Hepatitis B in liver transplant recipients
Abstract
Key Concepts:
- 1
The use of low-dose immunosuppressive therapy along with pre- and posttransplantation nucleos(t)ide therapy and posttransplantation hepatitis B immunoglobulin (HBIG) has yielded marked improvements in survival.
- 2
Lamivudine (Epivir-HBV), adefovir (Hepsera), entecavir (Baraclude), tenofovir (Viread), emtricitabine (Emtriva), and the combination drugs tenofovir + emtricitabine (Truvada) and abacavir + lamivudine (Epzicom) are effective nucleos(t)ide antiviral agents that, in some cases, may help reverse liver disease sufficiently to avoid transplant.
- 3
In posttransplantation patients, virus suppression with some combination of HBIG and the nucleos(t)ide agents may prevent graft loss and death or the need for a second transplant.
- 4
In both the pre- and posttransplantation setting, the goal of hepatitis B virus management is complete virus suppression.
- 5
The use of low-dose intramuscular HBIG is evolving, with studies showing that dosing and cost can be reduced by 50–300% with a customized approach.
- 6
Elimination of HBIG from the treatment paradigm is currently under evaluation and may be possible with the use of newer medications that have no or low resistance rates.
- 7
Although there is growing evidence that some types of combination therapy may decrease the chance that drug resistance will develop and increase the likelihood of long-term success in preventing graft loss and death, additional research will be required to determine which combinations will work well in the long term, and which will not. Liver Transpl 12:S54–S64, 2006. © 2006 AASLD.
A 44-year-old white man was diagnosed with chronic hepatitis B in 1999 by his primary-care physician. Analysis of laboratory results revealed the following: aspartate aminotransferase (AST) 144 IU/L, alanine aminotransferase (ALT) 82 IU/L, hepatitis B surface antigen (HBsAg) positive, hepatitis B e antigen (HBeAg) positive, anti-HBs negative, and hepatitis B virus (HBV) DNA 7,000,000 IU/mL. The source of HBV was thought to be from use of intravenous drugs 20 years before his evaluation. He is heterosexual and described no other risk factors for acquiring HBV. He did not drink alcohol. No antiviral treatment was started.
In 2001, the patient sought care from a gastroenterologist for increased abdominal distension and increasing fatigue. An ultrasound of the abdomen showed a cirrhotic liver, with a large amount of ascites. An esophagogastroduodenoscopy revealed grade 2 varices with portal hypertensive gastropathy. His renal function was normal. Therapy with the following was initiated at the time of his medical evaluation: lamivudine (100 mg daily), Aldactone (100 mg daily), Lasix (40 mg daily), and propranolol (20 mg twice daily).
After 1 year of therapy, laboratory values were as follows: AST 101 U/L, ALT 56 U/L, bilirubin 2.4 mg/dL, albumin 2.9 g/dL, and international normalized ratio of prothrombin time 1.4. HBV serologies were unchanged. He was lost to follow-up for the next 2 years, but he stated that he continued lamivudine therapy.
In June 2003, he was transferred to a transplant center as a result of increased lethargy, encephalopathy (grade 2), and worsening ascites. Physical examination revealed mild confusion. Vital signs were as follows: heart rate 120 beats/min, blood pressure 100/65 mm Hg, and temperature 38.0°C. He had scleral icterus and severe muscle wasting. Abdominal examination showed moderate tense ascites. Table 1 lists laboratory data.
| Characteristic | Value |
|---|---|
| Platelet count | 26 × 103/μL |
| White blood cell count | 9.2 × 103/μL |
| Hemoglobin | 12 g/dL |
| Sodium | 127 mmol/L |
| Creatinine | 1.6 mg/dL |
| International normalized ratio of prothrombin time | 3 |
| Total bilirubin | 18 mg/dL |
| Aspartate aminotransferase | 102 U/L |
| Alanine aminotransferase | 61 U/L |
| Albumin | 2 g/dL |
| Alpha-fetoprotein | 4 ng/mL |
| HBsAg | Positive |
| Anti-HBs | Negative |
| HBeAg | Positive |
| Anti-HBe | Negative |
| Hepatitis B virus DNA | 8,000,000 IU/mL |
- Abbreviations: Ag indicates antigen; HBe, hepatitis B e; and HBs, hepatitis B surface.
The patient's Model for End-Stage Liver Disease score was 34. Because paracentesis revealed ascitic fluid white count of 6.3 × 103/μL with 72% segmented neutrophils, ceftriaxone and albumin infusions were started. Ultrasonography showed a cirrhotic liver with massive ascites, splenomegaly, no intrahepatic masses, and patent portal vein and hepatic artery.
Evaluation for liver transplantation (LT) was completed, and he was deemed a transplant candidate. Because of increased creatinine levels, lamivudine was dose-adjusted to 100 mg every other day, and adefovir was added in a dose of 10 mg every other day. An analysis of the patient's resistance pattern by Inno-LiPA revealed both the 180 and 204 mutations that lead to lamivudine resistance.
Ten days later, an orthotopic liver transplant was completed. The liver was from a 24-year-old donor who was anti–HBV core positive. The patient's immediate transplant course was complicated by atrial fibrillation, new-onset diabetes mellitus, and development of acute renal failure that required dialysis for 2 weeks. He was discharged 3 weeks after LT with Prograf and prednisone as immunosuppressive therapy. Hepatitis B treatment included hepatitis B immunoglobulin (HBIG) infusion of 10,000 IU during the anhepatic phase and then daily infusions for the next 6 days to achieve an anti-HBsAb level of >500 IU/L. Adefovir was continued after LT and dose-adjusted as the patient's renal function first worsened and then sequentially improved. The anti-HBsAb titer was checked every week for 1 month, then monthly. Discharge laboratory data included the following: creatinine 1.4 mg/dL, AST 15 U/L, ALT 12 U/L, and bilirubin 0.8 mg/dL. He received adefovir at a dosage of 10 mg every other day.
Prednisone was discontinued at 6 months. He was maintained on Prograf as his only immunosuppressive medication. HBIG levels were monitored monthly to keep a level of >500 U/L. At 1 year, HBIG therapy was stopped. The patient received adefovir during this time.
One year later, rising liver test values (AST 200 U/L, ALT 233 U/L, and gamma-glutamyl transferase 424 U/L) led the managing physicians to check HBV DNA and HBsAg. The patient was HBsAg positive and had an HBV DNA level of 106 IU/mL. He did not come to the clinic for his next 2 appointments. Laboratory tests that were returned in the interim showed that he had developed an HBV-resistant virus with point mutations at the 180, 181, 204, and 236 nucleotides in the HBV polymerase region. He was now HBeAg negative, indicating that he also had a precore mutant virus. At his next appointment, the laboratory tests revealed the following: ALT 400 U/L, gamma-glutamyl transferase 800 U/L, and bilirubin 10 mg/dL. A liver biopsy was performed.
Antiviral treatment before and after LT of patients with the diagnosis chronic HBV infection requires up-to-date knowledge of transplant medicine, immunology, and virology. The extremely high rate of graft infection and early graft failure shortly after LT, as well as frequent patient death, led to restricted use or abandonment of LT in the early 1990s for patients with chronic HBV. Marked improvements in long-term survival in the mid-1990s resulted from the use of low-dose immunosuppressive therapy and administration of short-term and then long-term (indefinite) HBIG after LT with the subsequent introduction of nucleoside therapy both before and after LT.1-9
Historically, graft infection was proportional to the serum level of HBV DNA (replication) before transplantation, type of liver failure (fulminant versus chronic), and presence of hepatitis delta virus (HDV) infection before transplantation. Also, a strict requirement for compliance with HBIG injections with measurable serum titers of anti-HBs has been required for maximum efficacy, with HBIG dosing adjustments required relative to pre-LT virus levels. It is important to recognize that HBIG is a polyclonal antibody preparation initially derived from blood donors who have been exposed to and cleared HBV infection. It was designed to prevent de novo infection in individuals exposed to HBV in high-risk settings. In the case of LT, HBIG binds circulating virus and presumably prevents infection of the hepatocytes in the new graft. It may also prevent interhepatocyte virus transfer.
Genotyping of HBV infections is possible, but the effect of specific genotypes on pre- or post-LT outcomes is not yet clearly established. However, in recent studies, genotype D was associated with a high rate of fulminant hepatic failure,10 and HBV genotypes B and C were associated with different patterns of end-stage liver diseases, with genotype C shown to carry a greater risk of disease recurrence due to lamivudine-resistant mutants.11
TREATMENT OF HBV INFECTION BEFORE LT AND ITS IMPACT ON POST-LT MANAGEMENT
Lamivudine, adefovir, and entecavir all clearly decrease HBV replication in patients who are not immunosuppressed and may lead to “triple seroconversion” (HBeAg positive to negative, anti-HBe negative to positive, and HBV DNA suppression to undetectable levels) in patients with wild-type viral infection as well as good control of virus replication in patients with precore and core promoter mutations (Fig. 1).
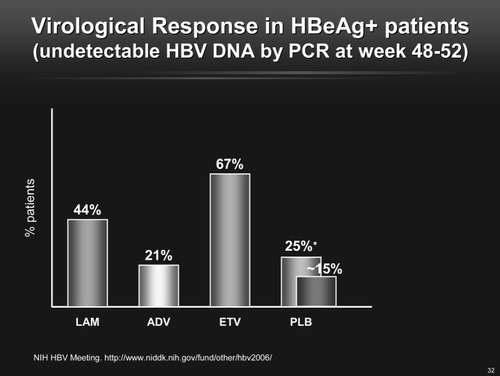
Virological response in HBeAg-positive patients (undetectable HBV DNA by polymerase chain reaction at weeks 48–52).
Exciting reports of the use of lamivudine and adefovir before LT revealed that some patients with cirrhosis could have a marked reduction in HBV DNA serum levels and reversal of fibrosis. Lowering of Model for End-Stage Liver Disease scores has also been reported with some patients being removed from the liver transplant list or being made inactive12 (Fig. 2).
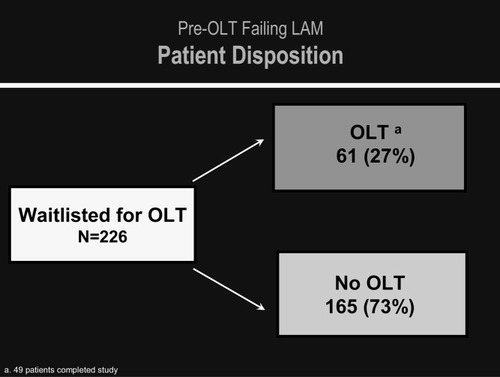
Pre-OLT failing lamivudine patient disposition.
Further reports have demonstrated that liver disease activity could be controlled after transplantation with either of these 2 agents, with improved serum levels of liver enzymes and improved liver synthetic tests; in some cases, both liver enzymes and liver function tests returned to normal.13 When this occurs before LT, it may eliminate the need for transplantation; after transplantation, it may prevent graft loss and death or the need for a second transplant.14-23
The key to management of HBV in the pretransplantation setting is complete virus control, defined as no measurable circulating virus. This control of virus replication also ensures better outcomes and simpler management after LT. This focus on complete virus suppression with the most potent agents available is now possible with the addition of newer therapies to our armamentarium.
There is also an emerging role for combination therapy with oral medications before or after LT. However, recent research has made it clear that although some combinations may work well, others may not. Favorable results were recently seen with treatment-naive patients in a randomized trial comparing adefovir monotherapy to the combination of adefovir with emtricitabine (FTC) in which the latter was shown to yield faster and greater virus suppression, with the faster response associated with enhanced T cell reactivity.24 In a trial of patients with lamivudine-resistant virus, the addition of adefovir either before or at transplant, with indefinite continuation, resulted in complete virus suppression, both in those patients who also received HBIG and those who did not, for a median follow-up of almost 2 years.25 A recent retrospective review looked at liver transplant recipients with HBV who were converted from HBIG with lamivudine to adefovir with lamivudine. During 11–20 months of follow-up from conversion, DNA status remained negative in all patients, with no increase in serum levels of ALT or AST.26
However, not all combination therapy results have been as promising. In a recent trial that compared the efficacy of treatment in 157 patients given either the combination of emtricitabine (FTC) with clevudine or monotherapy with FTC, after 24 weeks of therapy, no marked differences were seen in antiviral activity, serologic responses, or development of resistance,27 probably as a result of similarities in the antiviral medication profiles of FTC and clevudine. A similar lack of increased efficacy was seen in a trial that compared monotherapy with either telbivudine or lamivudine to combined therapy with both drugs in patients with compensated chronic hepatitis B and found no advantage to the combination.28 These mixed results make it clear that additional research will be required to fine-tune our understanding of which agents may be successfully combined for long-term virus suppression, and which may not. It must be emphasized that combination treatment studies need to use medications with different binding characteristics to the HBV polymerase and different resistance profiles. Also, there must be a focus on choosing to combine the agents that individually have the most potency and the least resistance risk.
The rule of 1-log virus suppression every 3 months must be applied to the pretransplantation patient as well.29 Persistent, long-term virus replication is the ideal setting for viral resistance to occur as well as graft reinfection, aggressive hepatitis, and fulminant liver failure either before or after LT.30 Clearly, decreased rates of resistance are seen in patients for whom combination therapy is applied, as well as with single agents such as entecavir, where no resistance at 2 years has been reported.31
In the earlier years of LT for HBV, lamivudine was the initial nucleoside medication used before transplantation. However, development of mutations and subsequent virus replication posed risks to patients before their transplantation. Fortunately, newer, more potent nucleos(t)ide medications are available. For nucleoside-naive patients, the most potent treatment at this time is entecavir,32, 33 with 81 and 94% of patients remaining at less than 60 IU/mL at 2 years of treatment.32-38 Adefovir is a less potent viral medication than either lamivudine or entecavir, but extensive data in the liver transplant patient population show that it clearly plays a role as a single agent and can be used with lamivudine if lamivudine resistance is discovered.25, 39-45 The addition of adefovir to lamivudine once viral rebound has occurred can result in marked virus suppression. Only one case of adefovir resistance has been seen where there is an “add” rather than a “switch” prescription.30, 46 If adefovir and lamivudine are started concurrently, there is no evidence of viral resistance to date. There is a risk of renal toxicity from adefovir in this patient population because there is already preexisting renal disease both before and after LT.
Importantly, there is no head-to-head comparison of adefovir and entecavir in patients with cirrhosis or transplant recipients. However, a study that compared the 2 drugs in chronically infected nucleoside-naive patients has shown clear evidence that entecavir results in a higher likelihood of negativity and more favorable viral kinetics.47 The use of entecavir in patients with preexisting lamivudine resistance must be taken with caution in the pre- and post-LT setting because this medication is weakened in terms of virus suppression and has much lower rates of yielding undetectable virus in a short period of time (<1 year). Resistance risk with entecavir occurs only if there is preexisting lamivudine resistance, with the additional requirement of 2 more point mutations. In this setting, the rate of entecavir resistance with virus rebound is 9% within 2 years.48 Rebound (an increase in virus load of 1 log from nadir or becoming nucleic acid test positive after being negative) with any medication in a patient with cirrhosis could be life-threatening, and could be problematic after transplant in immunosuppressed patients who are not being closely monitored.
Tenofovir treatment may also be an alternative option for patients as either primary therapy or with lamivudine resistance. Ultimately, depending on the data, it may be useful as a first-line therapy for patients with cirrhosis or after receipt of a liver transplant.49, 50 Tenofovir is a more potent nucleotide than adefovir and can be added to lamivudine. Alternatively, Truvada, a drug that combines a fluorinated version of lamivudine, emtricitabine (FTC), with tenofovir, can be used. Doses of each of these medications must be adjusted for renal insufficiency (Fig. 3, Table 2).
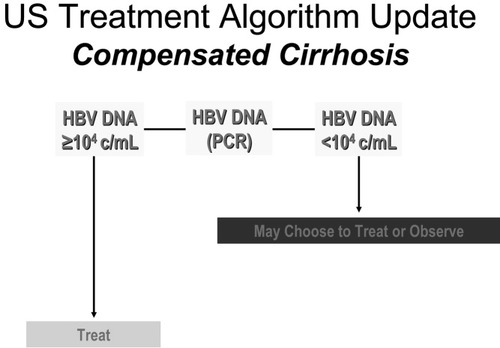
Treatment algorithm update for compensated cirrhosis.
| Hepatitis B status | Recommendation |
|---|---|
| HBeAg-positive chronic hepatitis B | IFN, ETV, and ADV first-line therapy |
| HBeAg-negative chronic hepatitis B | ETV, ADV, or IFN preferred because of need for long-term therapy |
| Compensated cirrhosis | ADV or ETV |
| Decompensated cirrhosis | ETV or ADV; refer to and coordinate with transplant center |
| IFN nonresponse or contraindicated | ADV or ETV |
| LAM resistance | Switch to ETV, or add ADV or IFN |
| ADV resistance | LAM or ETV |
- Abbreviations: ADV, adefovir dipivoxil; ETV, entecavir; HBeAg, hepatitis B e antigen; IFN, interferon; and LAM, lamivudine.
FOCUS ON THE PREVENTION AND TREATMENT OF HBV INFECTION AFTER LT
Samuel and coworkers were the first to describe the use of HBIG in a large clinical trial to prevent recurrent liver disease after LT in patients with liver failure due to HBV infection.7, 8 The concept was to bind the circulating virus during the anhepatic phase when the recipient's liver, as the critical source of HBV replication, was removed and then administer and maintain high serum levels of anti-HBs to continuously bind all circulating HBV.51 Compared with historical controls, overall survival and disease-free survival rates were markedly improved, and other centers subsequently demonstrated improved survival (>80%) at 1 year after liver transplantation.51-54 Thus, HBIG administration became the standard of care at most centers worldwide that provide LT. The high cost of HBIG ($30–50,000/yr) and the increasing per-unit costs relative to the transplant cost itself that have occurred over the last 5 years, have stimulated discussions about replacement therapies.51-54
One major question to answer has been to discover if and when patients can be discontinued from HBIG therapy—that is, once most circulating virus is bound, is the risk of viral infection of hepatocytes or graft lower in the long term? We know that the recurrence rate of HBV infection in the liver graft exceeds 60% with short-term HBIG (< 6 months) and 10–20% if HBIG is stopped more than 6 months to 1 year after orthotopic liver transplantation (OLT), even with the continuation of a nucleoside such as lamivudine.5, 55, 56 In some cases, HBIG was not used at all, with only modest recurrence rates.57 The use of low-dose intramuscular HBIG is also evolving. Studies, including those at California Pacific Medical Center, have shown that dosing and cost can be reduced by 50–300% with a customized approach to dosing intramuscularly and changing doses in response to anti-HBs titers.9, 58-63 Further emphasizing the point that transplant patients are a special population, several studies that used a focused polyclonal synthetic HBIG preparation resulted in the emergence of HBV with surface antigen mutations.64-66 In addition, patients with lamivudine resistance mutations have changes in the HBsAg, which can result in escape from vaccine and HBIG.67, 68
DETAILS ON DIRECT ANTIVIRAL THERAPY USING NUCLEOSIDE AND NUCLEOTIDE ANALOGUES AFTER LT WITH OR WITHOUT HBIG
First and foremost in the prevention of recurrent disease after LT is the use of preoperative nucleos(t)ide analogues. The second step is intraoperative and postoperative HBIG and concomitant oral nucleos(t)ide therapy to prevent or modulate graft reinfection. If disease occurs after LT, it may be due to underdosing of HBIG, noncompliance, resistance, or de novo disease such as donor-derived or blood product–derived chronic hepatitis B (CH-B). From a historical perspective, ganciclovir (DHPG, Cytovene; Roche, Nutley, NJ) was the first nucleoside to treat post-OLT HBV graft infection.4 Ganciclovir is no longer used to treat HBV patients in any clinical setting. More recently, lamivudine and adefovir therapy was shown in a major clinical trial to decrease HBV DNA levels in patient awaiting LT, resulting in excellent control (suppression) of serum levels of HBV DNA after OLT.12, 69-72 Thus, the primary objective is to have all patients awaiting LT on nucleos(t)ide therapy. Also, it now appears possible that patients who develop de novo or recurrent HBV infection after OLT (HBIG untreated or failure to respond to treatment) can also be treated with nucleos(t)ide analogues.4, 71, 73-77 Stopping all antiviral therapy after LT is fraught with the danger of severe liver disease and death and must be avoided.78 This combination of pretransplantation nucleos(t)ide therapy with indefinite posttransplantation nucleos(t)ide and HBIG combination therapy is probably the best current approach until we can define who can have HBIG therapy stopped.
Cost modeling has demonstrated that lamivudine + adefovir may be much cheaper because of the high cost of HBIG.63 As already discussed, a recent retrospective review of liver transplant recipients with HBV who were converted from HBIG with lamivudine to adefovir with lamivudine showed that during 11–20 months of follow-up from conversion, DNA status remained negative in all patients, with no increase in transaminases. The cost savings were substantial, with an average monthly cost of only $602.92 for the nucleoside combination, compared with $9225 for the HBIG-lamivudine therapy.26
Nucleos(t)ide monotherapy, especially in the setting of immunosuppression, has a high infection “recurrence” rate (>20%) of the new graft,79 even in current times. There is also a very high replication rate, with a concomitant higher mutation rate, once graft infection has occurred, leading to a high rate of nucleos(t)ide resistance with monotherapy with either lamivudine or adefovir.30, 80
Virus replication in patients that occurs after 2 years of antiviral therapy after LT can lead to severe liver disease and death, or need for retransplantation.71, 72, 81-91 Thus, patient compliance and monitoring is mandatory for effective long-term patient management and, more importantly, for survival. Although conversion from HBIG-nucleos(t)ide to nucleos(t)ide monotherapy has been considered an alternative option, it is important to recognize that recurrence rates are approximately 10–20%, and any recurrence could be deemed unacceptable with the high risk of progressive liver disease.55, 56 Combination nucleos(t)ide therapy may potentially eliminate the need for long-term HBIG use while keeping the risk of resistance below 1–5%.92 Although it has not yet been defined who can safely discontinue HBIG therapy, the best candidates are probably patients with undetectable HBV DNA before LT who use combination therapy with medications that have low risk of resistance.
Unlike patients who are not undergoing immunosuppression who develop resistance to lamivudine, patients with lamivudine-resistant mutants after transplantation may have aggressive liver disease.85, 90, 91 This has also now been reported with adefovir therapy.30 Low-dose intramuscular HBIG is useful in combination with lamivudine for patients with the precore mutations who undergo LT.89
Once activation of virus replication takes place, aggressive hepatitis and subsequent rapid development of liver failure may develop (a syndrome described as fibrosing cholestatic hepatitis, or FCH). FCH is defined as a rapidly progressive liver disease with cholestasis, jaundice, hepatic fibrosis, and liver failure, often complicated by sudden and severe multiorgan dysfunction.93-99 Lamivudine, adefovir, and ganciclovir have been used to treat FCH.4, 100, 101 With the current potency of individual agents and the use of appropriate dosing of HBIG and/or combination of oral nucleos(t)ides, FCH is an extremely rare condition and should not be seen unless there is patient noncompliance. Retransplantation has been performed in patients with FCH, but those who have rapid liver failure shortly after OLT have much worse survival.102, 103 If recurrence of hepatitis B infection is suspected after OLT, immunoperoxidase staining for the HBV core (HBc) and surface (HBs) antigens is useful to document disease severity and to differentiate recurrent HBV disease from rejection.51, 104
INTERFERON THERAPY
From a historical perspective, interferon just before and continued after transplantation may modify the aggressive postoperative course of HBV.105, 106 However, use of interferon before and after transplantation is fraught with side effects, including thrombocytopenia and neutropenia, and it increases the risk of rejection. Therefore, interferon is rarely used.
ALTERING THE IMMUNE SYSTEM THROUGH MODIFICATIONS IN IMMUNOSUPPRESSION
Management of liver transplant recipients who have chronic HBV infection can also be enhanced by the use of low-dose immunosuppressive therapy.5, 107-114 This includes overall lower doses of tacrolimus, elimination of OKT3 use, minimizing corticosteroid use, and obviating corticosteroid treatment of rejection. Interestingly, patients who have chronic HBV disease have undergone viral manipulation of their immune systems and appear to be at a lower risk of rejection, leading to this innovative approach of posttransplantation management (low-dose immunosuppression).
A recent interesting proposal that has not been used often is simultaneous transplantation of liver with bone marrow from a vaccinated donor or immune donor.115 One must be careful in defining “immunity” because patients who are both anti-HBc and anti-HBs positive have been reported to transmit HBV to organ transplant recipients. This method of transferring cellular immunity was originally pioneered in bone marrow transplant recipients,116, 117 leading to HBV clearance in the recipient of the bone marrow transplant.
USE OF HBSAG-POSITIVE DONORS
Because of the scarcity of organ donors, a few centers have used HBsAg-positive donors and have had some success. It appears that HBIG will play only a small role in this setting, similar to the anti-HBc–positive donor model. With the use of a lamivudine-adefovir combination, it may be possible to control recurrent disease in a majority of these patients.118
COINFECTION
HCV
There are few data in prospective studies that provide absolute guidance for treatment of both HCV and HBV in the clinical setting of transplantation. Most practitioners start by treating the dominant disease as determined by serum levels of virus. In the setting of cirrhosis or after LT, keeping the HBV suppressed to undetectable in the long term is essential, and prophylactic treatment of any HBsAg-positive patient must be the long-term goal in the management of such patients.
Human Immunodeficiency Virus (HIV)
Coinfection with HBV and HIV provides a complex clinical setting that demands interaction between the hepatologist, surgeon, and infectious disease specialist. Close monitoring of all resistance patterns for both viruses is essential. Combination therapy with tenofovir and lamivudine or emtricitabine would be a possible first step to treat both infections.119-121 A third anti-HIV drug would be required to fully suppress that virus, and addition of entecavir could be considered if perfect HBV viral control is not reached.
Hepatitis Delta Infection
Patients who undergo LT with HDV infection are interesting from the perspective that they often have low or very low serum levels of HBV (low replication) and have an overall good survival rate after LT as a result of the “antiviral” effect of delta on HBV replication. Suppression of HBV replication by delta hepatitis has led to better posttransplantation survival in coinfected patients.51, 122-124 The importance of the use of HBIG is also well defined in patients coinfected with HDV-HBV. Most centers would use a peri- and post-OLT protocol that included the use of HBIG and a nucleoside or nucleotide analogue to serve as triple therapy to minimize the risk of HBV reactivation, although these 2 treatments will have no effect on delta replication.
ANTI-HBC–POSITIVE DONORS AND DE NOVO HBV AFTER TRANSPLANTATION
If a perfect donor liver is not immediately available, the use of a donor who is anti-HBc positive (high-risk donor) is considered at many centers. However, donors who are anti-HBc positive pose a significant risk (ranging 34–86%) of transmitting HBV infection to the liver transplant recipient.125-129 The use of anti-HBc–positive donors presents a special problem to the transplant and infectious disease clinicians and is an interesting model of the compartmentation of HBV infection.130-132 Patients who are anti-HBc positive often have molecular evidence of virus replication including covalently closed circular DNA (cccDNA) and pregenomic RNA in liver but have no circulating HBV by the most sensitive molecular tests.129, 133 Unfortunately, the recipient may develop very aggressive liver disease if preemptive treatment is not initiated, and lifelong nucleotide or nucleoside therapy is currently recommended.134
The role of HBIG is not defined and should not have any specific benefit because the liver is already infected and there is no benefit to binding circulating virus. Because patients have developed fulminant HBV in this setting, even with the use of lamivudine, combination therapy may be the best option. Another possibility is the use of entecavir to which resistance has not been reported in patients with no evidence of resistance to lamivudine for durations of follow up beyond 2 years.48 All immunosuppressed patients should have liver tests monthly and virus replication studies every 6 months or with any change in liver function.
VACCINATION FOR HBV
The administration of standard HBV vaccine to liver transplant candidates or recipients who are infected with HBV has had no consistent effect on virus replication or clearance,135 although some authors claim that lower doses of HBIG can be used or eliminated in patients who are surface antigen negative.135-137 There have been some case reports of virus clearance after LT in some patients with HBV vaccination.136, 138 Newer vaccines may be able to aid in T cell reconstitution that is focused on HBV-directed immune responses.
SUMMARY
By recognizing the special models of chronic viral disease, great advances in the management of HBV infection have taken place in the treatment of patients who are undergoing OLT for both acute and chronic liver disease due to HBV. Combination therapy with nucleoside analogues and HBIG has emerged as the current standard of care, even with the lengthy duration of therapy, especially for HBIG, required to minimize recurrent disease. The emergence of new oral medications, especially nucleos(t)ide analogues such as adefovir and entecavir, are further advancing our ability to control HBV disease both before and after LT. Lessons learned from customizing immunosuppression and preemptive peritransplant treatment with oral nucleos(t)ide therapy have allowed us to achieve superb long-term survival in this group of patients that were nearly abandoned as transplant candidates in the early 1990s (Table 3).
| Clinical setting | Primary treatment option | Other options |
|---|---|---|
| ESLD, pre-LT treatment naive | Entecavir | Combination therapy: |
| lamivudine with adefovir | ||
| lamivudine with tenofovir | ||
| ESLD, pre-LT drug resistance to lamivudine | Add tenofovir or adefovir | Change to entecavir |
| ESLD, pre-LT drug resistance to adefovir | Add entecavir | Add entecavir or lamivudine |
| Change to tenofovir | ||
| ESLD, pre-LT drug resistance to entecavir | Add tenofovir | Add adefovir |
| ESLD, pre-LT drug resistance to tenofovir | Add entecavir | Add lamivudine |
| Change to Truvada (combination of tenofovir and emtricitabine) | ||
| Post-LT prophylaxis of recurrence | HBIG (IV with immediate transition to IM therapy) with nucleoside or nucleotide adjusted for renal function | Combination of nucleoside and nucleotide |
| Entecavir and tenofovir | ||
| Tenofovir and emtricitabine | ||
| Tenofovir and lamivudine | ||
| Adefovir and lamivudine | ||
| Active CH-B post-LT treatment naive | Entecavir | Combination therapy |
| Active CH-B post-LT resistance | Combination therapy | … |
- Abbreviations: ESLD, end-stage liver disease; LT, liver transplantation; HBIG, hepatitis B immunoglobulin; IV, intravenous; and IM, intramuscular.




