Improvement of impaired albumin binding capacity in acute-on-chronic liver failure by albumin dialysis
Abstract
Extracorporeal albumin dialysis (ECAD) enables the elimination of albumin bound substances and is used as artificial liver support system. Albumin binding function for the benzodiazepine binding site specific marker Dansylsarcosine was estimated in plasma samples of 22 patients with cirrhosis and hyperbilirubinaemia (ECAD: n = 12; control: n = 10) during a period of 30 days in a randomized controlled clinical ECAD trial. Albumin Binding Capacity (ABiC) at baseline was reduced to 31.8% (median; range 24%-74%) and correlated to the severity of liver disease. Within two weeks a significant improvement of ABiC and a reduction of the albumin bound markers bilirubin and bile acids were observed in the ECAD group. During single treatments a significant decrease of albumin bound substances (bilirubin and bile acids) as well as an increase in ABiC was observed. In the control group, baseline ABiC was significantly lower in patients who died during study period (34.2% vs. 41.7%; P < 0.028), whereas no significant differences were observed for CHILD, coagulation factors, albumin, bile acids nor bilirubin. At baseline 13 patients had a severely impaired ABiC (<40%), improvement of ABiC was more frequent in the ECAD group (5/6) than in the SMT group (2/7). Reduced albumin binding function is present in decompensated liver failure and is related to severity and 30 day survival. ABiC can be improved by ECAD. The beneficial effect of this treatment may be related to the improvement of albumin binding function more than to the elimination of specific substances. Characterization of albumin function by the ABiC test may help to evaluate different liver support systems and other therapeutic measures. Liver Transpl 14:1333–1339,2008. © 2008 AASLD.
Human serum albumin as the major plasma protein not only is responsible for the maintenance of the colloid osmotic pressure but also provides ligand binding and transport properties, antioxidant functions, and enzymatic activities.1-3 A variety of endogenous substances and metabolites as well as drugs are transported by albumin from the intestine to the liver and to other tissues and organs.
Under physiological conditions, one of the primary transport roles of the molecule is to carry fatty acids, up to 6 mol/mol of albumin.4
In addition to at least 3 to 4 binding areas for long chain fatty acids, 2 other major binding sites have been identified.5, 6 Although site I (according to Sudlow)6 is seen as the primary binding area for large heterocyclic components or dicarboxylic acids (such as eicosanoids, warfarin, and bilirubin), ligands of site II are often aromatic carboxylic structures, such as diazepam, tryptophan, and other indols.2, 3
Albumin binding of a drug may be influenced by other ligands bound to the same binding site. Competitive drug-drug interactions are known, and binding sites of new drugs can be characterized by the displacement of a site-specific marker from albumin. Furthermore, interactions between drugs and endogenous substances can be observed, as has been described for bilirubin and drugs bound to site I, such as valporate, warfarin, furosemide, and salicylate.3 The binding of dansyl sarcosine (DS), a site II–specific marker, is influenced by the binding of substances such as octanoate, diazepam, ibuprofen, and bile acids.7, 8
Reduced albumin binding of drugs can be observed in hepatic insufficiency.3, 9-11 This may be related to either the reduced albumin concentration or the accumulation of drugs, metabolites, or endogenous inhibitors.
Recently, the albumin binding capacity (ABiC) as a method for the assessment of the binding site II–specific binding capacity and thus for the characterization of the functional status of the albumin molecule has been reported.12, 13 In a first clinical evaluation, a lower ABiC was observed in patients with an acute decompensation of a chronic liver disease in comparison with healthy volunteers, and ABiC was correlated to the severity of liver failure.11, 13
Extracorporeal albumin dialysis (ECAD) is a therapeutic option for the elimination of albumin-bound substances and has been shown to be able to improve cholestasis, renal and liver functions, hepatic encephalopathy (HE), and hemodynamic situations effectively, and this has resulted in improved survival.14-18
As the ABiC test characterizes the binding site II–specific albumin load and as ECAD eliminates albumin-bound substances, an improvement of ABiC by ECAD can be assumed.
In the present study, the ABiC test was applied to plasma samples collected during a controlled, randomized clinical ECAD trial to investigate the time course of ABiC in patients with and without extracorporeal elimination of albumin-bound substances and to characterize changes in ABiC during ECAD treatments.
Abbreviations
ABiC, albumin binding capacity; CHILD, Child-Pugh-Turcotte classification; DS, dansyl sarcosine; ECAD, extracorporeal albumin dialysis; HE, hepatic encephalopathy; max, maximum; min, minimum; NS, not significant; SD, standard deviation; SMT, standard medical therapy.
PATIENTS AND METHODS
The study was performed at the liver transplant centers of the Universities of Essen and Rostock in an open-label, randomized, controlled parallel group design.
Twenty-four patients presenting cirrhosis and a superimposed acute liver injury leading to decompensation and severe hyperbilirubinemia (total bilirubin in serum > 20 mg/dL) with a further increase during the prephase with conventional therapy were enrolled into this study. Hepatorenal syndrome and decreasing bilirubin levels during the prephase were exclusion criteria. Patients were randomly assigned either to a group with standard medical therapy (SMT; n = 12) or to a group with SMT and up to 10 additional ECAD treatments (n = 12) with the MARS® system (Teraklin AG, Rostock, Germany) within the first 12 days with a mean duration of 6 hours daily. ECAD treatment was performed if bilirubin was greater than 15 mg/dL and was discontinued when the patient reached the endpoint (responder: bilirubin < 15 mg/dL for 3 consecutive days without ECAD treatment).
Plasma samples were collected on a daily basis until response, death, or the end of the study period and for responders during weekly main visits in both therapy groups, and they were stored at −80°C until analysis. In addition, plasma samples were taken before and after ECAD treatments.
Analysis of the ABiC was performed in 22 patients of the per protocol group. One of the enrolled patients was excluded from per protocol analysis as a biopsy revealed a prolonged cholestatic drug reaction due to an anabolic drug. For another patient of the SMT group, no baseline ABiC data were available. Source data verification of laboratory data as well as other clinical parameters was performed by an independent monitor. If data at the weekly main visits were missing, data from the day before or after were used instead. With respect to a mortality of 50% in the control group, all analyses were performed in addition by the last observed value being carried forward to avoid a bias by the reduction of patients with the most severe clinical status in one group.
The design of the study, patient characteristics, and primary outcome measures have been reported in detail already.19
The protocol complied with the ethical guidelines of the Declaration of Helsinki and was approved by the medical ethics committees of both universities. Informed consent was obtained from all subjects or their closest relatives.
Assessment of ABiC
Methodological aspects of the ABiC test have been described in detail previously.13 Briefly, this method is based on the measurement of the fraction of a site-specific fluorescence marker not bound by the albumin in the sample (unbound fraction). By the comparison of the unbound fraction of this marker to the same amount of a reference albumin, ABiC of the albumin in the sample can be expressed as a percentage of the binding capacity of the reference albumin. For the ABiC assessment, all samples were diluted to the same albumin concentration of 150 μmol/L. The same volume and same concentration of a binding site II (benzodiazepine binding site) specific fluorescence marker (DS; Sigma Chemical) were added to each sample. The unbound marker molecules were separated by ultrafiltration (Centrisart I, Sartorius Göttingen; cutoff = 20,000 Da). Multiple aliquots of the albumin-free ultrafiltrate were added to a 96-well plate, and fluorescence was measured (excitation = 355 nm, emission = 460 nm; Fluoroscan, Labsystems, Finland) after the addition of an albumin solution as a fluorescence amplifier. In parallel, the same procedure was performed with a standard albumin for reference. A standardized virus-inactivated human serum preparation from pooled human plasma (Biseko, Biotest® Pharma GmbH, Dreieich, Germany) was used as a reference for ABiC.
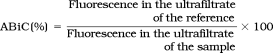
Statistical Analysis
Statistical analysis was performed with SPSS 12 software, and P < 0.05 was considered significant. With respect to the small numbers, the Fisher exact t test was applied where appropriate. Differences between groups were tested by the use of the nonparametric Mann-Whitney U test for 2 independent samples, whereas differences before and after a single treatment were analyzed by means of the paired Student t test. Frequencies were compared by use of Fisher's exact test. The 2-sided Spearman rho test was used to assess correlations between ABiC and the HE grade, Child-Pugh-Turcotte classification (CHILD) score, renal function (creatinine), liver function [prothrombin time (Quick) and albumin], white blood cell count, platelet count, C-reactive protein, levels of strongly albumin-bound substances (bilirubin and bile acids), and their molar ratio with respect to albumin for the entire study group at baseline. To assess the impact of baseline ABiC on the 30-day survival, the same parameters were used in a subgroup analysis of the patients without extracorporeal elimination of albumin-bound substances (the SMT group).
RESULTS
In the study cohorts, ABiC was reduced to 38.1% (range 24%-74%) in comparison with the reference albumin obtained from healthy blood donors. Baseline ABiC did not significantly differ between the two study groups (43% for ECAD + SMT versus 38% for SMT; not significant).
The reduced ABiC at baseline correlated significantly with the severity of liver disease (CHILD score: r = −0.55, P < 0.008; HE grade: r = −0.50, P < 0.019). The molar ratio of bilirubin to albumin was inversely correlated to ABiC (r = −0.72, P < 0.001), whereas the bilirubin concentration without adjustment for albumin was not significantly correlated.
To evaluate the relationship between the documented parameters and mortality, baseline data were analyzed for survivors and patients who died during the study period. As only in the SMT group was the clinical course not influenced by the elimination of albumin-bound substances, only patients assigned to the SMT group were considered for this subgroup analysis.
The median baseline ABiC of the survivors was 41.7% (range 36.8%-69.0%, n = 5) and significantly higher than that of patients who died during the course of the study (34.2%, range 23.5%-38.1%, n = 5, P < 0.028).
In contrast, no significant differences between survivors and patients who died were detected for liver function (albumin and prothrombin time), severity of liver disease (CHILD and HE grade), accumulated strongly albumin-bound substances (bilirubin and bile acids), or the loading of the albumin molecule (bilirubin/albumin molar ratio).
ABiC was lower than 40% in 13 patients (ECAD: 6 patients; SMT: 7 patients). After the first week, an improvement above this level was observed in 6 survivors, whereas this was observed in only 1 patient who did not survive. In contrast, in the majority of the patients who died, no improvement of ABiC to a value higher than 40% was achieved (Table 1). In this study, significant differences in survival were observed with respect to ABiC values below or above 40% (baseline P = 0.046; after 1 week, P < 0.001).
| ABiC | Nonsurvivor (n) | Survivor (n) | |
|---|---|---|---|
| Baseline | <40% | 6 | 7 |
| >40% | 0 | 9 | |
| After 7 days | <40% | 5 | 1 |
| >40% | 1 | 15 |
- Abbreviation: ABiC, albumin binding capacity.
Improvement was more frequent in the ECAD group (5/6) than in the SMT group (2/7).
During the first week, a significant improvement of ABiC from baseline was observed in the albumin dialysis–treated group (+20.8%) in comparison with the control group (+3.8%).
In parallel, a decrease of plasma levels of strongly albumin-bound endogenous substances such as bilirubin (−10.8 mg/dL) and bile acids (−155.7 μmol/L) was observed in the ECAD group (Figs. 1-3). Even though the last ECAD treatment was performed between study days 3 and 12, the difference between groups was significant also at day 15 (Table 2).
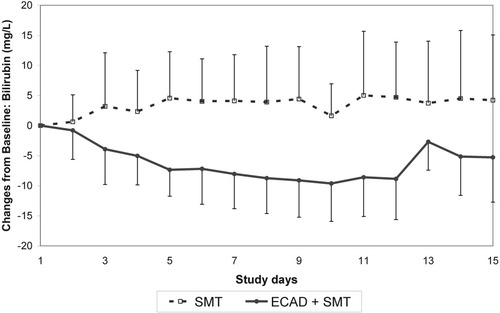
Time course of bilirubin concentrations in the albumin dialysis (ECAD)–treated and control groups (mean ± standard deviation). Abbreviations: ECAD, extracorporeal albumin dialysis; SMT, standard medical therapy.
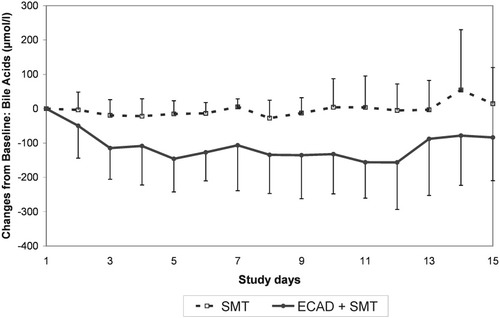
Time course of bile acid concentrations in the albumin dialysis (ECAD)–treated and control groups (mean ± standard deviation). Abbreviations: ECAD, extracorporeal albumin dialysis; SMT, standard medical therapy.
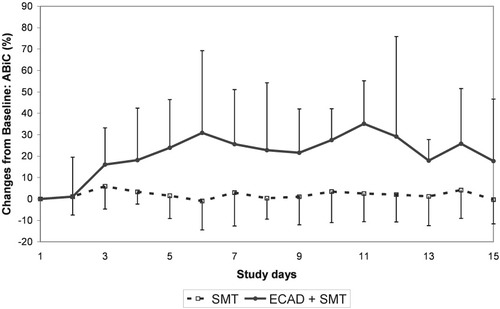
Time course of ABiC in the albumin dialysis (ECAD)–treated and control groups (mean ± standard deviation). Abbreviations: ABiC, albumin binding capacity; ECAD, extracorporeal albumin dialysis; SMT, standard medical therapy.
| Bilirubin (mg/dL) | Bile Acids (μmol/L) | ABiC (%) | |||||
|---|---|---|---|---|---|---|---|
| ECAD + SMT (n = 12) | SMT (n = 10) | ECAD + SMT (n = 12) | SMT (n = 10) | ECAD + SMT (n = 12) | SMT (n = 10) | ||
| After week 1 | Median (min, max) | −10.8 (−16.3, 1.1) | −0.2 (−4.1, 26.3) | −155.7 (−316.0, 20) | −15.5 (−136.0, 50.0) | 20.8 (−21.8, 96.7) | 3.8 (−14.7, 13.9) |
| P value | 0.001 | 0.014 | 0.021 | ||||
| After week 2 | Median (min, max) | −2.7 (−18.2, 3.7) | 3.2 (−6.7, 30.5) | −116.0 (−266.0, 215) | −10.5 (−179.0, 175.0) | 10.7 (16.9, 80.1) | 3.6 (−28.7, 11.6) |
| P value | 0.049 | 0.007 | 0.018 | ||||
| After week 3 | Median (min, max) | 0.6 (−20, 9.3) | 1.2 (9.7, 31.1) | −78.5 (−272.0, 527.0) | − 12 (−179.0, 333.0) | 14.1 (−14.7, 45.2) | 5.6 (−38.5, 40.8) |
| P value | NS | NS | NS | ||||
| After week 4 | Median (min, max) | −−0.1 (−22.0, 9.3) | 1.2 (−13.2, 14.9) | −73.5 (−277.0, 411.0) | −18.0 (−179.0, 349.0) | 5.5 (−21.8, 45.2) | 5.2 (−14.0, 40.5) |
| P value | NS | NS | NS | ||||
- Abbreviations: ABiC, albumin binding capacity; ECAD, extracorporeal albumin dialysis; max, maximum; min, minimum; NS, not significant; SMT, standard medical therapy.
An analysis of the levels before and after each single treatment revealed a significant improvement of ABiC (8.2%) and a reduction of bilirubin (−5.7 mg/dL) and bile acids (−62.8 μmol/L; Table 3 and Fig. 4).
| Bilirubin (mg/dL; n = 85) | Bile Acids (μmol/L; n = 82) | ABiC (%; n = 80) | |
|---|---|---|---|
| Before | 25.8 ± 8.5 | 146,7 ± 100.0 | 69.7 ± 25.1 |
| After | 20.0 ± 6.2 | 80.0 ± 67.2 | 78.5 ± 20.4 |
| P value | <0.001 | <0.001 | <0.01 |
- Abbreviations: ECAD, extracorporeal albumin dialysis; SD, standard deviation.
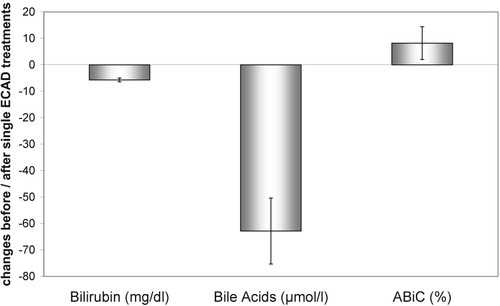
Reduction of albumin-bound substances during single albumin dialysis treatments is associated with an improvement of ABiC (mean ± 95% confidence intervals). Abbreviations: ABiC, albumin binding capacity; ECAD, extracorporeal albumin dialysis.
DISCUSSION
In this study, the binding capacity of albumin binding site II (benzodiazepine/indol binding site) was assessed in 22 patients with severe liver disease treated in a randomized, controlled clinical trial with conventional therapy or additional extracorporeal liver support.
All patients had elevated levels of albumin-bound substances (bilirubin and bile acids) at study enrolment. The binding of the site II–specific fluorescent marker DS and the calculated ABiC were reduced. More severe liver failure was associated with a more reduced ABiC, as documented by the correlation between ABiC and CHILD, HE, or albumin at baseline. Furthermore, binding of the ABiC marker DS was more reduced in patients who died during the study. This relation between reduced ABiC and severity of liver disease has been previously reported for a larger group of patients.13 However, in this study, a significant relation between impaired ABiC and reduced survival is reported for the first time.
Protein binding capacity can be influenced by (1) decreasing concentrations of albumin or (2) simultaneous binding of other interacting drugs or endogenous compounds. In addition to competitive mechanisms, binding can be influenced by electrostatic effects and/or binding-induced conformational changes in the protein.3
In liver failure, reduced albumin concentrations are common and have been identified as an independent prognostic predictor.20, 21 In addition, albumin-bound substances such as bilirubin and bile acids are elevated and are used for the assessment of severity of disease.20-22 Therefore, a higher molar ratio of ligand to albumin can be detected in more severe hepatic insufficiency. If the binding capacity of the albumin molecule is saturated because of the high load in liver failure, displacement of albumin-bound substances and changes in the unbound fraction might occur.
A correlation of the unbound fraction of a drug and the pharmacological activity as well as drug toxicity is generally assumed. For strongly albumin-bound substances, a slight decrease in the protein binding rate would be associated with a pronounced increase in the unbound fraction. A decrease in the protein binding from 98% to 96% doubles the pharmacologically active unbound fraction.23 Monitoring of the unbound fraction rather than the total concentration is in better agreement with the clinical effects and the avoidance of toxic effects and is recommended for drugs with varying protein binding, such as phenytoin.11, 24 Also, the action of hormones such as thyroid hormones is related to the unbound fraction, which can be influenced by drugs bound to the same albumin binding site, such as phenytoin.25
In the present study, no correlation between plasma levels of albumin ligands such as bilirubin and the binding capacity for the ABiC marker has been found at baseline, but the molar ratio of the accumulated substance to the transport protein albumin is correlated to ABiC significantly. Impaired ABiC can be explained by an overload of the available binding sites of the albumin molecule because of an accumulation of albumin-bound endogenous substances, which might be strengthened by a reduced albumin level in chronic liver disease. As for the ABiC test, all samples were adjusted to the same concentration before the ABiC marker was added; the decreased binding of DS indicates an impaired binding function of the albumin molecule itself and not an impaired albumin concentration.
In vitro ABiC can be reduced by an increase in the load of albumin binding site II, and an increase of the unbound fraction of diazepam was found in albumin samples with reduced ABiC.12, 26
It can be hypothesized that not the plasma level of pathogenic substances in liver failure but rather the unbound fraction, which is determined by the available binding capacity of the albumin molecule, correlates to clinical course and prognosis.
Following the assumption that distinguished albumin-bound toxins which accumulate in liver failure are directly responsible for the severity of the disease, we would expect a correlation of plasma levels of those substances and the severity of liver disease and consequently mortality. In this study, no significant differences were observed for prognostic parameters such as bilirubin, prothrombin time, HE grade, albumin, and CHILD between patients who died and those who survived the 30-day period. However, in patients who died during the trial, ABiC was significantly reduced. As for the ABiC test, all samples were adjusted to the same albumin concentration before the ABiC marker was added; the decreased binding of DS indicates an impaired binding function of the albumin molecule itself and not an impaired albumin concentration.
Between the two therapy groups, no differences with respect to albumin-bound substances and ABiC were observed at baseline. As the recommendations for the standard medical care for both groups were the same, the decreases in bilirubin and bile acids as well as a concomitant improvement of the ABiC are effects of the additional extracorporeal therapy in the ECAD group. In addition to the improvement in the ECAD group over time, a decrease in bilirubin and bile acids as well as an improvement of ABiC was observed during single ECAD treatments.
Elimination of bilirubin and other albumin-bound substances during ECAD has been reported by several authors.27-31 However, this is the first report of an improvement of albumin binding function by the elimination of albumin ligands during albumin dialysis.
In the ECAD system, patient's blood and albumin solution are separated by a high-flux membrane. Transport of albumin across this high-flux membranes is only minimal. No significant decrease of albumin-bound bilirubin was found in in vitro experiments with a high-flux membrane, whereas adding albumin to the dialysate was followed by a significant reduction of albumin-bound substances.31 Cumulative albumin loss to the dialysate during 4-hour dialysis treatments with a standard high-flux membrane was found to be less then 1 g.32 A decrease of patient albumin levels from 31.3 ±1.4 to 29.8 ± 1.4 g/L before and after ECAD treatments was reported by Evenepoel et al.,30 although this decrease was not significant. Therefore, an improvement of ABiC by passover of albumin from the dialysate to a patient's blood during ECAD treatments seems to be unlikely.
Although these results should be interpreted with caution as our numbers are small and an additional effect of other covariates, not investigated in this study, cannot be ruled out completely, a potential role of albumin binding function in decompensated liver disease can be hypothesized.
Information on the functional status of the binding site, regardless of which substances might be bound in which quantity, already may be obtained by the ABiC test. Furthermore, ABiC allows a comparison of different plasma samples at the same level because the test is adjusted for the albumin concentration.
In conclusion, ABiC is reduced in patients with an acute deterioration of a chronic liver disease and correlates to the severity of liver failure. The importance of albumin function is highlighted by the significant differences between survivors and nonsurvivors at baseline and by the improved survival in patients with an improvement of ABiC.
ECAD enables the elimination of albumin-bound substances, and an improvement of the ABiC was observed during single treatments and during the treatment period. It can be hypothesized that not the elimination of single albumin-bound substances but the improvement of the albumin binding function may be responsible for the positive effects of ECAD treatments in liver failure.
The ABiC test can be used to characterize the available binding capacity without the necessity of a prior analysis of the ligand already bound to albumin. Thus, this test may provide more information than the plasma concentration of single substances. The ABiC test may further be used to characterize the therapeutic effects of different liver support systems or other therapeutic measures in liver failure and has to be evaluated in appropriate clinical investigations.
Acknowledgements
Special thanks are due to H. Weiss-Reining and S. Vogt for their excellent technical assistance.




