Advances in the diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis
Primary sclerosing cholangitis (PSC) is an idiopathic chronic cholestatic liver disease characterized by inflammatory destruction of the biliary tree. It slowly progresses, resulting in biliary fibrosis and obliteration leading to end-stage liver disease. The most feared complication of PSC is the development of cholangiocarcinoma (CCA).1 The prevalence of CCA in patients with PSC ranges 4.8-36.4%.2-7 PSC is the most common known predisposing factor for CCA in the United Kingdom (lifetime risk 5-15%).8 It has been reported that the incidence of developing CCA in patients with PSC is 0.6-1.5% per year.9, 10 The highest incidence is the first 2 years after the diagnosis of PSC. Indeed, in 37-50% of patients with PSC who develop CCA, the diagnosis of malignancy was made within the first year after the diagnosis of PSC.10, 11 Either only a certain subset of patients with PSC are at risk for the development of CCA and do so early in the course of their disease, or many patients have symptomatic, difficult-to-diagnose CCA at presentation. CCA in patients with PSC has an onset about 3 decades earlier than CCA in patients without PSC.12 The cholangiographic findings and clinical symptoms of CCA are nonspecific and are difficult to differentiate from benign dominant bile duct strictures, which are common in PSC.13 Therefore, CCA in the setting of PSC remains difficult to diagnose. It is often found at autopsy as an advanced stage of disease, or unexpectedly at the time of liver transplantation.10, 12, 14
EPIDEMIOLOGY AND RISK FACTORS
Several risk factors have been proposed to increase the occurrence of CCA in patients with PSC: smoking,15 alcohol,16 older age at onset of PSC,10 and a longer history of inflammatory bowel disease before diagnosis of PSC.11 Unlike the risks of ulcerative colitis and colon cancer, the risk of developing CCA does not appear to be related to the duration of PSC. However, patients with PSC with a history of colorectal dysplasia or cancer in the setting of concomitant inflammatory bowel disease are at increased risk for developing CCA. Ursodeoxycholic acid therapy may reduce the risk of CCA in PSC, as it does for colorectal neoplasia in inflammatory bowel disease.17 In a Swedish study, patients with PSC had an increased risk of pancreatic carcinoma compared with the general population. None of the patients had a known history of chronic pancreatitis.10 However, this finding has not been replicated, and it is difficult to differentiate distal bile duct CCA from pancreatic cancer. Thus, the risk of pancreatic cancer in PSC is unclear.
Abbreviations
PSC, primary sclerosing cholangitis; CCA, cholangiocarcinoma; CA, carbohydrate antigen; MRI, magnetic resonance imaging; DIA, digital image analysis; FISH, fluorescence in situ hybridization.
DIAGNOSIS OF CCA IN PATIENTS WITH PSC
Clinical Features
Extra- and intrahepatic CCA have distinct clinical presentations. Extrahepatic CCAs arise in the left and right hepatic ducts, common hepatic ducts, and common bile ducts, and they present with features of biliary obstruction. Such an identifiable stricture causing biliary obstruction in a patient with PSC is often referred to as a “dominant stricture,” and the problem is differentiating a malignant from a benign inflammatory stricture. Intrahepatic CCA occurs within the hepatic parenchyma, develops as an intrahepatic mass lesion, and frequently presents with advanced signs and symptoms (abdominal pain, weight loss, anorexia). In patients with PSC, CCA usually develops as an extrahepatic tumor rather than as an intrahepatic mass.1 From a cohort of 394 patients with PSC, the mean age at diagnosis of PSC was comparable in patients with CCA (40.7 ± 12.7 years) and without CCA (38.4 ± 15.1 years) (P = 0.192). Jaundice, pruritus, fatigue, and abdominal pain were markedly more frequent among patients with CCA.11 Weight loss, fever, cholangitis, hepatomegaly, splenomegaly, and ascites were present but were not markedly more frequent compared with patients who did not develop CCA.11 On the basis of clinical symptoms alone, it is difficult to differentiate advanced PSC from PSC with superimposed CCA. Many experienced clinicians, however, base their suspicion of CCA on the rate of development of the symptoms described above. The sudden onset of jaundice, malaise, pruritus, and weight loss is more worrisome for CCA that the slow, insidious onset of such symptoms over time.
Serum Tumor Markers
There is no specific tumor marker for the diagnosis of CCA in patients with PSC. The best serum test is the carbohydrate antigen 19-9 (CA 19-9). A CA 19-9 with a cutoff of 129 U/mL provides a sensitivity of 78.6%, specificity of 98.5%, adjusted positive predictive value of 56.6%, and negative predictive value of 99.4%. In this study, only 2 patients with CCA were candidates for curative therapy.18 Thus, a markedly elevated serum CA 19-9 value usually signifies advanced malignant disease. Further, bacterial cholangitis results in elevated serum CA 19-9 values, which diminishes the specificity of this test. CEA alone and in combination with CA 19-9 have been examined in patients with PSC with CCA. Most of these studies concluded that CEA is of little value for the detection of cancer because of low specificity, low sensitivity, or both.19-22 Indeed, CEA can also be increased in inflammatory bowel disease, biliary obstruction, other tumors, and severe liver injury.8 CA-50 and CA-242 have also been studied in a prospective cohort of patients with PSC. The number of patients with CCA when tumor markers were analyzed was too small to permit calculation of sensitivity and specificity, but the authors concluded that tumor markers were of little value in identifying patients with CCA.19 Other serum tumor markers, such as CA-125, CA-195, IL-6, DU-PAN-2, and trypsinogen-2, have been related to CCA, but the clinical role remains to be further determined.8
Imaging Studies
Although ultrasonography remains the first-line investigation for suspected biliary obstruction, in patients without PSC, ultrasonography seldom identifies CCA in PSC.8 For example, it has been reported that definite or probable tumor was seen by ultrasound examination in 5 (25%) of 20 patients who underwent imaging studies within 12 months of pathologic diagnosis. Doppler ultrasonography may identify features of neoplastic involvement, such as loss of blood flow in portal vein branches and/or hepatic arterial branches as a result of cancer encasement of the vessels. Computed tomographic scans may provide better views of CCA mass lesions than ultrasonography. Segmental bile duct dilatation, lobar atrophy, and vascular encasement can all be readily identified by computed tomographic scan. However, like ultrasonography, it may not identify the cancer, and it usually does not define the extent of CCA; abdominal lymphadenopathy is common in PSC and does not necessarily indicate malignant change.23
Magnetic resonance imaging (MRI) studies are considered the optimal noninvasive initial investigation for suspected CCA8 (Fig. 1). MRI has the advantage of being able to depict the biliary tree by MRI cholangiography, vascular anatomy (MRI angiography), and cross-sectional imaging of the liver with a single technique.24 On cross-sectional MRI, intrahepatic CCA appears as a hypointense lesion on T1-weighted images and as moderately intense signal on T2-weighted images.1, 25 We have found that Feridex MRI studies with delayed images after intravenous gadolinium to be very helpful for detecting CCA in patients with PSC (J. Fidler and C.D. Johnson, personal communication). The Feridex renders the liver dark on T1 images, thus permitting identification of desmoplastic tumors. Delayed gadolinium enhancement of the tumor mass has also proven useful for distinguishing between inflammatory vs. neoplastic thickening of the bile duct. Therefore, we consider MRI imaging to be the best test for identifying CCA in patients with PSC.
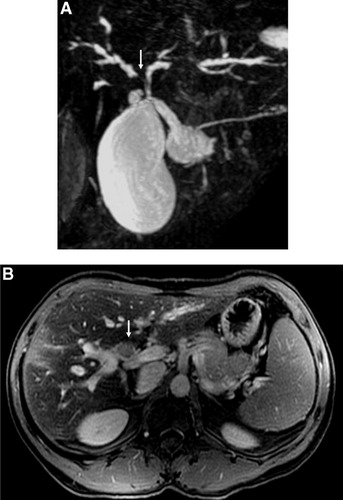
Magnetic resonance studies of a patient with CCA and PSC. (A) Magnetic resonance cholangiogram showing dominant stricture of the right hepatic duct (arrow). (B) Cross-sectional MRI study showing a tumor mass (below arrow).
Cytology
Direct cholangiography with cytologic acquisition is essential for early diagnosis of CCA. Endoscopic retrograde pancreatography allows bile sampling for cytology. Evaluating biliary cytology in patients with PSC is problematic because of the marked inflammatory and reactive changes that can be present.26 In our institution, we prospectively analyzed cytology results in 86 patients with PSC. The overall sensitivity was 17% and the specificity 100%. If we suspected malignancy, samples were also considered positive the sensitivity increased to 39% and the specificity dropped slightly to 97%. In contrast, Furmanczyk et al.27 reported an overall sensitivity and specificity of biliary cytology for malignancy in patients with PSC to be 62.5% and 100%, respectively. If bile duct brushings thought to be suspicious were considered positive, the sensitivity increased to 87.5% and the specificity decreased to 86.2%. The differences between studies may be related to different study populations and methodologies. Nonetheless, routine cytology is not a sensitive technique for the diagnosis of CCA in the setting of PSC.
New diagnostic methods have been developed to improve on cytology results: digital image analysis (DIA) and fluorescence in situ hybridization (FISH) (Fig. 2). They have a higher sensitivity and specificity for the diagnosis of malignant strictures as compared with routine cytology. DIA uses a Feulgen dye to stain DNA. Computer-assisted technology is then used to measure the relative DNA content of individual cells. This technique is thus able to quantitate aneuploidy in small populations of cells. It has been previously reported that DIA is far more sensitive than cytology for the detection of malignancy in suspicious biliary strictures in patients with and without PSC.28
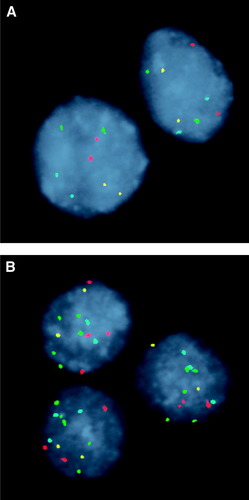
Representative examples of FISH-negative and -positive cases. (A) FISH-negative case in which cells exhibit 2 signals for each of the 4 probes. (B) FISH positive for polysomy with 2 or more copies for 2 or more of the 4 probes. CEP 7 (green), CEP 3 (red), CEP 17 (aqua), and LSI 9p21 (gold).
At Mayo Clinic, we prospectively evaluated 86 patients with PSC; of these, 17 patients (20%) had cancer. DIA had a 43% sensitivity and 87% specificity. We also analyzed the sensitivity and specificity of DIA, in samples where routine cytology was neither positive nor suspicious, because in our practice, this is a common clinical scenario. In this analysis, DIA had a sensitivity of 14% and a specificity of 88% (Fig. 3). Thus, an additional 14% of tumors would be identified by the routine use of DIA while preserving the specificity of routine cytology. FISH represents fluorescently labeled DNA-based probes to detect chromosomal aberrations in cholangiocytes. The available commercial set contains probes that hybridize to the centromere of chromosomes 3, 7, and 17 and to the chromosomal band 9p21 (p16 locus). After hybridization, the cells are stained with the nuclear counterstain (4,6-diami-dino-2-phenylindole), and fluorescence microscopy is used to screen the slide for abnormal cells. A FISH assay is considered positive when 5 or more cells display gains of 2 or more chromosomes (Fig. 2), or 10 or more cells demonstrate a gain of a single chromosome.29
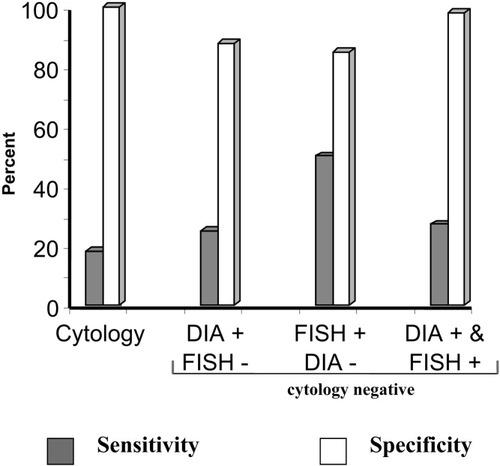
Sensitivity and specificity of cytology, and combination of DIA and FISH-positive or -negative findings when routine cytology is negative for the diagnosis of malignancy in PSC-dominant strictures.
In the same cohort of patients with PSC, we also evaluated FISH. In PSC, FISH had a 70% sensitivity and 86% specificity. We also evaluated these results when routine cytology was neither positive nor suspicious, yielding 60% sensitivity and 87% specificity. When combining FISH and DIA for the analysis, the sensitivity was low, but the specificity was very high—even higher than cytology (positive or suspicious) results (DIA 15%, FISH 98%) (Fig. 3).
SUMMARY
In patients with PSC, CCA is the most dreaded complication. The survival of patients with PSC plus CCA is greatly diminished. Neither a specific serum tumor marker nor any imaging studies have been proved to be 100% accurate in the detection of CCA. However, more recently developed techniques such as DIA and FISH have shown to improve on the results in patients with negative routine cytology. On the basis of these data, we now routinely use these studies for the diagnosis of CCA in PSC (Table 1). Patients should be followed yearly with MRI studies. If there is a rapid change in a focal stricture and/or the rapid onset of jaundice, we advise direct cholangiography (usually by endoscopic retrograde pancreatography), and brushings and biopsies of the dominant stricture. Where available, advanced cytologic studies for aneuploidy should be performed.
| Definitive diagnostic criteria |
| • Biopsy |
| • Positive cytology |
| • Mass lesion |
| • Dominant stricture and CA 19.9>100 U/mL and/or FISH polysomy and/or DIA >1.89 |
| Indeterminate diagnostic criteria |
| • FISH trisomy 7 |
| • Dysplasia |
| • DIA >1.11 and <1.89 |
| • FISH polysomy in absence of dominant stricture |
| • Dominant stricture in absence of mass, positive cytology, biopsy, elevated CA 19.9, or FISH polysomy |
- Abbreviations: CA 19.9, carbohydrate antigen 19-9; DIA, digital image analysis; and FISH, fluorescence in situ hybridization.




