Clinical characteristics of thrombotic microangiopathy following ABO incompatible living donor liver transplantation
Abstract
Thrombotic microangiopathy (TMA) may develop after living donor liver transplantation (LDLT), but the mechanism is not fully understood. We retrospectively analyzed all patients undergoing LDLT at our center, including TMA patients, to elucidate the clinical characteristics and presentation and to determine which patients have a higher risk of occurrence of TMA. In all, 57 adult patients were reviewed after LDLT at our institution. TMA was diagnosed by sudden and severe thrombocytopenia, followed by hemolytic anemia with fractionated erythrocytes in the blood smear. Clinical features were compared between the TMA group and the non-TMA group. Of the 57 patients, 4 were diagnosed with posttransplantation TMA. ABO blood group (ABO)-incompatibility, cyclophosphamide (CPA), and recipient blood group (type O) were closely correlated with the occurrence of TMA. Thrombocytopenia appeared 1 to 5 days before hemolytic anemia. Coagulative function markers stayed at the same level after TMA, while marked elevation was shown in fibrinolytic function markers such as plasminogen activator inhibitor type 1 (PAI-1). TMA occurred at a higher prevalence in ABO-incompatible graft recipients. Additional factors associated with ABO-incompatible transplantation, such as an overdose of immunosuppressants, may affect the likelihood of TMA. Sudden and severe thrombocytopenia presented before hemolytic anemia and the serum levels of PAI-1 correlated well with the clinical course of TMA. In conclusion, early recognition of thrombocytopenia and elevation of PAI-1 is crucial to diagnose TMA especially in ABO-incompatible LDLT. Liver Transpl 13:1455–1462, 2007. © 2007 AASLD.
Thrombotic microangiopathy (TMA) is a microvascular occlusive disorder induced by endothelial damage and primary platelet aggregation.1, 2 This complication is a well-recognized disorder that may occur in up to 6% of patients following bone marrow transplantation.3 TMA stands out as an infrequent but severe life-threatening complication, often requiring intense therapy.2 According to previous reports, TMA develops after solid-organ transplantation with an incidence of 0.5 to 3%.4, 5 This complication is most prevalent in kidney transplant recipients, but it has also been reported in liver transplant recipients.6, 7 Possible causative factors of TMA following liver transplantation include calcineurin inhibitors (CNI)8, 9 and infections,10 including with hepatitis C virus.11 However, the specific pathophysiological mechanism of TMA is not yet fully understood. TMA occurs more commonly in cadaveric transplants, which suggests that prolonged cold ischemia time and reperfusion injury may cause endothelial injury.12, 13 However, TMA also can occur in living donor liver transplantation (LDLT) and, to date, there have been sporadic reports of TMA in LDLT recipients.11, 14, 15
A total of 106 patients underwent LDLT for various terminal liver diseases at Keio University Hospital (Tokyo, Japan). Of the 106 patients, 4 (3.7%) presented with TMA after LDLT in our institution. Of these, 2 patients recovered from this difficult situation, while the other 2 patients had a fatal outcome.
In this article, we describe our experience of TMA in adult LDLT. From its clinical behavior and the patient clinical characteristics, we have attempted to elucidate the risk factors and initial clinical signs to be able to predict this lethal complication beforehand and to initiate appropriate therapy promptly.
Abbreviations
TMA, thrombotic microangiopathy; LDLT, living donor liver transplantation; ABO, ABO blood group; PAI-1, plasminogen activator inhibitor type 1; CNI, calcineurin inhibitors; CPA, cyclophosphamide; PE, plasma exchange; vWF, Von Willebrand factor; IV, intravenous; IgM, immunoglobulin M; IgG, immunoglobulin G; DNA, deoxyribonucleic acid; Ag, antigen.
PATIENTS AND METHODS
Patients
Between April 1995 and November 2005, a total of 106 patients underwent LDLT for various terminal liver diseases at Keio University Hospital. Of the 106 patients, 49 were pediatric and 57 were adult recipients. A total of 9 adults received ABO blood group (ABO)-incompatible grafts. The clinical records of the recipients were reviewed and revealed 4 patients who with presented TMA, all of whom had received ABO-incompatible grafts. These patients were studied in detail. All patients with TMA had undergone adult-to-adult LDLT. Living donors were all relatives and selected after considering their age, blood type, graft size, and liver function, and confirming their wish to volunteer. Surgical techniques and use of immunosuppression for LDLT were carried out carefully according to the standard methods described elsewhere.16-18 A new therapeutic strategy for ABO-incompatible liver transplantation using intraportal administration of methyl-prednisolone, gabexate mesilate (FOY, protease inhibitor; Ono Pharmaceutical, Tokyo, Japan), and prostaglandin E1 has been introduced since November 1998 in our institute and all the ABO-incompatible recipients received this novel regimen.19 This protocol includes bolus intravenous methyl-prednisolone for the first 7 days posttransplantation (1 gm intraoperatively, 2 mg/kg/day on days 1-3, 1 mg/kg/day on days 4–6, and 0.5 mg/kg/day on day 7), which was replaced with 0.5 mg/kg/day of oral prednisolone from day 8. The target trough level of tacrolimus was 20–25 ng/mL on days 0–7, 15–20 ng/mL on days 8–21, and 5–10 ng/mL thereafter. Cyclophosphamide (CPA) (50–100 mg/day) or mizoribine (200 mg/day) was administered from a few days before transplantation, until day 21 posttransplantation, and then switched to 50 mg/day of oral azathioprine. In addition to the systemic antirejection therapy, we inserted an intraportal catheter during transplantation, and performed continuous intraportal infusion of methyl-prednisolone (125 mg/day on days 0–7, 50 mg/day on days 8–14, then tapered and discontinued on day 21), prostaglandin E1 (0.01–0.02 μg/kg/minute on days 0–14), and gabexate mesilate (1,000 mg/day on days 0–14). Serial monitoring of the antihemagglutinin titers was performed from 7 days before transplantation and plasma exchange (PE) was performed preoperatively until the levels of immunoglobulin M and immunoglobulin G were decreased at least below 1:16. The antihemagglutinin titer monitoring was continued postoperatively for 3 more weeks and PE was performed appropriately to keep the levels of immunoglobulin M and immunoglobulin G below 1:32.
Diagnosis and Treatment of TMA
The criteria for TMA were as defined previously2, 11, 20 and included: thrombocytopenia and microangiopathic hemolytic anemia with no clinically apparent alternative explanations. More specifically, the presence of thrombocytopenia (platelet count <5.0 × 104/mm3) or a progressive decline in platelet counts (decrease of >3.0 × 104/mm3 within 24 hours) and microangiopathic hemolytic anemia (hemoglobin <8.0 gm/dL), suggested by sharply elevated serum lactate dehydrogenase levels (typically >500 IU/L) and the presence of fractionated erythrocytes in the blood smear, were considered sufficient to establish a clinical diagnosis of TMA. Differential diagnosis between disseminated intravascular coagulation was made on the basis of the lack of laboratory findings suggestive of the consumption of clotting factors with normal or near-normal international normalized ratio of prothrombin time (<1.5). For patients who received ABO-incompatible grafts, serum immunoglobulin G measurement and direct Coomb's tests were performed routinely to differentiate TMA from graft-vs.-host–related hemolysis.
The severity of TMA in each case was evaluated according to the grading system (grade 0-4) advocated by Zeigler et al.21 based on the lactate dehydrogenase level and percentage of fragmented red blood cells. Plasma infusion was performed for patients with grade 1 and PE was initiated immediately for patients with grade 2 or higher. CNI modification was performed as a part of treatment, as described elsewhere.4, 7, 8 PE with fresh frozen plasma (0.08 L/kg) was continued on a daily basis until steady recovery was indicated by a stable decrease in fractionated red blood cells and serum lactate dehydrogenase levels. An analog of prostacyclin, prostaglandin I2 (iloprost) (50 μg/day over 8 hours) or antithrombin III (1,500 units per day) was administered concurrently as a part of treatment.
Measurement of Hemostatic Parameters
Routine blood coagulation tests; namely, activated partial thromboplastin time, prothrombin time, fibrinogen assay, antithrombin, and platelet counts were performed in all patients. In addition, for Patient 4, other specific hemostatic parameters also were evaluated. Von Willebrand factor (vWF) was measured by an enzyme-linked immunosorbent assay; values were expressed as percentages. Plasminogen activator inhibitor type 1 (PAI-1) was determined by a commercially available enzyme-linked immunosorbent assay; values were expressed as nanograms per milliliter (ng/mL). These hemostatic parameters were only investigated for Patient 4 retrospectively after the diagnosis of TMA, measuring the serum preserved at the clinical laboratory. Reference intervals were determined according to the guidelines described in the National Committee for Clinical Laboratory Standards (document C28-P).22 A total of 100 healthy Japanese subjects (50 women and 50 men; aged 20 to 57 yr, mean, 37 yr) were used as controls.
RESULTS
Patient Profile
A total of 57 patients who underwent adult-to-adult LDLT were divided in 2 groups: patients with TMA (n = 4) and patients without TMA (n = 53). Patient clinical characteristics of both groups are listed in Table 1. All the TMA cases received ABO-incompatible grafts. The recipient blood type was type O in all the TMA cases. FK506 was preferentially used in the TMA cases and CPA was the highly prevalent additional immunosuppressive agent in the TMA cases. Serum levels of immunoglobulin G were kept low and direct Coomb's tests were negative in all the TMA patients.
| Patients with TMA (n = 4) | Patients without TMA (n = 53) | |
|---|---|---|
| Mean age | ||
| Recipients (yr) | 38.8 (range 23–48) | 44.3 (range 21–64) |
| Donors (yr) | 48.5 (range 45–53) | 40.7 (range 19–65) |
| Gender | ||
| Males | 2 | 30 |
| Females | 2 | 23 |
| Primary liver disease | ||
| Cholestasis | 1 | 18 |
| Hepatitis/cirrhosis/HCC | 2 | 21 |
| Metabolic | 0 | 3 |
| Fulminant hepatic failure | 1 | 9 |
| Others | 0 | 2 |
| Mean HLA mismatches | 3.00 (range 2–4) | 3.36 (range 2–5) |
| ABO blood group compatibility | ||
| Identical | 0 | 33 |
| Compatible | 0 | 15 |
| Incompatible | 4 | 5 |
| Recipient blood type | ||
| Type O | 4 | 21 |
| Non-O | 0 | 32 |
| CNI | ||
| FK506 | 4 | 31 |
| Cyclosporin | 0 | 22 |
| Additional immunosuppressive agents | ||
| CPA | 3 | 7 |
| Non-CPA | 1 | 46* |
- Abbreviations: HCC, hepatocellular carcinoma; HLA, human leukocyte antigen; MMF, mycophenolate mofetil; CNI, calcineurin inhibitor.
- * Non-CPA includes: mizoribine, 18 patients; azathioprine, 10 patients; MMF, 5 patients; and none, 13 patients.
Patient 1
A 46-yr-old man underwent LDLT for end-stage liver disease due to hepatitis B virus. The trough level of tacrolimus was over 30 mg/mL incidentally at 5 days posttransplantation. Tacrolimus was reduced immediately. The patient had severe thrombocytopenia, hemolytic anemia with many fragmented red blood cells, and elevated lactate dehydrogenase levels. Intensive therapy consisting of PEs and a low-dose daily prostacyclin analog infusion were started. However, the patient required surgical intervention twice for abdominal hemorrhage at day 7 and day 8 after transplantation. Finally, the patient died of a cerebral hemorrhage on the 16th postoperative day.
Patient 2
A 23-yr-old woman underwent LDLT for fulminant hepatic failure due to a flare-up of autoimmune hepatitis. Duct-to-duct biliary reconstruction was performed. The patient suffered duct-to-duct anastomosis leakage and uncontrolled bile infection around the reconstructed common bile duct. The level of immunosuppression was required to be kept low. A high fever continued over 20 days at 3 months after transplantation. TMA finally developed and PEs and a low-dose daily prostacyclin analog infusion were initiated immediately. However, the patient died of multiple organ failure at 4 months after transplantation. The autopsy revealed severe intimal hyperplasia of the hepatic arterioles and portal venules, perivascular fibrosis of the central veins, degeneration of the hepatic ducts, and hemorrhagic necrosis in the grafted liver, which suggested chronic rejection.
Patient 3
A 48-yr-old man underwent LDLT for end-stage hepatic cirrhosis and focal hepatocellular carcinoma due to hepatitis C virus. The postoperative course was uneventful, but fragmented red blood cells were detected on the 14th postoperative day. The trough level of FK506 was set lower and CPA was switched to mizoribine. Prostacyclin analogue, antithrombin III, and fresh frozen plasma were administered immediately. TMA was controlled under subclinical basis and showed no clinical symptoms. He was discharged on the 45th postoperative day.
Patient 4
A 39-yr-old woman underwent LDLT for end-stage liver disease due to primary biliary cirrhosis. The trough level of FK506 was over 30 mg/mL due to accidental administration of an increased dosage. Fragmented red blood cells were detected on the 12th postoperative day and the FK506 dose was reduced. Daily PE was performed and antithrombin III was administered. Although the patient showed neurological symptoms, including epilepsy, during the TMA period, gradual resolution of thrombocytopenia and anemia ensued, and no further recurrences of TMA or neurological disorders were noted.
These clinical profiles and immunosuppressive regimen of the TMA patients are summarized in Table 2 and Table 3.
| Patient | TMA-onset (POD) | Duration of TMA (days) | Maximum value of fragmented red blood cell (%) | Zeigler grade | Complication due to TMA | ACR | Humoral rejection | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 9 | 24.3 | 4 | Cerebral hemorrhage | – | – | Dead |
| 2 | 102 | 20 | 11.9 | 4 | High fever, MOF | + | – | Dead |
| 3 | 14 | 14 | 1.1 | 1 | None | – | – | Alive |
| 4 | 11 | 37 | 4.7 | 2 | Epilepsy | – | – | Alive |
- Abbreviations: POD, postoperative day; ACR, acute cellular rejection; MOF, multiple organ failure.
| Patient | Immunosuppressive regimen | Level of immunosuppressants | Tcellreatment | ||
|---|---|---|---|---|---|
| Changes in CNI | Change in additional immunosuppressive agent | Other | |||
| 1 | FK+CPA | Over-dosed | FK reduced | CPA withdrawal | PE+PGI2 |
| 2 | FK+CPA | Kept low | FK kept low | CPA withdrawal | PE+PGI2 |
| 3 | FK+CPA | Adequate | FK reduced | CPA switched to mizo | PI+PGI2+ATIII |
| 4 | FK+mizo | Over-dosed | FK reduced | Mizo reduced | PE+ATIII |
- Abbreviations: FK, FK506 (tacrolimus); mizo, mizoribine; PI, plasma infusion; PGI2, Prostaglandin I2; ATIII, antithrombin III.
TMA Onset and Therapy
A sharp drop in platelet counts (decrease of >3.0 × 104/mm3 within 24 hours) was shown in 2 TMA patients while the other 2 showed a sharp decline in platelet count of 2.0 × 104/mm3 within 24 hours. Only 6 patients out of 53 non-TMA patients presented a sharp drop in platelet counts. Thrombocytopenia (platelet count <5.0 × 104/mm3) was present in all the 4 TMA patients while 40 patients out of 53 also presented thrombocytopenia in non-TMA patient. Erythrocyte fragmentation, accompanied by marked elevation of lactate dehydrogenase levels and a decrease in hemoglobin, followed 2 to 8 days after the onset of thrombocytopenia. A sharp drop in platelet counts was apparent before hemolytic anemia (Fig. 1).
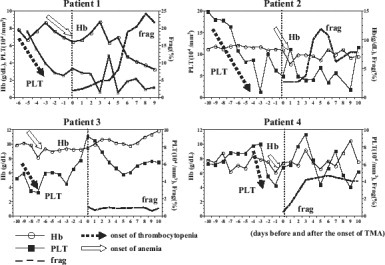
Difference in timing of onset of thrombocytopenia and hemolytic anemia. A sharp drop in platelet counts appears 2 to 8 days before hemolytic anemia.
A total of 3 patients presented with fever during disease onset. Two patients had impaired renal function and 1 had neurologic symptoms. The time of occurrence varied from immediately posttransplantation up to 3 months after transplantation. The duration of the disease ranged from 9 to 37 days (median, 17 days). Trough levels of CNI at the time of disease occurrence were managed within an acceptable therapeutic range only for Patient 3, while 2 patients (Patients 1 and 4) received increased amounts of CNI and the other (Patient 2) was limited to lower levels of immunosuppressant due to persistent infection. PAI-1, a fibrinolytic function marker, had the most parallel correlation with the course of TMA event, while coagulative function markers such as international normalized ratio of prothrombin time or vWF were independent of the TMA event (Fig. 2).
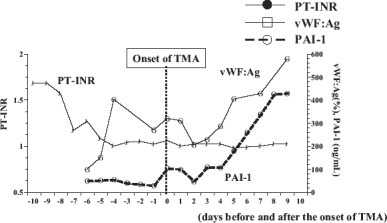
Changes in coagulation molecular markers before and after the onset of TMA in Patient 4. Fibrinolytic function marker (PAI-1) correlated well with the course of TMA event compared with coagulative function markers (international normalized ratio of prothrombin time [PT-INR], vWF:antigen [vWF:Ag])
PE was indicated for patients with greater than grade 2 according to the Zeigler grading system; it was performed in 3 patients and initiated within 1 to 2 days of disease occurrence. The number of PE sessions required ranged from 2 to 5. For the patient with grade 1, plasma infusion therapy alone was performed without necessitating PE. A total of 5 units were required for a total of 2 days in Patient 3. CNI dose was reduced for the 3 patients and kept low for Patient 2. Additional immunosuppressive agents were withdrawn in 2 cases, reduced in 1 case, and switched in 1 case. Low-dose daily prostacyclin analog or daily antithrombin III was administered until cessation of the event.
DISCUSSION
TMA is a potentially life-threatening complication after solid-organ transplantation. Without treatment, the disease is associated with a mortality rate of greater than 90%.23 Early recognition, diagnosis, and initiation of appropriate therapy are crucial to overcome this critical state. In our experience, thrombocytopenia occurs before hemolytic anemia. It is believed that primary platelet aggregation after endothelial damage initially triggers the cascade of this disorder. Endothelial damage stimulates primary platelet aggregation followed by hemolytic anemia due to microvascular occlusion.4, 9 Therefore, early recognition of thrombocytopenia, which occurs before hemolytic anemia, would be of considerable importance. Although thrombocytopenia is not an unusual condition after liver transplantation, a sudden drop in platelet count, especially in cases with normal coagulation profiles, should be observed carefully because it could be a key predictive sign of TMA.
To diagnose TMA after liver transplantation, histologic evidence is not necessarily needed. Zeigler et al.21 defined the criteria for TMA only including thrombocytopenia and microangiopathic hemolytic anemia with no clinically apparent alternative explanations. The kidney is the organ most commonly affected by TMA. Widespread occlusion by fibrin thrombi of renal cortical vessels and glomerular capillaries is usually shown.24 However, renal biopsy is not suitable and it is risky only to diagnose TMA under a thrombocytopenic condition. The microcirculation of the central nervous system may also be involved by TMA, but histological proof of the central nervous system is also rather difficult. Other organs are rarely affected by the microvascular lesions.25 Therefore, liver grafts themselves would hardly demonstrate the typical microvascular changes, which make the histological diagnosis of TMA quite difficult.
The incidence of TMA following renal transplantation was much higher in ABO-incompatible recipients compared with ABO-compatible recipients26 and recipients of liver transplants across the blood group barrier also might be at greater risk.27 Because TMA had never occurred in ABO-identical or ABO-compatible cases in our experience, ABO incompatibility certainly could be an important causative factor. Although TMA occurs more commonly in cadaveric transplants,12, 13 special attention should be directed to all those who receive ABO-incompatible grafts. Interaction between antidonor hemagglutinin and the endothelial cells of a graft may play an important role in TMA associated with ABO-incompatible liver transplantation.14 Antidonor hemagglutinin induces compliment activation, resulting in formation of microthrombi in the microcirculation and could readily be inhibited by administration of a protease inhibitor (gabexate mesilate), via the portal vein, to ABO-incompatible LDLT recipients according to our protocol.22 Although the precise mechanism of TMA is not yet well established, certain stimuli facilitate endothelial cell injury and invoke the cascade of the disorder. The causative stimuli have been reported elsewhere and include CNI, immunosuppressive agents, sepsis, infection, and ABO-incompatibility.8-11, 14 In our series of LDLT patients, only ABO-incompatible LDLT recipients presented posttransplantation TMA. While none of the TMA patients showed an activated humoral state, antidonor hemagglutinin levels were kept low and no humoral rejection was observed. Although whether ABO-incompatibility itself invokes TMA or not remains unknown, other additional factors associated with ABO-incompatible transplantation may lead to endothelial cell injury.
In general, immunosuppressive agents, such as cyclosporine A and tacrolimus, play a key role in the pathogenesis of TMA.28 As for the cases of ABO-incompatible transplants, the amount of immunosuppressive agent inevitably tends to be “over-dosed” to regulate the hyperimmunological state. In particular, CPA showed a close association with the incidence of TMA, compared with other additional immunosuppressive agents, including azathioprine and mizoribine. Although azathioprine and mizoribine act as antilymphocyte agents, CPA, which is classified as an antineoplastic agent, acts by destroying rapidly proliferating cells.29 Therefore, CPA can cause direct endothelial damage, because the endothelium, which has a high mitotic index, is one of the sites most susceptible to antineoplastic agents. Because CPA can disrupt carbohydrate, lipid, amino acid, protein, and nucleic acid metabolism,30 it is supposed that actions other than deoxyribonucleic acid alkylation may have greater importance in damaging the endothelium.
The other notable factor in our series of TMA patients was that a coagulation molecular marker, PAI-1, had a close correlation with the course of TMA event and, along with thrombocytopenia, should be a good indicator of the disease. In general, the change in coagulation factors in TMA is moderate compared with that in disseminated intravascular coagulation.31 Severe secondary fibrinolysis occurs in disseminated intravascular coagulation patients but not in those with TMA.32 Microcirculation disturbances due to microthrombi in various organs stimulate fibrinolysis to increase production of fibrinogen/fibrin degradation product.31, 33 However, the elevation of PAI-1 in the acute phase of TMA was far higher than in disseminated intravascular coagulation. As a result, the elevation of fibrinogen/fibrin degradation product in TMA might have been limited to a moderate degree compared to that in disseminated intravascular coagulation. In a disorder with severe thrombocytopenia due to intense platelet activation, a huge amount of latent PAI-1 might be released, not only from platelet agglutinates but also from injured endothelial cells. PAI-1 elevation may regulate the radical secondary fibrinolysis to help prevent the hyperfibrinolytic state and hemorrhage in organs. But this speculation is subject to the limitation that PAI-1 was examined only for 1 patient retrospectively after the diagnosis of TMA. Therefore, further investigation in other patients would be necessary to confirm the phenomenon.
All the TMA patients in our series were in blood group O. This coincidence could be incidental but might somehow have influenced the pathogenesis of TMA. Many studies have showed lower vWF levels in blood group O.34-36 It has been suggested that the differences in glycosylation of the vWF molecule in patients with different blood groups affect their clearance, such that the vWF molecules containing blood group A and group B sugars in patients with non-group O blood are cleared more slowly.35 In the pathogenesis of TMA, endothelial injury results in the release of endothelial ultralarge vWF multimers. Ultralarge vWF multimers increase platelet adhesiveness and thus promote the formation of platelet-fibrin thrombi in the microvasculature.10 Patients with TMA have been found to have a deficiency of, or an inhibitor to, the vWF-cleaving protease.10 This factor has been proposed to play a major role in the pathogenesis of TMA. Nakazawa et al.15 hypothesized that the severity of the vWF-cleaving protease deficit might be involved in the occurrence of TMA in LDLT recipients because vWF-cleaving protease is exclusively synthesized in the liver and the small-for-size graft in LDLT might have a reduced capacity to produce the enzyme. However, the vWF-cleaving protease level is higher in healthy individuals of blood group O than those of non-O blood groups.37 Therefore, blood group O may not be a risk factor for TMA in terms of vWF-cleaving protease. The other possible explanation is that blood group O recipients have a higher prevalence of ABO-incompatibility situation because a non-O graft automatically results in ABO-incompatibility for a blood group O recipient.
There are some limitations of our study. First, our results are subject to the limitations inherent in any retrospective uncontrolled study in an institution. Second, as the TMA event itself is rather a rare event and the incidence rate of TMA is quite small, with only 4 cases of TMA, chance will dominate. Nevertheless, a multiinstitutional study is needed to evaluate a much larger population to elucidate the exact risk factors causing TMA.
In summary, we had 4 patients with TMA among the 9 adults who underwent ABO-incompatible LDLT, and we failed to save 2 of them. It remains to be determined whether ABO-incompatible transplantation itself had any influence on the occurrence of TMA. Other additional factors, such as “over-dosed” immunosuppressive agents, also may influence this phenomenon. Sudden and severe thrombocytopenia was presented before hemolytic anemia and should be an important predictive sign for TMA, and the serum levels of PAI-1 correlated well with the clinical course of TMA compared to other coagulative factors.