time to evaluate the patency of treated biliary strictures, we assessed patency 4 weeks after drainage tube positioning above the stricture in combination with clinical symptoms, laboratory data, and cholangiographic findings. In comparison, most investigators deemed the main bile duct to be patent when less than 20%-30% of the duct contained residual stenosis on the basis of routine follow-up cholangiography and they did not perform temporary retrieval of the drainage tubes.9-14 However, we regarded a cholangiogram obtained immediately after the removal of a drainage tube as not displaying the actual status of the treated biliary strictures. Actually, 24 (31.6%) of our 76 patients from whom drainage tubes were finally removed needed several additional months of drainage tube replacement because of significant restenosis seen on the follow-up cholangiogram obtained 4 weeks after drainage tube positioning above the stricture (Fig. 5). In addition, 5 patients who showed clinical success also had to maintain their drainage tubes because of early restenosis. Sunku et al.15 reported that a single course of balloon dilation followed by the placement of 6- to 14-Fr drainage tubes for 3 months was unsuccessful in treating biliary strictures in 66% of 35 patients.
Our findings indicate that a sufficient interval of large-diameter drainage tube interposition followed by a sufficient interval without a tube is needed to minimize the incidence of early recurrence following drainage tube removal. We found that the 1- and 3-year primary patency rates were 95.3% ± 2.7% and 80.9% ± 5.2%, respectively. These patency rates were comparable to previously reported 1- and 3-year primary patency rates of 58.8%-97.3% and 52.0%-82.3%, respectively.9, 10, 13
We found that the interval between LDLT and PTBD (>6 months) was the only independent factor associated with early drainage tube removal. In contrast, Sung et al.10 reported that the type (simple type) and location (extrahepatic) of biliary strictures were independent factors for tube independency. Further investigations will be needed to determine the independent factors associated with tube independence following percutaneous transhepatic management.
Our study had several limitations. First, the treatment duration was excessive. We assumed, however, that long-term tube placement effectively reduced the recurrence rate following tube removal, and our low recurrence rate may validate our practice. Although surgical revision is absolutely the definitive modality for treatment of anastomotic biliary strictures with early recovery, in our opinion, surgical revision is a more invasive procedure and may not be simple in LDLT recipients because the various types of biliary stricture that may occur following LDLT differ from those following orthotopic liver transplantation. Actually, 36% of our patients had complex-type biliary strictures following LDLT. Therefore, in our opinion, surgical revision should be considered in patients who have simple-type biliary strictures. Uncovered metallic stent placement has been used to treat biliary strictures refractory to balloon angioplasty9, 16, 17 with excellent immediate success rates; however, these stents had limited patency and frequently required repeat intervention, with a primary patency rate of only 27% at 1-year follow-up.9 We also considered covered retrievable stent placement; however, in almost all our patients, the segment of bile duct from the anastomotic stricture to the intrahepatic bile duct branches was too short, and complex-type anastomotic strictures were frequent in our patients. These configurations prohibited covered retrievable stent placement because of the risk of branched intrahepatic duct occlusion. Yazumi et al.5 also reported that 57 (76%) of 75 patients with biliary strictures following LDLT had fork-shaped, trident-shaped, or multibranched strictures. It is likely, however, that covered retrievable stent placement may be able to shorten the duration of treatment.18, 19 In addition, endoscopic treatment may be considered in the future as an initial, less invasive therapeutic modality with short treatment duration for treating HJ biliary strictures. Recently, several investigators have reported high success rates using endoscopic retrograde cholangiography or double balloon enteroscopy to treat posttransplant HJ biliary complications or small bowel diseases.20, 21
Second, our definition of “fluent passage” of the contrast medium on follow-up cholangiogram was subjective, although we referred to the presence of recurrent patient symptoms and changes in biochemical data. A more objective definition is needed to define the patency of treated HJ anastomotic strictures. Savader et al.22 performed biliary manometric perfusion testing prior to drainage tube removal following biliary stricture dilation, and they considered intraductal pressure less than 20 cm H2O to be acceptable.
In conclusion, we have found that our treatment protocol is effective for treating HJ anastomotic biliary strictures. However, further investigation is needed to minimize the duration of treatment and the recurrence rate following tube removal.
Acknowledgements
We thank Bonnie Hami, M.A. (United States), for her editorial assistance in preparing this article.




