Fatal GvHD as a complication of liver transplantation for undetermined fulminant hepatic failure and associated aplastic anemia
Abstract
Fulminant hepatic failure of unknown origin is the most common cause of fulminant hepatitis with high incidence of aplastic anaemia. Furthermore, the association of liver failure and aplastic anaemia has an increased mortality rate. In this report we describe a 16-month-old boy who presented with aplastic anaemia preceding a non-A, non-B, non-C fulminant liver failure. He developed severe graft versus host disease (GvHD) after liver transplantation, proven by the presence of donor cells in the peripheral blood and in the skin biopsy. He received conventional therapy (steroids, mycophenolate, anti-IL-2 monoclonal antibodies, anti-thymocyte globulin) without success. In an attempt to obtain T cell depletion and reduce the GvHD, he was treated with Alemtuzumab, a first time use for this indication. Aplastic anaemia was extensively investigated, especially exploring the possibility of primary immunodeficiency and reticular dysgenesis which were excluded based on clinical history. However, another form of primary immunodeficiency could be the cause of the uncontrollable proliferation of the donor lymphocytes derived from the liver transplant. Despite aggressive treatment GvHD progressed and the patient died of multiorgan failure. The majority of authors mention aplastic anaemia as a secondary event post liver transplant, whereas in our view this might be a haematopoietic stem cell disorder preceding fulminant hepatic failure. These patients also need to be evaluated extensively in order to exclude a primary immunodeficiency. The underlying disease will determine the choice of immunosuppressive treatment, especially in case of development of GvHD caused by the transplanted lymphocytes inhabiting the donor liver. Liver Transpl 12:1693–1697, 2006. © 2006 AASLD.
Fulminant hepatic failure of unknown origin is the most common cause of fulminant hepatitis,1-3 which can lead to liver failure requiring transplantation. These cases have a high incidence of aplastic anemia (AA),4 particularly in younger patients, in which the incidence rises to 33%.4 The association of liver failure and AA has an increased mortality rate. Non- or partial recovery of the bone marrow after liver transplant has been described before, mostly as a secondary event after orthotopic liver transplant.2, 4-8 In this report, we describe a patient in which the AA preceded fulminant liver failure and who developed a fatal graft vs. host disease (GvHD) after liver transplantation. We discuss the possible pathophysiological mechanisms of GvHD in this case and propose revision of the actual procedure.
Abbreviations
UFHF, undetermined fulminant hepatic failure; POD, postoperative day; GvHD, graft vs. host disease; AA, aplastic anemia, HLA, human leukocyte antigen.
MATERIALS AND METHODS
HLA class I typing of the patient and family members was performed by standard serology and HLA-DR typing by polymerase chain reaction–sequence-specific primers. The patient's HLA type was: A1,2; B8,51, DRB1*03,*11. For mixed chimerism analyses, deoxyribonucleic acid was extracted from whole blood, from CD3+ peripheral blood lymphocytes isolated by immunomagnetic beads, and from a skin biopsy specimen roughly 2 mm2 in size.
The liver donor was typed locally by the recruiting center as A2,29; B39,44; DR4,7.
Semiquantitative HLA-DRB1*04 donor-specific nested polymerase chain reaction was performed as described previously.9 For the blood and the CD3+ peripheral blood lymphocyte fractions, at least 3 individual deoxyribonucleic acid extractions were performed. From each patient's deoxyribonucleic acid sample at least 8 polymerase chain reactions derived from at least 3 separate deoxyribonucleic acid extractions were tested in parallel. DRB1*04-negative control deoxyribonucleic acids were from a pretransplantation patient's blood sample, as well as from an unrelated blood donor with the same HLA-DR phenotype (DRB1*03,*11). The technique was previously applied to analyze microchimerism after liver transplantation and allows the detection of 1 donor cell/105 recipient cells.9 Taking advantage of the HLA-DR incompatibility between patient (DR3,11) and donor (DR4,7), this nested polymerase chain reaction–sequence-specific primers technique specific for the DRB1*04 allele was used to determine the presence of cells of donor origin in peripheral blood after isolation of CD3+ lymphocytes (26 days posttransplantation) and in a skin biopsy (27 days posttransplantation).
Skin samples were fixed in 10% phosphate-buffered formaldehyde, embedded in paraffin, and processed for histological analysis. Sections were cut at 5 μm, mounted onto slides, and stained with hematoxylin-eosin according to standard procedures.
CASE REPORT
A previously healthy 16-month-old boy was transferred to a nontransplanting pediatric intensive care unit with pancytopenia and fulminant hepatitis of unknown origin. Second child of nonconsanguineous parents, he had presented 3 weeks before with jaundice, hepatosplenomegaly, and fever and was followed as an outpatient by his pediatrician for a fortnight. As the patient deteriorated, he was hospitalized in his local institution. There was no family history of jaundice. Serologies for hepatitis A, B, C, human immunodeficiency virus, human herpesvirus-6, human herpesvirus-8, adenovirus, and parvovirus B19 remained negative, while cytomegalovirus and Epstein-Barr virus were compatible with previous infections. Blood and urine were screened for toxic substances, which were all negative. Wilson's disease, α1-antitrypsin deficiency, autoimmune hepatitis, and cystic fibrosis were ruled out by the appropriate tests. Congenital disorders of glycosylation were excluded as serum transferrin level was normal and transferrin isoelectric focusing showed a normal pattern. The non-A, non-B, non-C hepatitis progressed rapidly to acute liver failure with severe coagulopathy, encephalopathy, and with associated AA. He then was transferred to our transplantation unit for evaluation for a liver transplant due to undetermined fulminant hepatic failure (UFHF). At admission, the patient presented with grade I encephalopathy and the following liver functions tests: aspartate aminotransferase, 489 U/L; alanine aminotransferase, 794 U/L; gamma glutamyltransferase, 37 U/L; bilirubin, 345 μmol/L; and factor V, 57%. Liver and bone marrow biopsies showed massive necrosis of the liver without recognizable normal liver structure and severely hypoplastic marrow. To understand the nature of the pancytopenia, bone marrow karyotyping and an immunological workup were done. The karyotype was normal. The peripheral blood lymphocyte profile was compatible with a severe combined immunodeficiency although the clinical history of the patient was uneventful since birth until the present episode (Table 1). At 4 weeks into the disease, he qualified for emergency liver transplantation, reaching the criteria for fulminant hepatitis (factor V: 17%, encephalopathy grade II) with associated AA. To try to improve the granulocytopenia, he benefited from daily subcutaneous granulocyte colony-stimulating factor injections (100 μg) without success. He received a split liver transplant from a 16-year-old girl. Immunosuppression consisted of triple therapy with mycophenolate mofetil (1 gm/1.73 m2), tacrolimus (2 mg/kg/j) and steroids. A single dose (10 mg) of basiliximab (Simulect) was given at postoperative day (POD) 0 and POD 4. Low-dose ganciclovir (5 mg/kg/day) was given for a fortnight as cytomegalovirus prophylaxis. The first 10 days following liver transplantation were marked by continuing aplasia and fever, for which he was intensively monitored and treated with wide spectrum antibiotics, and antiviral and antifungal agents. Candida was detected in stools, and E. cloacae in blood cultures. Severe internal hemorrhages due to severe thrombocytopenia, needing intensive daily platelet replacement complicated the transplant on POD 11. He was stabilized with recombinant factor VII (NovoSeven), fresh frozen plasma, and erythrocyte and platelet transfusions, and on POD 14 underwent an explorative laparotomy and a liver biopsy, which showed discrete steatosis with cholestasis, without any acute rejection. Oral tacrolimus was switched for intravenous cyclosporine. Although the liver tests showed a good function of the graft (aspartate aminotransferase, 190 U/L; alanine aminotransferase, 370 U/L; γ-GT, 89 U/L; bilirubin, 121 μmol/L; factor V, 96%), no improvement in the marrow cytology was seen on the second bone marrow biopsy performed on POD 17: severe hypoplastic bone marrow, with the presence of lymphocytes from both donor and recipient as evidenced by karyotype analysis were found. At this point, cyclosporine was stopped because of the recurrent fever and the high suspicion of infection. A bone marrow transplant was discussed and the members of the family were tested. On POD 22 the course was further complicated with tachypnea, hematemesis, diarrhea, measles-like skin rash, and severe coagulation problems. He remained febrile with a C-reactive protein of 136. GvHD was suspected, which was confirmed by a skin biopsy (Figs. 1), proving the presence of lymphocytes of donor origin, based on HLA donor type (Table 2). Bone marrow karyotype analysis showed an exclusive presence of lymphocytes with the XX karyotype (donor origin) in the bone marrow, and lymphocytes of the donor origin started to appear in the peripheral blood as well. Intensive treatment with daily high doses of steroids (2 gm/m2 [POD 12-28] and anti-interleukin-2 monoclonal antibodies [Zenapax] 2.5 mg/kg/day [POD 21-22]) were started. The latter was replaced by antithymocyte globulin after 2 days because of nonresponse (POD 22-26). Despite this treatment, GvHD progressed to several organ systems: skin, digestive gastrointestinal tract, and lungs. Alemtuzumab (Campath) at 0.2 mg/kg/day for 5 days replaced antithymocyte globulin on POD 25-29. The patient developed a capillary leak syndrome and end-stage renal failure for which he was started on hemodialysis on POD 28. He died of multiorgan failure on POD 30, 9 weeks into the illness.
| Patient | X-SCID phenotype | Normal values for age (range) | |
|---|---|---|---|
| Hemoglobin (gm/L) | 74 | 105-135 | |
| WBC (G/L) | 0.6 | 6-17 | |
| Platelets (G/L) | 42 | 168-392 | |
| Lymphocytes (G/L) | |||
| Total | 0.3 | <2 | 4-13,5 |
| T cells | 0.097 | 0-0.8 | >1.8 |
| B cells | 0.024 | 0-3 | 0.7-1.3 |
| NK cells | 0.026 | <0.1 | 0.7-1.3 |
| Antibodies IgG (UI/L) | |||
| Antitetanus | 2084 (N) | Very poor | Normal |
| Antidiphtheria | 405 (low) | Very poor | Normal |
| Antihemophilus | 2.7 (N) | Very poor | Normal |
| Immunoglobulin (gm/L) | |||
| IgG | 7.93 | Low | 2.93-10.30 |
| IgA | 1.25 | Very low | 0.12-0.88 |
| IgM | 0.95 | Very low | 0.26-1.16 |
| Other tests | |||
| Thymus shadow | Absent | Absent | Present |
- Abbreviations: X-SCID, X-linked severe combined immunodeficiency; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; NK, natural killer; G, giga; N, normal.
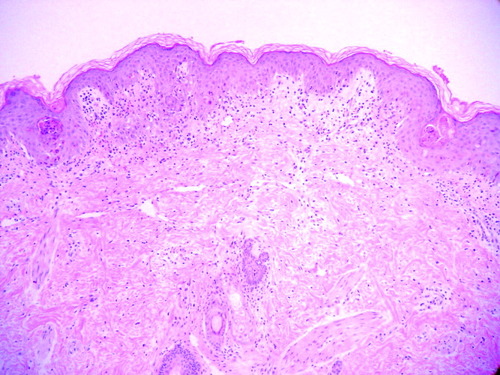
The epidermis shows orthokeratosis and scattered necrotic keratinocytes. In the superficial dermis there is an inflammatory infiltrate composed of lymphocytes mainly located at the dermoepidermal junction and around the hair follicles.
| Origin of DNA samples | HLA-DRB1*04-positive* |
| Pretransplantation patient's PBLs | 0+/8 |
| DRB1*03/*11 control unrelated DNA | 0+/2 |
| No DNA | 0+/1 |
| Posttransplantation patient's whole blood (day 26) | 12+/12 |
| Posttransplantation patient's CD3+ PBL (day 26) | 16+/16 |
| Posttransplantation patient's skin biopsy (day 27) | 8+/8 |
- * Microchimerism expressed as number of DRB1*04-positive PCRs divided by the total number of PCRs.
- Abbreviations: PCR, polymerase chain reaction; DNA, deoxyribonucleic acid; PBL, peripheral blood lymphocyte; HLA, human leukocyte antigen.
DISCUSSION
Our patient presented with concomitant AA and UFHF. The nature of the AA was extensively investigated. Among the possible etiologies, severe combined immunodeficiency and reticular dysgenesis were excluded because of the biology and the clinical history of the patient. However, we can not exclude that some form of preexisting congenital or acquired immunodeficiency, as well as a pluripotent stem cell disorder, was the cause of the uncontrollable proliferation of the liver transplant donor lymphocytes in the patient.10 In the ideal situation, in view of the preexisting possible immunodeficiency or AA, the donor lymphocytes could have eventually repopulated the bone marrow as well. An option was to perform allogenic hematopoietic stem cell transplantation once the patient was stabilized after liver transplantation. His brother was found to be HLA-compatible. Finally, the last possibility would have been a self correction of the hematopoiesis, since in some reports AA resolved spontaneously after the liver transplant.2, 7, 11
Although a majority of the previous literature mentions AA as a secondary event post-liver transplantation, according to Molina et al.,10 AA might precede the UFHF. This assumption is supported by the significant difference between the white blood cell and platelet counts between the patients who subsequently develop AA after liver transplantation and those who do not develop AA. Our patient seems to be a more severe case than was reported in the previous publications. He could be at the extreme end of the spectrum in the group of patients with significantly lower lymphocyte and platelet counts, leading to AA preceding UFHF.
In our patient, the liver transplant donor lymphocytes infiltrated the skin, bone marrow, lungs, and intestines causing severe multiorgan GvHD. Using a pretransplantation blood sample as a negative control we could clearly demonstrate the presence of donor cells (at least 1 donor cell in 3 × 104 recipient cells) in the peripheral blood, as well as in the skin biopsy. The standard procedure in a HLA nonidentical bone marrow transplantation is in vitro T cell depletion of the graft as well in vivo immunosuppression of the host for several months. An HLA-incompatible liver with nonimmunosuppressed lymphocytes was transplanted into a severely immunocompromised child with retrieval of immunosuppression at POD 17, therefore the nonsuppressed donor lymphocytes attacked the “nonself” organs, causing severe, uncontrollable GvHD with capillary leak, subsequent multiorgan failure, and death. It is of note that the liver had no signs of GvHD as the lymphocytes were not attacking a “self” organ. Conventional therapy with high-dose steroids and anti-interleukin-2 antibodies was given without success. Alemtuzumab was used in an attempt to obtain T cell depletion and reduce the GvHD. To our knowledge this is the first time that it has been administered for that specific use in a liver transplantation patient.
To prevent GvHD by the lymphocytes within the transplanted liver, in analogy to T cell depletion of bone marrow, the liver could well be pretreated with in vitro Campath before transplantation in a patient with AA and UFHF.
In conclusion, in a case of concomitant AA and UFHF at initial presentation, if the liver transplant is not depleted of lymphocytes and possibly stem cells, there might be high risk of GvHD progressing rapidly and leading to multiorgan failure and death, even before allogenic hematopoietic stem cell transplantation can be considered. Therefore, these patients should be evaluated extensively to exclude a primary stem cell dysfunction. Furthermore, it might be prudent not to reduce the immunosuppressive regimen after liver transplantation in a patient with AA. 2
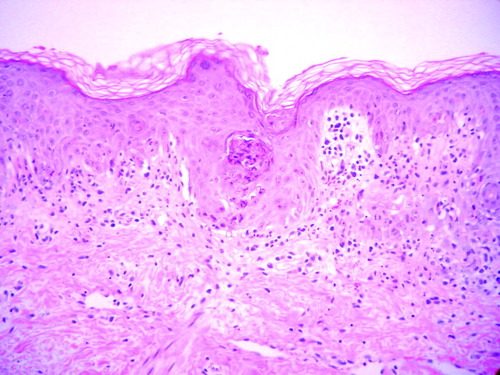
Numerous shrunken, eosinophilic keratinocytes with pyknotic nuclei occasionally accompanied by ≥2 lymphocytes, producing the picture of “satellite cell necrosis,” are seen at the basal and suprabasal layers of the epidermis. There is a sparse lymphocytic infiltrate at the dermoepidermal junction showing basal vacuolar change and small clefts, with exocytosis of some inflammatory cells into the epidermis.
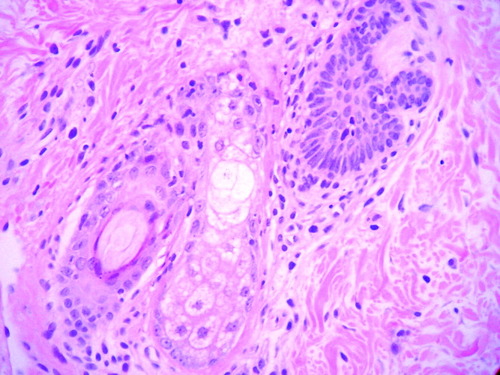
Perifollicular lymphocytic infiltrate with necrotic keratinocytes at all levels of the follicular epithelium and small clefts secondary to the basal damage.
Acknowledgements
We thank Dr. Florence Bettens for performing the microchimerism analysis by nested polymerase chain reaction.




