Dichloroacetate stabilizes the intraoperative acid-base balance during liver transplantation†‡
This work was conducted at Oregon Health & Science University and at the Portland Veterans Administration Medical Center.
The contents of this article were presented, in part, at meetings of the International Liver Transplantation Society, Berlin, Germany, July 11-13, 2001, and the International Anesthesia Research Society, San Diego, CA, March 16-20, 2002.
Abstract
Lactic acidosis occurs during orthotopic liver transplantation (OLT), especially during the anhepatic and early postreperfusion phases. Dichloroacetate (DCA) inhibits pyruvate dehydrogenase kinase-1, indirectly activating mitochondrial pyruvate dehydrogenase. This, in turn, markedly reduces systemic lactate production and, to a lesser extent, increases hepatic lactate uptake. The result is moderation of lactic acidosis in many clinical conditions. This study evaluated the efficacy of DCA in controlling lactic acidosis during OLT and improving perioperative outcome from OLT. After informed consent, 250 patients for OLT received either intraoperative DCA or placebo. DCA (40 mg/kg intravenously) or placebo was administered after anesthesia induction and repeated 4 hours later. Intraoperative measures were arterial blood gases, lactate, and Na+ and utilization of blood products, CaCl2, and NaHCO3. Outcome measures were time to tracheal extubation, intensive care unit length of stay, hospital length of stay, requirement for postoperative plasma transfusion, retransplantation, and perioperative mortality. DCA reduced the arterial lactic acid concentration by an average of 44% (1.8 mmol L−1, P < 0.001), stabilized the acid-base balance, and reduced NaHCO3 administration by 80% (P < 0.001). Postoperatively, DCA-treated patients required 50% less postoperative plasma transfusion (2 versus 4 units, respectively, P = 0.016), but the incidence of transfusion was similar in both groups (62% versus 60%, P = 0.381). DCA did not alter time to extubation, intensive care unit length of stay, or hospital length of stay. In conclusion, DCA attenuated lactic acidosis during OLT, stabilizing the intraoperative acid-base balance and decreasing NaHCO3 use. DCA decreased postoperative plasma transfusion requirement but otherwise had no measurable effect on perioperative outcome parameters. Liver Transpl 14:989–998, 2008. © 2008 AASLD.
Orthotopic liver transplantation (OLT) is the definitive treatment for patients with end-stage liver disease. Intraoperative metabolic acidosis is followed by a metabolic alkalosis that begins soon after graft reperfusion and persists for several days. The current standard treatment for severe acidosis during OLT is NaHCO3. NaHCO3 use has been criticized because it fails to increase (and may decrease) intracellular pH, even with alkalization of the plasma1-4; compromises myocardial performance because of induced metabolic disturbances4-6; exacerbates plasma lactic acid accumulation7-9; and (in large doses) can induce hypernatremia, increasing risk for central nervous system demyelination.10-12
Dichloroacetate (DCA) is a treatment for lactic acidosis that, rather than neutralizing serum H+, decreases the plasma lactic acid concentration. DCA, by inhibiting pyruvate dehydrogenase kinase-1, indirectly stimulates mitochondrial pyruvate dehydrogenase in many tissues, including the liver.13-16 It consequently reduces the plasma lactate concentration in patients undergoing liver transplantation,17 as well as those with sepsis,18 after severe burn injury19 and in healthy volunteers.19-22 We recently demonstrated that DCA decreases the plasma lactic acid concentration in both healthy humans and patients with end-stage liver disease by inhibiting its extrahepatic production, rather than increasing its clearance.23 The mechanism involves both increasing pyruvate oxidation to CO2 via the pyruvate dehydrogenase reaction and inhibiting glycolysis.23 In contrast to NaHCO3, DCA promotes intracellular acid-base homeostasis in the heart and liver and preserves myocardial function during metabolic acidosis.1, 13, 24, 25
The purpose of this study was to confirm that prophylactic DCA treatment attenuates metabolic acidosis in patients undergoing OLT. Furthermore, because liver pyruvate dehydrogenase is indirectly activated by DCA, we sought to determine if intraoperative DCA improves perioperative outcome by accelerating recovery of hepatic function. Treatment with DCA accelerates and improves myocardial function after ischemia, both in vitro and in vivo.14, 26-28 Because the magnitude of hyperlactatemia during OLT is reduced in DCA-treated patients and clearance of excess lactate is accompanied by a stoichiometric (1:2) consumption of H+, we sought to determine whether DCA treatment attenuates postoperative metabolic alkalosis and accelerates extubation and discharge from the intensive care unit and the hospital.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAH-B, chronic active hepatitis B; CAH-C, chronic active hepatitis C; DCA, dichloroacetate; EtOH, ethanol; FFP, fresh frozen plasma; INR, international normalized ratio; JI, jejunoileal; OLT, orthotopic liver transplantation; RBC, red blood cells; SD, standard deviation.
PATIENTS AND METHODS
Patients
Our protocol was approved by institutional review boards at Oregon Health & Science University and the Portland Veterans Administration Medical Center as well as the US Food and Drug Administration (R.E.S., IND #35,790). After written informed consent, 250 patients scheduled for OLT at the Portland Veterans Administration Medical Center were randomly assigned to either intraoperative DCA (n = 120) or an equal volume of saline (n = 130). Both the anesthesia and surgical teams caring for the patient were blinded to the treatment group. DCA (40 mg/kg intravenously) or saline was infused over 60 minutes immediately after anesthesia induction and again 4 hours later. DCA infusate was prepared as described previously.17 Severity of liver disease was assessed by the Pugh-Childs scoring system.29
Procedure
All patients fasted for 8-12 hours prior to surgery. Patients were monitored intraoperatively by noninvasive blood pressure cuff, electrocardiogram, pulse oximetry, mixed venous oximetry, exhaled gas analysis, and continuous monitoring by indwelling pulmonary and radial artery catheters. A contralateral radial artery catheter was used for blood sampling. Venovenous bypass was not employed.
Anesthesia was induced with fentanyl (1-3 μg kg−1) and thiopental (2-4 mg kg−1), with succinylcholine (1.5 mg kg−1) used to facilitate intubation. Maintenance anesthesia consisted of fentanyl, isoflurane, and pancuronium as needed. Before skin incision, methylprednisolone (1 g), ampicillin (1 g), and cefotaxime (1 g) were administered intravenously, and antibiotics were readministered at 6-hour intervals. No other immunosuppressive drugs or subsequent methylprednisolone was administered in the operating room.
Graft livers were preserved in ice-cold University of Wisconsin solution (Viaspan, Dupont Pharmaceuticals, Wilmington, DE) and flushed with several liters of heparinized 6% hetastarch (Hespan, Dupont Pharmaceuticals) before warming and anastomosis. Shed blood was replaced via a rapid-infusion system (Haemonetics, Braintree, MA). The rapid-infusion system was used to transfuse banked blood products and salvaged erythrocytes (Cell-Saver, Haemonetics). Blood was transfused through 2 large-bore (7-French) intravenous catheters. Packed erythrocyte transfusion was guided by hematocrit, plasma transfusion was guided by the international normalized ratio (INR) and platelet transfusion was guided by the platelet count. CaCl2 was administered as necessary to maintain stable hemodynamics in the setting of ionized hypocalcemia. A balanced salt solution (Normosol, Dupont Pharmaceuticals) was infused as necessary. Intraoperative metabolic acidosis in both groups was treated according to the clinical judgment of the attending anesthesiologist.
Sampling
Heart rate, central venous pressure, and systemic and pulmonary arterial blood pressures were measured continuously and recorded at 5-minute intervals throughout the procedure. Cardiac output, systemic vascular resistance, and pulmonary vascular resistance were calculated at 30- to 60-minute intervals throughout the operation.
Arterial blood was collected immediately after anesthesia induction (basal), immediately before skin incision, 2 hours and 1 hour before portal vein clamping, at portal vein clamping (hepatectomy), during the anhepatic phase at 30 and 60 minutes; and following portal vein unclamping at 15 minutes, 30 minutes, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 24 hours, and 36 hours. Blood samples for arterial blood pH, PCO2, PO2, base excess, hematocrit, and plasma concentrations of lactic acid and Na+ were collected in heparinized tubes and analyzed immediately. Postoperatively, blood was collected at 6-hour intervals for 48 hours to determine coagulation status (INR and partial thromboplastin time), and plasma concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and bilirubin. All tubes contained heparin, except those for hematology (which contained ethylene diamine tetraacetic acid) or coagulation status (which contained sodium citrate). An INR value of 1.5 or higher triggered plasma transfusion per the intensive care unit postoperative care team's defined critical pathway.
Arterial blood gas (pH, PCO2, PO2, HCO3, and base excess) and chemistry (lactate and electrolytes) were analyzed with a Bayer (East Walpole, MA) RapidLab 865 autoanalyzer. Postoperative liver function tests—AST, ALT, and bilirubin—and electrolytes were measured with a Roche (Indianapolis, IN) Integra 800 autoanalyzer. Coagulation values (INR and partial thromboplastin time) were determined with a Diagnostico Stago (Gennevilliers, France) compact autoanalyzer. Complete blood count, including platelets, was determined with a Beckman Coulter (Miami, FL) LH 750 analyzer.
Sample Size Calculation
The primary outcome variable was plasma lactate concentration. Using the standard deviation for plasma lactate concentration of 2.75 mmol L−1 for both patient groups, as determined in our previous samplings of liver transplant patients,15, 17 and assuming a high correlation of 0.9 for repeated measures within a patient with the lactate concentration measured at 15 different time points for each patient, we determined that 108 patients per group were needed to detect a group difference of 1 mmol L−1 at a significance level of 0.05 with 80% power when a 2-sided test accounting for the correlation among the repeated measures was used. When the correlation was smaller than 0.9, fewer patients per group were needed to detect a 1 mmol L−1difference. Such a sample size has 99% power to detect a difference of 1.5 mmol L−1 between 2 groups, assuming a correlation of 0.9.
Statistical Analysis
Data are expressed as the mean ± standard deviation when there is a normal (nonskewed) distribution, as determined by the Shapiro-Wilk test for normality and Q-Q plot, or as the median and 25th to 75th percentile range for nonnormal data. Variables for intraoperative comparisons were duration of surgery; hemodynamic values; blood products administered (including autologous salvaged and packed erythrocytes, plasma, and platelets); administered crystalloid, CaCl2, and NaHCO3; and serum Na+ concentration. Variables for perioperative comparisons were arterial blood gas data and plasma lactate concentration. Variables for postoperative comparisons were time to tracheal extubation; length of stay in the intensive care unit, in the ward, and in the hospital; peak postoperative values for plasma bilirubin, AST, and ALT concentrations; requirement for plasma transfusion; requirement for prostaglandin E2, acetazolamide, or HCl therapy; incidence of retransplantation at 30 days; and mortality at 7 and 30 days.
Data on plasma concentrations of lactic acid and arterial acid-base variables (pH, PCO2, HCO3, and base excess) were collected at multiple time points for each patient and thus correlated within each patient. We used a mixed effects model to compare difference in plasma lactic acid concentration and arterial acid-base variables between groups, as well as among different time points within groups. In our mixed effects model, group (control versus DCA treatment), time points, and the interaction between group and time points were all treated as fixed effects, while the variable of the unique patient identifier was treated as the random effect. The random effects incorporated the correlation among observations for each patient into the analysis and accounted for the repeated-measure nature of the data. For the 3 fixed effects, a post hoc test for multiple comparisons between groups or time points was performed with Tukey's adjusted P value when overall differences were at P < 0.05.
Single mean values were compared between the 2 groups by an independent t test for normally distributed data and by the Mann-Whitney U test for nonnormally distributed data. Binary and other discrete data were analyzed with Pearson's chi-square test, or Fisher's exact test was substituted when the cell size was small. The mixed effect model was fit with PROC MIXED from SAS Institute, Inc. (Cary, NC), and other statistical tests were performed with a specialized software program (Crunch 4, Crunch Software, formerly of Oakland, CA). Differences were considered statistically significant at P < 0.05, and all tests were 2-sided.
RESULTS
Table 1 shows that the 2 groups did not differ in age, gender, or severity of underlying liver disease. The most common etiology of liver disease in both groups was chronic hepatitis C infection concurrent with ethanol-induced cirrhosis. Hepatitis C virus and ethanol-induced cirrhosis either alone or in combination accounted for over half of all patients in both the control and DCA groups.
| Control (n = 130) | (+) DCA (n = 120) | Difference (P) | |
|---|---|---|---|
| Age (years) | 47 ± 9 | 49 ± 9 | 0.26 |
| Male (%) | 72 | 72 | 0.46 |
| Pugh-Childs score | 10 (9–12) | 10 (8–12) | 0.69 |
| Etiology of liver disease | 0.51 | ||
| CAH-C + EtOH cirrhosis | 38 | 33 | |
| EtOH cirrhosis | 19 | 20 | |
| CAH-C cirrhosis | 23 | 17 | |
| 1° sclerosing cholangitis | 9 | 11 | |
| Cryptogenic cirrhosis | 9 | 11 | |
| 1° biliary cirrhosis | 7 | 10 | |
| Fulminant hepatic failure | 7 | 5 | |
| CAH-B cirrhosis | 3 | 4 | |
| Autoimmune cirrhosis | 5 | 3 | |
| Alpha-1 antitrypsin deficiency | 3 | 2 | |
| Non-EtOH steatosis | 0 | 2 | |
| JI bypass–induced failure | 3 | 1 | |
| 2° sclerosing cholangitis | 1 | 1 | |
| Alagille syndrome | 1 | 0 | |
| Klatsin tumor | 1 | 0 | |
| Congenital hepatic fibrosis | 1 | 0 |
- NOTE: Age is presented as mean ± SD and compared by the Student t test. Pugh-Childs score is presented as median (25th to 75th percentile range) and compared by the Mann-Whitney U test. Gender distribution and etiology of liver disease were compared by Pearson's chi-square test. There were no differences between groups.
- Abbreviations: CAH-B, chronic active hepatitis B; CAH-C, chronic active hepatitis C; DCA, dichloroacetate; EtOH, ethanol; JI, jejunoileal; SD, standard deviation.
Durations of cold, warm, and total graft ischemia did not differ between groups (Table 2). Table 2 shows that surgical times for the dissection, anhepatic, and reperfusion stages did not differ between groups.
| Control (n = 130) | (+) DCA (n = 120) | Difference (P) | |
|---|---|---|---|
| Graft ischemia | |||
| Cold | 9.2 (6.9–11.5) | 9.0 (7.0–11.5) | 0.77 |
| Warm | 0.88 (0.80–1.00) | 0.90 (0.79–1.00) | 0.66 |
| Total | 10.2 (7.9–12.4) | 9.9 (8.0–12.4) | 0.78 |
| Surgery | |||
| Period I* | 3.1 (2.4–3.8) | 2.9 (2.3–3.4) | 0.07 |
| Period II† | 1.1 (0.9–1.2) | 1.1 (1.0–1.2) | 0.45 |
| Period III‡ | 4.8 (3.8–5.6) | 4.5 (3.6–5.6) | 0.49 |
| Total | 9.1 (7.6–10.7) | 8.5 (7.2–10.3) | 0.12 |
- NOTE: Data are expressed as median (25th to 75th percentile range). All values were compared by the Mann-Whitney U test. There were no differences between groups.
- Abbreviation: DCA, dichloroacetate.
- * Hepatectomy (skin incision to portal vein clamping).
- † Anhepatic stage.
- ‡ Reperfusion (portal vein unclamping to skin closure).
Basal plasma lactate concentration in the control and DCA groups did not differ (Fig. 1). In controls, plasma lactate concentration did not change during the 80 minutes between anesthesia induction and surgical incision. A progressive increase in plasma lactate concentration occurred during the dissection and anhepatic stages; it reached a peak at 30 minutes after graft reperfusion. Plasma lactate concentration remained increased during the next 6 hours and decreased to near the baseline value at 24 hours after reperfusion. In DCA-treated patients, plasma lactate concentration trended toward a decrease from baseline at surgical incision, although the decrease was not statistically significant (P = 0.63). Skin incision occurred about 20 minutes after completion of the first DCA infusion. DCA-treated patients exhibited a progressive intraoperative increase in plasma lactate concentration; it reached a maximum at 15 minutes after graft reperfusion and returned to the baseline value at 24 hours. When DCA-treated patients were compared to controls, they showed an overall 44% (1.8 mmol L−1) decrease in plasma lactate concentration during the 7-hour intraoperative period (P < 0.0001), which was also present at different time points (P < 0.0001; Fig. 1). Interaction between the intervention group and time points was significant (P < 0.0001), indicating that the change in mean plasma lactate concentration with time was different for the control and DCA groups. The increment in plasma lactate concentration during the anhepatic stage was 1.1 mmol L−1 in controls (P < 0.001) and only 0.5 mmol L−1 in DCA-treated patients (P = 0.43). The difference between groups in lactate concentration at the end of the anhepatic period (60 minutes) was 2.1 ± 0.2 mmol L−1 (P < 0.0001), and the maximum difference between the 2 groups occurred 2 hours after reperfusion (2.9 ± 0.2 mmol L−1, P < 0.0001). At 24 hours after portal reperfusion, the difference in plasma lactate concentration between the DCA-treated and control groups was 0.2 ±0.3 mmol L−1, which was both statistically and clinically insignificant.
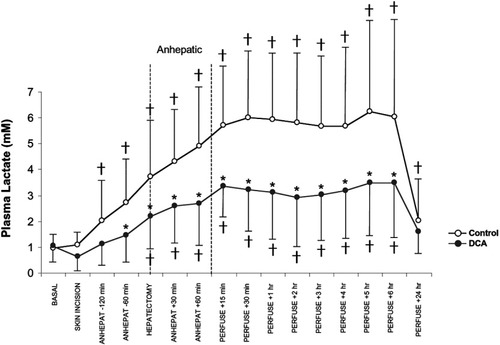
Effect of DCA on arterial plasma lactate during and after orthotopic liver transplantation. Controls (n = 130) are shown by open circles, and DCA-treated patients (n = 120) are shown by closed circles. The first DCA infusion was completed before skin incision in all patients, and the second DCA infusion was completed by the early anhepatic phase. Differences between controls and DCA-treated patients (P < 0.05) are noted by asterisks. Differences from baseline within groups (P < 0.05) are noted by crosses. Abbreviation: DCA, dichloroacetate.
Changes in arterial acid-base parameters with time for the control and DCA-treated patients are shown in Fig. 2. For arterial pH, HCO3, and base excess, both the 2 main effects (intervention group and time points) and the interaction between intervention groups and time points were significant (P < 0.001). For PCO2, the main effect (time points) and the interaction between intervention groups and time points were significant (P < 0.0001), but there was no overall significant difference between the DCA-treated and control groups (P = 0.97). Basal arterial acid-base parameters did not differ between groups. Both groups exhibited a progressive metabolic acidosis throughout the dissection and anhepatic periods, which reached a nadir shortly after graft reperfusion and paralleled the plasma accumulation of lactic acid (Fig. 1). Within 1 hour after graft reperfusion, there was an alkalizing trend, despite a persistently high plasma lactate concentration, which continued postoperatively. In controls, arterial pH became acidemic at skin incision, worsened during the dissection and anhepatic stages, reached a nadir at 15 minutes after graft reperfusion, and remained acidemic for 1 hour after portal reperfusion (Fig. 2A). Arterial pH returned to baseline by 2 hours after reperfusion and remained unchanged from baseline through 24 and 36 hours. In DCA-treated patients, arterial pH became acidemic versus baseline later during the dissection stage, worsened during the anhepatic stage, reached a nadir at 15 minutes after reperfusion, and recovered to the baseline value by 2 hours after reperfusion. Arterial pH became slightly above baseline at 7 hours but was close to baseline at 24 and 36 hours. When control and DCA-treated groups were compared, arterial pH in DCA-treated patients was higher than that in controls at 15 minutes, 30 minutes, and 1 hour after reperfusion (P < 0.001), after which there was no difference between groups. DCA-treated patients exhibited a decrease of 0.044 pH units between 60 minutes anhepatic and 15 minutes after portal vein unclamping, which was less pronounced than the corresponding 0.067 unit decrease in controls (P < 0.0001).
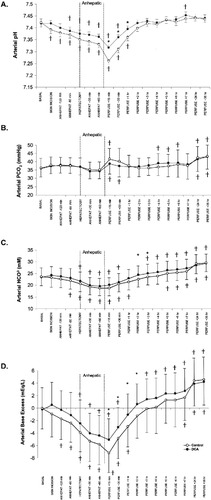
Effect of DCA on arterial blood gas data during and after orthotopic liver transplantation: (A) pH, (B) PCO2, (C) [HCO3], and (D) base excess. Controls (n = 130) are shown by open circles, and DCA-treated patients (n = 120) are shown by closed circles. The first DCA infusion was completed before skin incision in all patients, and the second DCA infusion was completed by the early anhepatic phase. Differences between controls and DCA-treated patients (P < 0.05) are noted by asterisks. Differences from baseline within groups (P < 0.05) are noted by crosses. Abbreviation: DCA, dichloroacetate.
Arterial PCO2 (Fig. 2B) in both patient groups trended toward a decrease below baseline during the anhepatic stage, although the decrease did not reach statistical significance, and then it increased above baseline from graft reperfusion to the postoperative period at 24 and 36 hours after reperfusion. The 2 groups exhibited similar PCO2 values throughout the study, and there was no significant difference in PCO2 between the 2 groups at any time.
Arterial HCO3 concentration (Fig. 2C) of both patient groups decreased from baseline during the anhepatic stage (P < 0.0001) and reached a nadir at 15 minutes post-reperfusion. Later, arterial HCO3 concentration began to increase after reperfusion and rose above baseline in both groups 5 hours after reperfusion. In controls, HCO3 was lower than baseline from 60 minutes before the anhepatic stage through 1 hour after portal reperfusion, with a maximum decrease of 4.8 mmol L−1 from baseline at 15 minutes after reperfusion (P < 0.0001). From 5 to 36 hours after reperfusion, arterial HCO3 was higher than the baseline value, and the concentration reached a maximum of 29.4 ±3.7 mmol L−1 at 36 hours after reperfusion, an increase of 5.9 mmol L−1 (P < 0.0001). In DCA-treated patients, arterial HCO3 fell below baseline at hepatic clamping and recovered to baseline by 60 minutes after reperfusion. The maximum HCO3 decrease from baseline, which occurred at 60 minutes into the anhepatic phase, was 3.8 mmol L−1 (P < 0.001). From 3 to 36 hours after reperfusion, arterial HCO3 in DCA-treated patients was increased above baseline, with a maximal concentration of 29.2 ±3.9 mmol L−1 at 36 hours, an increase of 5.8 mmol L−1 (P < 0.0001). Compared to controls, DCA-treated patients exhibited higher arterial HCO3 concentration at 2 and 3 hours after reperfusion with a difference of 1.8 mmol (P = 0.004) and 1.6 mmol L−1 (P = 0.04), respectively.
Figure 2D shows the change of base excess with time. Both groups exhibited a progressive base deficit through the dissection and anhepatic phases, which reached a nadir at 15 minutes after reperfusion, with a net decrease of 7.2 mEq L−1 in controls (P < 0.0001) and 4.9 mEq L−1 in DCA-treated patients (P < 0.0001). All patients subsequently accumulated base equivalents to yield a neutral base content by 5 hours after reperfusion in controls and by 2 hours in the DCA group. Both groups exhibited a maximal and similar base excess at 36 hours after reperfusion. In comparison with controls, DCA attenuated the arterial base deficit early after portal reperfusion (15 minutes through 2 hours). Subsequently, from 3 to 36 hours after reperfusion, there was no difference in arterial base excess between groups. The overall time-averaged increase in base excess for the DCA-treated group was 1.2 mEqL−1 (P = 0.0001). To summarize Table 2, DCA caused more stable intraoperative acid-base balance by attenuating the development of metabolic acidosis without altering the acid-base balance in the postoperative setting.
The 2 groups had similar replacements of packed erythrocytes, plasma, platelets, salvaged erythrocytes, crystalloid, and CaCl2 (Table 3). In contrast, NaHCO3 use in DCA-treated patients was only 20% of that for controls. DCA-treated patients exhibited better acid-base balance, which was accomplished with one-fifth the NaHCO3 dose of controls. Only 30 of 120 DCA patients (25%) received any NaHCO3 versus 82 of 130 controls (63%, P < 0.001). Both groups exhibited an increase in serum Na+ concentration through surgery (P < 0.001), although neither the initial nor final intraoperative serum Na+ concentrations of DCA-treated patients (134 ± 5 and 140 ± 5 mEqL−1, respectively) differed from corresponding values in controls (135 ± 5 and 140 ± 6 mEqL−1, with P = 0.75 and P = 0.17, respectively). The incidence of hypernatremia (Na > 145 mEqL−1) in the DCA group (13/120 = 11%) did not differ from that in controls (20/130 = 15%, P = 0.29).
| Control (n = 130) | (+) DCA (n = 120) | Difference (P) | |
|---|---|---|---|
| Packed RBC (units) | 11 (7.5–16.5) | 14 (8–16) | 0.79 |
| FFP (units) | 25 (18–35) | 28 (18–45) | 0.28 |
| Platelets (units) | 30 (10–50) | 30 (20–50) | 0.16 |
| Salvaged RBC (L) | 2.2 (0.8–3.6) | 2.5 (0.8–4.6) | 0.37 |
| Crystalloid (L) | 5.0 (4.0–7.0) | 4.7 (3.5–6.6) | 0.50 |
| CaCl2 (g) | 5 (3–7) | 5 (3–9) | 0.63 |
| NaHCO3 (mEq) | 100 (0–200) | 0* (0–200) | 0.001 |
- NOTE: Data are expressed as median (25th to 75th percentile range). Values were compared by the Mann-Whitney U test.
- Abbreviations: DCA, dichloroacetate; FFP, fresh frozen plasma; RBC, red blood cells (packed erythrocytes, citrate phosphate dextrose–preserved).
- * Difference from control value.
Patients in both groups exhibited a hyperdynamic circulation, with low vascular resistance and high cardiac index. Cardiac index was reduced during the anhepatic stage and returned to baseline after graft reperfusion for the remainder of the operation. There was no difference between controls and DCA-treated subjects in heart rate, central venous pressure, mean arterial pressure, pulmonary artery pressures, cardiac index, or vascular resistance at any time.
Table 4 shows that 60% of patients in both groups required postoperative plasma transfusion triggered by abnormal coagulation times, although the transfusion requirement of DCA-treated patients who received plasma was half of that of controls (2 versus 4 units, respectively). There was no difference in peak liver function tests (serum bilirubin, AST, or ALT) between DCA-treated and control patients. Furthermore, the 11% incidence of prostaglandin E2 therapy for poor postoperative hepatic function in DCA-treated patients did not differ from the 15% incidence in the control group. Although intraoperative acid-base status differed between groups, postoperative acid-base status was similar, with no difference in incidence of receiving either acetazolamide (14% for DCA-treated, 13% for controls) or HCl infusion (6% for DCA-treated, 8% for controls). DCA did not alter the time from surgical intensive care unit arrival to tracheal extubation or lengths of stay in the surgical intensive care unit, ward, or total hospital (Table 5). The incidence of retransplantation and mortality at 1 week and 1 month did not differ between groups (Table 5).
| Control (n = 130) | (+) DCA (n = 120) | Difference (P) | |
|---|---|---|---|
| Peak serum total [bilirubin] (mg/dL) | 6.7 (4.1–12.1) | 7.0 (4.9–8.5) | 0.70 |
| Peak serum [AST] (U/L) | 842 (539–1893) | 924 (473–1458) | 0.36 |
| Peak serum [ALT] (U/L) | 472 (282–1155) | 537 (285–1060) | 0.98 |
| FFP transfusion (% incidence) | 60 | 62 | 0.38 |
| Quantity of FFP transfused (units) | 4 (2–7) | 2* (1–4) | 0.016 |
- NOTE: Data, except for the incidence of FFP transfusion, are expressed as median (25th to 75th percentile range). Bilirubin, ALT, AST, and quantity of FFP transfused were compared by the Mann-Whitney standardized U test. Incidence of FFP transfusion was compared by Pearson's chi-square test.
- Abbreviations: ALT, alanine aminotransferase (serum glutamate pyruvate transaminase); AST, aspartate aminotransferase (serum glutamic oxaloacetic transaminase); DCA, dichloroacetate; FFP, fresh frozen plasma.
- * Difference from control value.
| Control (n = 130) | (+) DCA (n = 120) | Difference (P) | |
|---|---|---|---|
| Time to extubation (hours after SICU admittance) | 29.2 (23.2–48.0) | 26.8 (22.1–46.7) | 0.51 |
| SICU length of stay (days) | 3 (2–5) | 3 (2–4) | 0.32 |
| Ward length of stay (days) | 12 (8–21) | 13 (10–21) | 0.06 |
| Hospital length of stay (days) | 15 (11–26) | 15 (12–27) | 0.32 |
| Mortality at 1 week (incidence) | 2 (1.5%) | 5 (4.2%) | 0.27 |
| Mortality at 30 days (incidence) | 8 (6.2%) | 10 (8.3%) | 0.51 |
| Retransplant at 30 days (incidence) | 1 (0.8%) | 2 (1.7%) | 0.61 |
- NOTE: Time data are expressed as median (25th to 75th percentile range) and compared by the Mann-Whitney U test. Incidences of mortality at 1 week and retransplantation (rare events) were compared by Fisher's exact test. Incidences of mortality at 30 days were compared by Pearson's chi-square test. There were no differences between groups.
- Abbreviations: DCA, dichloroacetate; SICU, surgical intensive care unit.
DISCUSSION
This study confirms previous reports of biphasic intraoperative lactic acidemia and subsequent metabolic alkalosis in patients undergoing OLT.17, 30-32 It confirms our previous finding that DCA attenuates accumulation of lactic acid, stabilizes acid-base equilibrium, and reduces use of NaHCO3 during OLT.17
Healthy awake humans turn over about 14 μmol of lactate per kilogram per minute, a value not appreciably changed in the presence of end-stage liver disease.23 The liver is the principal organ clearing lactic acid from the circulation, accounting for 40%-50% of whole-body clearance under basal conditions.7 Kidneys are responsible for clearing another 30%, and 20% is removed by peripheral tissues.7, 8, 33 Lactic acid taken up by the liver can either be metabolized through the Krebs cycle or be utilized for gluconeogenesis.34-37 By either route, lactate metabolism consumes 2 free protons. A complete round of the Cori cycle is proton-neutral because the 2 H+ ions produced during the formation of lactate from glucose are removed during the synthesis of glucose from lactate.
Four mechanisms underlie the development of lactic acidosis during OLT. First, peripheral tissues in patients with cirrhosis produce excessive lactic acid in response to a glucose challenge during, for example, a blood transfusion.38 Second, clearance of a lactate challenge is prolonged in patients with cirrhosis because of impaired reserve capacity of the liver.39, 40 Third, transfusion of erythrocytes during OLT is an exogenous source of lactic acid.17, 41 Fourth, surgical manipulation may cause visceral ischemia, concomitantly increasing endogenous lactic acid production and compromising its hepatic and renal uptake. Lactic acid accumulation is most pronounced during the anhepatic stage. It is accompanied by the molar equivalent of 2 H+, causing metabolic acidosis. Severe metabolic acidosis impairs not only coagulation42 but also myocardial contractility and vascular tone, which together can induce hypotension.4, 43-45
The 44% intraoperative decrease in plasma lactic acid concentration after DCA evident during OLT in the present study is consistent with our previous smaller series.17, 46, 47 Unlike our previous studies, in which a strict algorithm dictated NaHCO3 use, we left therapy in the present study to the discretion of the individual anesthesiologist. Control patients in the present study received less NaHCO3 than those in our earlier studies. Nevertheless, a difference in NaHCO3 use between DCA-treated and control patients persisted, and the ability of DCA to stabilize the intraoperative acid-base balance became even more apparent than it was previously.17 Less NaHCO3 use in controls in the present study, coupled with the equalizing effect of transfused plasma containing a higher sodium concentration than that in our patients' plasma, may have prevented a reproduction of the lower incidence of intraoperative hypernatremia in DCA-treated patients previously reported.17
We have shown that DCA does not cause any difference in intraoperative hemodynamic values (mean arterial pressure, heart rate, cardiac index, and systemic or pulmonary vascular resistance) between groups at any intraoperative time.17, 47 We similarly did not find any hemodynamic differences between groups in the present study.
We anticipated that DCA would decrease the severity of postoperative metabolic alkalosis because it decreased intraoperative plasma lactate concentration by 44%, whereas the early postoperative values were indistinguishable. Our observed accelerated lactate clearance from plasma, whether followed by lactate oxidation or gluconeogenesis, consumed 2 H+ Eq/mol. Intravenous lactate challenge increases both arterial pH and HCO3 concentration in humans.8 DCA, however, failed to attenuate the severity of postoperative metabolic alkalosis in our patients. It has been suggested that the citrate load secondary to plasma transfusion could underlie postoperative metabolic alkalosis.48 Metabolic clearance of citrate is associated with the effective molar consumption of 3 H+ Eq. The time course, however, of elevated plasma citrate concentration, like that of lactate, does not match the time course for postoperative metabolic alkalosis. Our peak metabolic alkalosis occurs on the second postoperative day, whereas the plasma citrate concentration, like the plasma lactate concentration, returns to near the baseline value by postoperative day 1.49 We examined urea synthesis in a subset of patients to determine whether an occult decrease in urea synthesis, an HCO3-consuming process, contributes to postoperative metabolic alkalosis.50 The observed urea synthesis rate, however, was stimulated almost 10-fold at 48 hours postoperatively, perhaps driven by metabolic alkalosis but certainly not responsible for it. It is possible that the metabolic alkalosis in our patients is due to the infusion of dopamine and furosemide that has been in our postoperative regimen to return patients to preoperative body weight.
We hypothesized that the stabilizing effect of DCA on the intraoperative acid-base balance and a potential protective effect on graft ischemia-reperfusion injury would speed extubation time, reduce hepatic injury–induced enzyme leakage, and shorten intensive care unit stay, with no effect on longer term ward stay. Our data show that, in terms of liver function, the only sign of improved outcome was a decreased plasma transfusion requirement, whereas the hepatic injury indexed by enzyme leakage did not differ. DCA had no apparent effect on the measured outcome parameters. Our study was limited, however, in that it did not possess adequate power to detect a statistical difference in many outcome measures. For instance, to detect a 1-day difference in length of stay with 80% power at P < 0.05 would have required enrolling 210 patients per group. With our population size, we would have needed a length of stay difference of 1.4 days between groups. Our mortality data trended toward a worsened outcome in DCA-treated patients at both 1 week and 30 days postoperatively. To demonstrate a statistically significant difference, however, with 80% power at P < 0.05 would have required a study of 596 patients per group for mortality at 1 week, 2393 patients per group for mortality at 30 days, or 2392 patients per group for retransplantation. On the side potentially favorable to DCA use, we would have needed 884 patients per group to validate the 4% difference in the incidence of hypernatremia between groups and 2100 patients per group to validate the difference of 2.4 hours in time to tracheal extubation. It is debatable whether any of these comparisons would achieve clinical significance, given the data set that we obtained. Our values for hospital lengths of stay and time to extubation were indistinguishable. A larger study of the effect of DCA on outcome from liver transplantation does not appear to be warranted. In conclusion, DCA markedly attenuates the magnitude of lactic acidosis during OLT, stabilizing the intraoperative acid-base balance and decreasing NaHCO3 use. DCA simplified intraoperative OLT anesthetic management. With the exception of a decrease in postoperative plasma transfusion, however, DCA did not alter either liver function or other outcome variables following OLT.
Acknowledgements
The authors are indebted to Drs. John Rabkin, Henry Casson, Grace Chien, John Cooney, Daniel Curtis, Richard Davis, John Evans, and Steven Fiamengo for their assistance in enrolling patients for the study. Thanks are due to John Hromco, Scott Paffenroth, Roberta Ruimy, Sue Gann, and Jeremy Graham for their help in collating and analyzing the data. Thanks are also due to TCI America (Portland, OR) for its generous provision of raw DCA.




