Acute rejection in HCV-infected liver transplant recipients: The great conundrum
Abstract
Key Concepts:
- 1
In hepatitis C virus (HCV)-infected patients, treatment of acute rejection is associated with worse outcomes (increased risk of allograft cirrhosis and mortality).
- 2
Whether patients with HCV are at higher risk for rejection remains controversial.
- 3
The mechanisms mediating acute rejection and recurrence of HCV are distinct, and as such, it should be possible to develop techniques based on these molecular differences that are diagnostically useful.
- 4
Liver biopsy is considered the gold-standard for diagnosing acute rejection and recurrent HCV; however, given histopathological similarities between the two conditions, discrimination can be extremely difficult.
- 5
At the present time, there are no reliable, noninvasive tools available to distinguish between HCV recurrence alone and acute rejection plus HCV recurrence.
- 6
Mild rejection per se is not associated with graft loss and treatment of rejection with steroids and OKT3 is associated with worse outcome in HCV; thus, it seems logical that we should no longer treat mild rejection. Liver Transpl 12:S38–S47, 2006. © 2006 AASLD.
Despite the significant improvements in patient and liver allograft survival over the past 2 decades, the long-term use of increasingly potent immunosuppression results in many side effects, including increasing metabolic complications (hypertension, hypercholesterolemia, and diabetes) and associated risk for cardiovascular disease, renal toxicity from calcineurin inhibitors, de novo malignancy, and acceleration of recurrent disease. Recurrence of hepatitis C virus (HCV) following liver transplantation (LT) represents the most significant issue facing transplant physicians and surgeons because of the high prevalence of HCV as an indication for LT and the fact that ∼30% of recipients develop progressive allograft injury, including cirrhosis.1-3 The critical problem is the distinction between acute cellular rejection (ACR) and HCV recurrence because treatment of ACR with corticosteroids or T cell depletion has been consistently associated with significant increase in mortality.4 As reflected by the high priority assigned by the recent National Institutes of Health Liver Action plan,5 the identification of reliable, noninvasive biomarkers for ACR is paramount. In this review, we summarize the current understanding of optimal immunosuppression and treatment of rejection as it pertains to HCV-infected liver transplant patients, mechanisms mediating ACR and HCV-related allograft injury, and methods to differentiate these two processes, as well as the risk of rejection following treatment of HCV recurrence. Unfortunately, there are too many gaps in our current knowledge base to provide evidence-based algorithms for management.
BASAL AND MAINTENANCE IMMUNOSUPPRESSION IN HCV-INFECTED RECIPIENTS
Induction therapy is not typical administered in LT; however, its use has increased from 7% in 1997 to 20% in 2003.6 The explanation for this trend is likely due to increasing prevalence of renal insufficiency due largely to the contribution of serum creatinine in determining priority under Model for End-Stage Liver Disease allocation and desire to avoid renal toxicity from calcineurin inhibitors. Current maintenance immunosuppression prior to discharge consists largely of corticosteroids (82% in 2003), tacrolimus (89% in 2003), and mycophenolate mofetil (54% in 2003).6 There does not appear to be a significant difference between outcomes with respect to the use of calcineurin inhibitors, tacrolimus, and cyclosporine.4, 7-12 More severe fibrosis was significantly associated with triple- or double- rather than single-agent initial immunosuppression.13 Recipients transplanted for viral-induced cirrhosis are generally felt to benefit the most from steroid withdrawal and reduction of immunosuppression. It has been known for over a decade that LT leads to a marked increase (∼20-fold) in circulating viral titers by 1 month.14, 15 HCV ribonucleic acid (RNA) increased rapidly in patients receiving corticosteroids as part of their immunosuppressive therapy, whereas it continued to decrease in most patients who did not receive corticosteroids. Given the association with treating HCV-positive patients with corticosteroids and the severity of recurrent HCV, one of the most hotly debated issues is how to optimally handle maintenance corticosteroids.12, 16-20 Data suggest that complete avoidance of corticosteroids may be beneficial for patients with HCV infection undergoing LT.20 By contrast, at least 4 studies suggest that “late” withdrawal of corticosteroids (beyond 3 months posttransplantation) may be detrimental to HCV-infected patients undergoing LT.16
Data on the effect of mycophenolate mofetil21, 22 and antibody induction on HCV recurrence are also mixed.16 Whether newer immunosuppressants adversely affect fibrosis progression in patients with recurrent HCV is unknown. Sirolimus is a relatively new immunosuppressant used in both renal transplantation and LT. In vitro, sirolimus inhibits hepatic stellate cell proliferation and may prove effective in limiting fibrogenesis in patients with HCV recurrence.23 Long-term favorable outcomes for HCV patients are most likely to be impacted by development of better therapies for recurrent HCV or to prevent reinfection rather than modifications to current immunosuppressive regimens.16
ACR in LT
ACR is a common problem following LT and may manifest as mild graft dysfunction or, rarely, if severe, graft loss. The histologic hallmark is portal inflammation, bile duct damage and endothelitis (attachment of inflammatory cells to the endothelial surface of portal or terminal hepatic veins).24 Liver biopsy is the gold standard for diagnosis; however, although the presence of cellular infiltrates represents an immunologic response, this does not by itself imply the graft is being rejected.25
The reported incidence of ACR varies widely (24–80%).26 A number of studies have investigated factors associated with ACR.27-31 Given the advances in immunosuppression, the relevance of these studies may be limited today as many were performed at least a decade or more ago. The largest of these studies, from Wiesner et al.,30 identified lower recipient age, creatinine less than 2.0 mg/dL without dialysis, fewer human leukocyte antigen–DR matches, cold ischemia time >15 hours, and donor age >30 yr as being independently associated with early rejection (<42 days after transplantation) Cholestatic liver diseases, particularly primary biliary cirrhosis and autoimmune hepatitis, have been implicated as increasing the risk for ACR.28, 29, 31, 32 Additionally, the type of immunosuppression used after transplantation has also been identified as a risk factor.30, 32, 33
Abbreviations
LT, liver transplantation; RNA, ribonucleic acid; HCV, hepatitis C virus; ACR, acute cellular rejection; IgM, immunoglobulin M.
INCIDENCE OF ACR IN HCV AND IMPACT OF ACR TREATMENT ON OUTCOME
Treatment of rejection during the first transplant year has decreased from 50% in 1995 to 18% in 2004.6 Whether this decrease represents an actual reduction in ACR or the actual treatment of ACR is not known. Potential explanations for this decrease include the introduction of new immunosuppressant medications reducing the risk of ACR, improved recognition of recurrent HCV versus rejection, as well as, a decreased likelihood of treating mild rejection in patient transplanted with diagnoses of HCV.34 However, as is illustrated in Figure 1A, there appears to be an overall reduction in treatment of ACR irrespective of HCV diagnosis. Corticosteroids remain the primary agent to treat ACR in the first year without any apparent differences with regard to HCV diagnosis (Fig. 1B). In contrast, Khalili et al.35 report the results of a survey of large (>45 LT/yr) North American transplant centers. In both 1998 and 2003, ∼40% of centers indicated that treatment of allograft rejection was different in HCV vs. non-HCV patients. In 1998, 75% used corticosteroid boluses to treat mild to moderate rejection, while in 2003 36% reported using corticosteroid boluses. Use of antibodies (OKT3, thymoglobulin, daclizumab) to treat rejection during the first year after transplantation ranges from 15 to 23% in recent years, with a tendency for their use to be higher in non-HCV diagnoses (Fig 1C). There has been a trend since 1994 for less use of OKT3—when it was the primary agent used 27% of the time—to about 5% in 2003.6
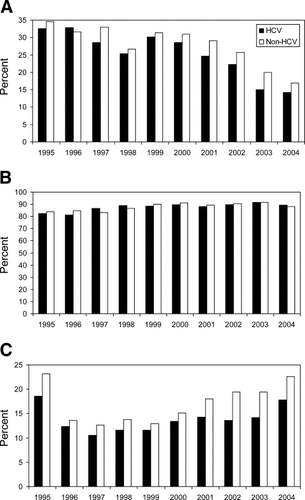
Trends in treatment of rejection 1 yr after LT for HCV (black bars) and non-HCV diagnoses (white bars), 1995–2004. (A) Antirejection treatment overall during first yr posttransplantation, (B) Use of corticosteroid boluses to treat rejection during first yr posttransplantation, (C) Antibody use (predominately OKT3, thymoglobulin, and daclizumab) to treat rejection during first posttransplantation yr. (Source: Analyses prepared by the Scientific Registry of Transplant Recipients, 6/14/06).
Whether patients with HCV are at higher risk for rejection is controversial.36 HCV-positive transplant patients are more likely to develop elevated liver enzymes, triggering more frequent biopsies, so a “biopsy bias” may exist. Wiesner et al.30 reported an incidence of early ACR (<6 weeks) for HCV at 52% in a large multicenter cohort of 762 patients from 1990–1995 (highest rates being for chronic hepatitis B-related liver disease and autoimmune liver diseases). In this study, HCV diagnosis was not independently associated with ACR. Farges et al.31 confirmed the higher rates of ACR in autoimmune diseases (∼60% at 6 months) with lowest rates in virally (HCV and chronic hepatitis B)-related cirrhosis and alcoholic cirrhosis (∼30%). When viral etiologies were broken down, the incidence in HCV cirrhosis was ∼40% at 6-months compared to ∼20% for chronic hepatitis B cirrhosis (contrary to the findings of Wiesner et al.30). Most of the differences between autoimmune vs. viral/alcohol and chronic hepatitis B vs. HCV occurred within the first 30 days of transplant.31 Neuberger et al.37 reported incidence of severe rejection on day 7 protocol liver biopsy in 69% of HCV patients, comparable to autoimmune diseases.
McTaggart et al.38 reported an overall incidence of one or more episodes of early ACR (<6 months, 93% biopsy proven) of 41% in a contemporary cohort of 285 patients at the University of California, San Francisco. The incidence of early ACR was 49% for HCV cirrhosis, comparable for patients transplanted for autoimmune and cholestatic diseases (49%); higher than for alcoholic liver disease (27%) and for chronic hepatitis B cirrhosis (19%). By multivariate analysis, HCV etiology remained a robust risk factor (hazard rate 1.71; 95% confidence interval, 1.17–2.52). Importantly, there was no impact of interferon therapy on the diagnosis of ACR in this cohort. The authors point out that the association between HCV and ACR may represent a situation in which recurrent disease (HCV) is being incorrectly diagnosed as ACR because of more frequent biopsies to evaluate liver enzyme abnormalities and histologic ambiguity. Alternatively, acute HCV reinfection of the allograft may precipitate an antidonor (allogeneic) response. In this regard, our group has demonstrated the presence of recipient-derived HCV-specific CTLs that are restricted by donor alleles.39
Gomez-Manero et al.40 developed a prognostic model to predict development of early (<45 days) ACR and also found HCV as a risk factor for rejection. The only factors independently associated with early ACR were recipient age (younger patients were at greatest risk), Child-Turcotte-Pugh class immediately prior to LT (class A at greatest risk), and etiology of liver disease (HCV cirrhosis, metabolic cirrhosis, and immunologically-mediated cirrhosis at 3-, 12-, and 16-times greater risk, respectively). In terms of misdiagnosis of early ACR instead of HCV recurrence, two-thirds of early ACR episodes occurred in the first 2 weeks post-LT, a time when HCV recurrence is uncommon.
Little doubt exists that treatment of ACR with corticosteroid boluses or OKT3 negatively impacts HCV-infected transplant recipients.41-46 Corticosteroid boluses lead to sudden and dramatic increases in HCV RNA (4–100× pretreatment levels).47 In a homogenous population of genotype 1b–infected patients who received cyclosporine, rejection was significantly more common among patients with allograft cirrhosis versus those without (83% vs. 48%; P = 0.02), with an association between the incidence of cirrhosis and the number of rejection episodes: 5%, 15%, and 50% in patients without rejection, 1 and 2 episodes, respectively (P = 0.001).43
Charlton and Seaberg,42 using the National Institute of Diabetes and Digestive and Kidney Diseases liver transplant database, reported similar rates of early rejection (<6 weeks) between non-HCV and HCV transplant recipients Although an episode of early ACR was protective (relative risk = 0.6; P < 0.007), i.e., associated with a lower cumulative mortality among HCV-negative transplant recipients, the opposite was true for HCV-infected transplant recipients, who experience an increased risk for mortality after an episode of early ACR (relative risk = 2.4; P = 0.03).
PATHOGENIC MECHANISMS OF ACR AND RECURRENT HCV
The mechanisms mediating ACR and recurrence of HCV are distinct, and as such, it should be possible to develop techniques based on these molecular differences that are diagnostically useful. The targets of activated lymphocytes in ACR are the bile duct epithelial cells and the endothelium of the veins and arteries within the liver allograft. Recipient T lymphocytes can recognize and target donor alloantigen through either of 2 pathways.48 In the direct pathway, host T lymphocytes recognize native major histocompatibility complex molecules expressed on graft-associated antigen presenting cells. It is likely that the direct pathway is the predominant mechanism of ACR of hepatic allografts; T cells derived from the direct pathway constitute up to 10% of the total T-cell peripheral pool. In the indirect pathway, host T lymphocytes recognize donor alloantigen-derived peptides in the context of self–major histocompatibility complex molecules expressed on recipient antigen-presenting cells. The activated T lymphocytes secrete cytokines and provide the necessary signals for the maturation of effector cytotoxic T cells.49 The indirect pathway amplifies the rejection process, especially in chronic rejection, as a variety of allopeptides are successively presented by self antigen-presenting cells.48 Although the portal infiltrate in ACR is predominantly comprised of activated T lymphocytes, it also includes B cells (CD20+) and plasma cells (CD138+).50
The mechanisms mediating HCV-related allograft injury are likely multifactorial. The intrahepatic transcriptome response in recurrent HCV following LT is qualitatively similar to the nonimmunosuppressed state with upregulation of Th1, fibrosis, interferon α, apoptotic, and proliferative genes.51 The finding that the signature gene expression of HCV recurrence is typically greater than pretransplant HCV disease has been interpreted to signify that the rapid progression of HCV post-LT is due to the same processes as in nonimmunosuppressed disease.51 However, there is also evidence that a direct cytopathic effect may also be involved in HCV-related allograft injury; in particular, the “cholestatic” variant of HCV recurrence,52 characterized by very high intrahepatic levels of replication and rapid fibrosis development is analogous to “forced overexpression” in experimental models. Recently, gene expression profiling has indicated that upregulation of patterns related to interferon-mediated innate response and antigen presentation was associated with a more benign course of HCV recurrence, and the absence of upregulated gene expression predicted rapid fibrosis development.53 These observations are congruent with the model that HCV-specific T cells serve an important protective function, i.e., are associated with minimal recurrence,54-56 whereas their absence is associated with rapidly progressive HCV recurrence.
DIFFERENTIATING HCV RECURRENCE ALONE FROM ACR PLUS HCV RECURRENCE
Liver Biopsy—Gold Standard?
ACR and recurrent HCV often display a similar clinical picture, with elevated serum transaminases and at times bilirubin in the absence of vascular or biliary complications. While ACR often occurs in the first month post-LT and recurrent HCV greater than a month post-LT, there is significant overlap. Liver biopsy is considered the gold-standard for diagnosing ACR and recurrent HCV; however given histopathological similarities between the two conditions57 (Table 1), discrimination can be difficult. In particular, bile duct injury, a component of ACR, has also been described in chronic HCV infection.58-60
| Recurrent HCV | Rejection | |
|---|---|---|
| Time post-transplant | Anytime; onset usually within first year | Usually in first 2 months |
| Portal inflammation | Most cases | Always |
| Lymphocytes | Bland, uniform | Activated |
| Aggregates | Usually | Occasionally |
| Follicles | 50% of cases | Very rarely |
| Eosinophils | Inconspicuous | Almost always |
| Steatosis | Often | Never |
| Acidophilic bodies | Common | Uncommon |
| Duct damage | About 50% of cases | Very common |
| Atypical features | Cholestasis, ballooning degeneration without significant inflammation, marked ductular proliferation mimicking obstruction, granulomas | Prominent periportal and lobular necroinflammatory activity without subendothelial venular inflammation |
- NOTE: From Rosen HR, Martin P. Liver Transplantation. In: Schiff ER, Sorrell MR, Maddrey WC, eds. Schiff's Diseases of the Liver, 8th ed. Philadelphia: Lippincott Williams and Wilkins, 1999;1589–1615, with permission.
A recent study of 102 consecutive liver biopsies in patients transplanted for HCV found relatively low intra- and interobserver agreement among experienced liver transplant pathologists (Kappa scores 0.20–0.24 for interobserver and from 0.19–0.42 for intraobserver agreement).61 When the distinction is not possible from histology, both ACR and recurrent HCV may be present simultaneously.62 The liver allograft is more tolerant of rejection in contrast to other allografts such as hearts and kidneys, in which episodes of rejection are clearly associated with reduced graft survival.63 Given the fact that corticosteroid boluses and OKT3 have significant deleterious effects in recipients with HCV and since liver allografts can recover from most rejection-related insults,64 some have proposed not treating mild rejection in setting of HCV infection.36, 65, 66 Consideration of the entire posttransplantation clinical course needs to be carefully reviewed, including liver enzyme results in the context of immunosuppression levels and possibly HCV RNA levels, although as described below, the level of replication is not useful in an individual patient.
Demetris et al.65 retrospectively analyzed the accuracy of histopathologic diagnoses and the pathophysiologic relationship between recurrent HCV and rejection. Problematic cases were those with bile duct damage or perivenular inflammation (involving ≤50% of bile ducts or central veins), a finding more prevalent than usually seen with recurrent HCV alone. Their opinion was that such cases should be considered as mild rejection at most and as a secondary diagnosis to recurrent HCV. Patients should be followed closely without changes in immunosuppression with rebiopsy recommended if liver enzymes continue to rise. They recommended that increase immunosuppression should be considered only when rejection is felt to be the primary process (obvious bile duct damage or perivenular inflammation with hepatocyte dropout). In such cases rejection is often graded as moderate to severe.
Hepatic HCV RNA Levels
Correlations between serum HCV RNA levels with recurrent HCV hepatitis have been mixed.14, 67-71 Gottschlich et al.72 retrospectively examined 72 liver biopsies from 36 patients who underwent LT for HCV-related liver disease to determine whether hepatic HCV RNA levels could distinguish recurrent HCV from ACR. They found statistically significant differences across groups (definite/probable ACR, intermediate and probable/definite HCV recurrence), although there was considerable overlap in levels (Fig. 2). The highest HCV RNA levels were found in the recurrent HCV groups, suggesting that low HCV RNA levels argue against recurrent HCV. Whether this information can be clinically useful given that the time to determine hepatic HCV RNA levels is about 48–96 hours compared to 8–24 hours for biopsy results at most transplant centers. Though data on serum HCV RNA is mixed (Fig. 3), Demetris et al.65 cautions about making diagnosis of moderate rejection (>60 days) in patients with HCV RNA levels >10 million IU/mL, as all but 1 (who had 11.8 million IU/mL) of 10 patients, out of 14 with available HCV RNA levels, had levels <10 million IU/mL.
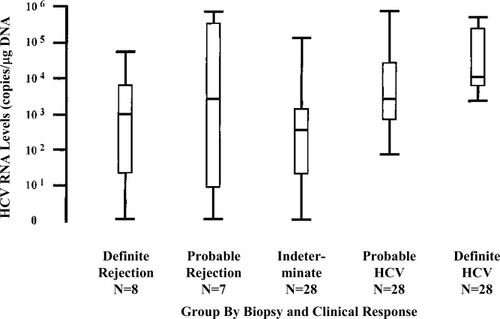
Hepatic HCV RNA levels expressed by group using dot-plots and box (25th to 75th percentiles)-and-whisker (minimum and maximum values) values. Solid bar represents median values. (Gottschlich et al.71 Reprinted with permission of Wiley-Liss, Inc, a subsidiary of John Wiley & Sons, Inc.)
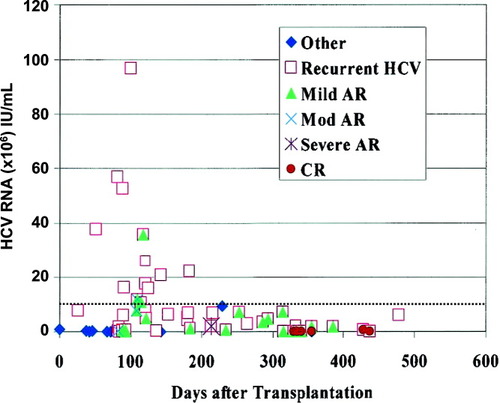
Correlation between quantitative HCV RNA levels and primary histopathologic diagnosis plotted against time after LT. HCV RNA levels obtained within 14 days before or 3 days after corresponding biopsy. HCV RNA levels greater than 10 million IU/mL (dotted line) are almost invariably associated with a histopathologic diagnosis of recurrent HCV alone and very high levels are associated with cholestatic hepatitis. (Demetris et al.64 Reprinted with permission).
Differential Gene Expression Analysis
Considering the mechanistically distinct events that lead to ACR and recurrent HCV, Sreekumar et al.73 used high-density oligonucleotide microarrays to determine the relative expression of more than 6,400 genes during ACR compared to recurrent HCV. They found of the 6,412 genes surveyed, 25 were relatively overexpressed by greater than 2-fold and 15 were relatively underexpressed in the ACR group, compared with the recurrent HCV group. Genes over expressed in ACR included those associated with major histocompatibility complex I and II, apoptosis, and a distinct subset of T cell activating genes. Whether the differential expression noted in this study can be used to distinguish between ACR and recurrent HCV will require further prospective study. However, the time required for this procedure will likely limit its clinical usefulness.
C4d
Another potential marker for differentiating ACR from recurrent HCV is C4d, the end-product of the activated classical complement cascade. Use of C4d has become an important tool in diagnosing ACR in kidney transplantation,74, 75 which has mechanisms involving humoral immunity, rather than the T cell mediated immunity that is thought to be central in LT.76 Traditionally, the liver has been regarded as resistant to antibody-mediated rejection, and postulated reasons include a large vascular bed, with a dual supply able to absorb antibody and complement, and secretion of soluble human leukocyte antigen molecules.77 Schmeding et al.78 reported the use of C4d in ACR after LT. In 34 patients diagnosed with ACR, C4d was positive in 23 (67.7%), whereas in 34 patients with recurrent HCV, C4d was positive in only 4 (11.8%); P < 0.001. With a specificity of 90% and sensitivity of only 68% for ACR, C4d is a supportive, but not a definitive marker of ACR in LT. Moreover, although these data might suggest a more important role for humoral immunity in hepatic allograft rejection than initially appreciated, it is important to recognize that C4d can be activated by nonantibody mechanisms; notably, C-reactive protein and the lectin pathway (mannan-binding lectin and ficolins).79
Anti-HCV Core Immunoglobulin M
Anti-HCV core immunoglobulin M (IgM) are detectable in acute and chronic HCV infection (range 45–90%) and associated with genotype 1, histological grade, alanine aminotransferase (ALT) level and subsequent failure to respond to interferon therapy.80, 81 In the transplant setting, the presence of anti-HCV core IgM has been shown to be associated with development of recurrent hepatitis, but not consistently with severity of recurrent HCV.82-84 Given the association with recurrent hepatitis, Ciccorossi et al.84 evaluated the usefulness of anti-HCV core IgM in differentiating between ACR and recurrent HCV, studying studied 98 consecutive patients transplanted for HCV-related liver disease. Liver biopsy was performed when ALT increased to twice the upper limit of normal. Of 86 liver biopsies, histology was consistent with ACR in 28, recurrent HCV in 44, and doubtful (overlap between ACR and recurrent HCV) in 14. Anti-HCV core IgM levels increased in 36 of 44 cases with recurrent HCV at time of ALT increase, but remained stable or lower in all 28 cases of ACR (P < 0.001). Anti-HCV core IgM increased in 10 of 11 doubtful cases later diagnosed as recurrent HCV. An increasing anti-HCV core IgM had a high degree of diagnostic accuracy, identifying hepatitis recurrence with 100% positive predictive value and specificity (Table 2). Confirmation of these findings in a large, prospective study is needed.
| Increasing IgM anti-HCV levels (Δ ≥ 0.18) Recurrent HCV (%) | Stable (Δ < 0.18) or decreasing IgM anti-HCV levels Rejection (%) | |
|---|---|---|
| Sensitivity | 82 | 100 |
| Specificity | 100 | 82 |
| PPV | 100 | 78 |
| NPV | 78 | 100 |
- Reprinted with permission by Blackwell Publishing Ltd. NOTE: IgM anti-HCV as measured one month before and at time of liver biopsy.84
- Abbreviations: PPV, positive predictive value; NPV, negative predictive value
What Degree of Rejection Should be Treated in Patients with HCV?
When adjusting for other risk factors for survival, early studies suggested that development of ACR was not significantly associated with mortality.30, 37 Since there does not appear to be mortality risk with ACR, particularly mild rejection, it might be best not to treat mild rejection. The diagnostic criteria for rejection and the threshold for treatment of rejection vary significantly between transplant centers.25, 36, 37, 86 McVicar et al.25 describe 41 patients who on protocol liver biopsies had either focal (≤20% portal tracts involved) or mild (20–50% portal tracts involved) rejection that was not treated. A total of 15% (6/41) went on to develop liver function abnormalities within 30 days, and needed treatment. Of the 35 not treated, 7 developed recurrent focal or mild rejection, again without liver function abnormalities. This approach avoided treatment of rejection in the majority (85%) of patients. While 2 of 6 (33%) developed steroid resistant rejection compared to 17 of 68 (25%) in the rest of the population, this difference was not statistically significant. This study points out the importance of distinguishing between “biological rejection” and “clinically significant rejection,”87 illustrating that not all rejection needs to be treated, yet since 15% went on to develop clinical rejection, close follow-up with hepatic enzymes and possibly repeat liver biopsy are warranted. Further support of this approach comes from the Birmingham group37 who found, in a review of 338 protocol 7-day liver biopsies, 109 (32%) with mild rejection (12 considered too unwell to tolerate increased immunosuppression). Of 97 patients with mild rejection, 50 were treated with prednisolone 200 mg daily for 3 days and 47 with no additional immunosuppression. There were no statistical differences in the incidence of subsequent ACR or chronic rejection between those with mild, treated and untreated rejection. The composite data suggest that it is the treatment of rejection with corticosteroids or T cell depletion that leads to diminished outcomes and not the presence of rejection per se. Although many experts have advocated treating only those patients with definitive and moderate-to-severe rejection (Banff score > 4)24 and carefully monitoring those patients with equivocal evidence of rejection or mild rejection by serial laboratory tests and repeat liver biopsy without treatment; the outcome of this approach has not been studied systematically. Moreover, the impact of another common strategy, i.e., increasing the target level of calcineurin inhibitor or adding mycophenolate mofetil, in the patient with definitive moderate rejection, is unknown.
TREATMENT OF HCV AND RISK OF ACR
A potentially serious and controversial complication of antiviral therapy in transplant patients is rejection. Three uncontrolled trials of treatment of recurrent HCV showed ACR rates between 11 and 30%,88-90 higher than randomized controlled trials91, 92 and other uncontrolled trials (0–5%).93-95 Some trials have reported remarkably low rates of rejection during combination therapy.96 The exact prevalence and severity of rejection is thus debatable, and it is unclear whether rejection is more common or severe with pegylated interferon. Concern exists that pegylated interferon may be associated with an increased risk of rejection because of its extended half-life and greater potency. Three uncontrolled trials of pegylated interferon and ribavirin yield conflicting results, with no cases of rejection in 2 studies,94, 95 and a rate of 25% in another.90 A controlled trial of pegylated interferon monotherapy as prophylactic therapy and for treatment of established disease showed no difference in rejection rates between treated and control patients.92 In recent report by Berenguer et al.,97 rejection occurred in 1 (3%) patient receiving standard interferon and in 5 patients (14%) receiving pegylated interferon. Though not statistically significant, this may suggest that use of pegylated interferon is a risk factor for rejection and requires further study. Moreover, data from the University of Colorado suggest that close monitoring of calcineurin inhibitor levels are necessary during and after antiviral therapy, as a greater proportion of virologic responders experienced a greater reduction in immunosuppression levels than nonresponders, presumably related to improved hepatic function and enhanced biotransformation, resulting in lower immunosuppression levels.98
SUMMARY
Immunosuppression in LT needs to be individualized, taking into account etiology of liver disease (immunologic or viral vs. other), risk of rejection, presence of renal dysfunction, and risk of infection. Long-term complications of immunosuppression start with the earliest dose of calcineurin inhibitors. If mild rejection is not associated with graft loss and treatment of rejection with steroids and OKT3 is associated with worse outcome in HCV, it seems simple that we should no longer treat mild rejection.
UNANSWERED QUESTIONS
Although a considerable amount of published material has addressed the issue of rejection and recurrent HCV, the data can be distilled simply: Treatment of ACR is associated with worse outcomes (allograft cirrhosis and mortality) in HCV-infected patients. Unfortunately, at the present time, there are no reliable, noninvasive tools available to distinguish between HCV recurrence (alone) and superimposed ACR, precluding establishment of evidence-based approaches to management. Indeed, liver biopsy, “the gold standard” is fraught with problems related to interpretation. Can the treatment of HCV recurrence that was misinterpreted as ACR lead to improvement of liver enzymes due to its nonspecific antiinflammatory properties? What is the outcome of not treating focal or mild rejection in a patient with HCV? Is there a benefit to avoiding steroids for the treatment of ACR and instead increasing the dose of calcineurin inhibitors or adding mycophenolate mofetil? No one knows the answers to these questions, and they will likely remain unresolved until prospective trials are conducted specifically to address these critically important issues.




