Impaired erythropoietin production in liver transplant recipients: The role of calcineurin inhibitors
Abstract
Anemia is common following liver transplantation. Because cyclosporine inhibits erythropoietin (Epo) production in experimental models, we investigated whether Epo production was impaired in liver transplant recipients receiving a cyclosporine- or tacrolimus-based immunosuppressive regimen. First, serum Epo levels were measured before and 1 year after transplantation in 35 liver transplant recipients. Second, serum Epo levels were compared in a large series of liver transplant recipients with stable graft and renal functions: 27 receiving a cyclosporine-based and 31 receiving a tacrolimus-based immunosuppressive regimen. A reference group was made up of 22 blood donors and 21 nontransplanted subjects with iron-deficiency anemia. Serum Epo levels were significantly lower after than before liver transplantation, especially in cyclosporine-treated patients. Serum Epo concentrations correlated with hematocrit values in both transplant recipients and control subjects. Using multiple linear regression models, the polynomial relationship between hematocrit and serum Epo values was similar to the control group in patients under tacrolimus, whereas Epo production was significantly reduced in patients under cyclosporine-based immunosuppression. Hematocrit values and the type of calcineurin inhibitor were the only parameters independently related to Epo levels. In conclusion, cyclosporine, but not tacrolimus, inhibits Epo production at the doses used in clinical practice. Liver Transpl 12:1649–1654, 2006. © 2006 AASLD.
Anemia is often observed following liver transplantation, and several factors may contribute to this disorder, including hypersplenism, renal failure, and inflammatory syndrome.1-3 Anemia may also be enhanced by drugs that impair bone marrow function, such as azathioprine, mycophenolate mofetil, or allopurinol. In addition, hemolysis may be superimposed when the antiviral drug ribavirin is used to treat recurrent hepatitis C.4 In patients transplanted for hepatitis C virus–related cirrhosis, viral reinfection is universal and a major cause of graft loss, liver failure, and retransplantation.5 During clinical trials, a substantial percentage of patients treated with interferon or pegylated interferon and ribavirin needed to withdraw because of symptomatic anemia.6 The cause of this anemia, usually more severe than in immunocompetent patients, has been poorly documented.
We hypothesize that immunosuppressive drugs themselves, and particularly calcineurin inhibitors, may increase the degree of anemia by impairing the production of erythropoietin (Epo). Epo production by the human Hep3B cell line was reduced when cyclosporine, but not tacrolimus, was incorporated in the culture medium, a significant amount of Epo remaining unsecreted within cells.7 In contrast, it was reported that deficient Epo production was not responsible for the anemia associated with cyclosporine therapy for insulin-dependent diabetes mellitus in a randomized, controlled trial.8 Epo production has rarely been investigated following liver transplantation.9
To determine whether Epo production was impaired in liver transplant recipients, serum Epo levels were measured before and after liver transplantation in patients receiving either cyclosporine or tacrolimus as the principal immunosuppressive drug. Serum Epo levels were significantly lower after than prior to liver transplantation, especially in cyclosporine-treated patients. These data thus prompted us to compare Epo levels in a larger series of patients receiving cyclosporine or tacrolimus with those seen in nontransplanted controls, either with normal hematocrit (Hte) levels (blood donors) or with iron-deficiency anemia.
Abbreviations
Epo, erythropoietin; Hte, hematocrit.
PATIENTS AND METHODS
Two series of measurements were made. In the first series, serum samples were obtained (with informed consent) from 35 consecutive stable liver transplant recipients recruited between May 2001 and March 2002 and presenting with stable liver and kidney function parameters. The first sample was collected prior to transplantation, and the second was obtained 1 year after transplantation (14 ± 6 months). Following transplantation, patients received initial therapy with methylprednisolone, 10 mg/kg/day intravenously, tapering to 1 mg/kg/day on day 7, and cyclosporine (Neoral) 10 mg/kg/day or tacrolimus (Prograf) 0.075 mg/kg/day, via a nasogastric tube. The cyclosporine dosage was then adjusted to maintain trough whole blood levels between 100 and 200 μg/L, and the tacrolimus dosage was adjusted to maintain trough whole blood levels between 10 and 15 μg/L, until the end of the first year. Fourteen patients were treated with cyclosporine, and 21 received tacrolimus. Seven patients also received mycophenolate mofetil, and 17 patients received an antihypertensive therapy. Data from these patients are given in Table 1.
| Cyclosporine, n = 14 | Tacrolimus, n = 21 | |
|---|---|---|
| Interval after liver transplantation (months) | 14 ± 7 | 15 ± 6 |
| Age (years) | 47.3 ± 9.1 | 49.1 ± 8.3 |
| Gender | 8 male, 6 female | 12 male, 9 female |
| Etiology of liver disease | 4 HCV-related cirrhosis | 6 HCV-related cirrhosis |
| 5 alcoholic cirrhosis | 7 alcoholic cirrhosis | |
| 2 HCV + alcoholic cirrhosis | 2 HCV + alcoholic cirrhosis | |
| 1 HCC + HCV-related cirrhosis | 2 HCC + HCV-related cirrhosis | |
| 2 others | 4 others | |
| Dose of glucocorticoids (mg/day) | 7.1 ± 2.2 | 4.7 ± 1.5 |
| Dose of calcineurin inhibitor (mg/day) | 225 ± 75 | 4.3 ± 3.1 |
| Use of antimetabolite | MMF: 3 | MMF: 4 |
| Antihypertensive drugs | 1-drug: 5; 2-drug: | 1-drug: 7; 2-drug: |
| 2; 3 and 4-drug: 0 | 2; 3 and 4-drug: 1 |
- Abbreviations: HCV, hepatitis C virus; HCC, hepatocellular carcinoma; MMF, mycophenolate mofetil.
In an enlarged series of 58 consecutive transplant patients recruited between February 2003 and July 2004, serum Epo levels were compared in 27 liver transplant recipients under cyclosporine and 31 patients receiving a tacrolimus-based immunosuppressive regimen. Evaluations were performed at a mean of 20 ± 7 months after liver transplantation. Data from these patients are shown in Table 2. The control group was made up of 22 blood donors and 21 subjects with iron-deficiency anemia (serum ferritin level <10 μg/L). Among liver transplant recipients, 30 patients were being treated for arterial hypertension, (monotherapy in 19 cases, bitherapy in 7 cases, and tritherapy or quadritherapy in 4 cases); 18 patients were receiving a calcium channel blocker, 10 a beta-blocker, 14 an angiotensin converting enzyme inhibitor, and 4 were receiving other antihypertensive drugs. No patients received interferon or ribavirin during the present study. All patients and controls had given their informed consent.
| Cyclosporine, n = 27 | Tacrolimus, n = 31 | |
|---|---|---|
| Interval after liver transplantation (months) | 21 ± 7 | 19 ± 5 |
| Age (years) | 52.3 ± 9.6 | 54.0 ± 6.1 |
| Gender | 16 male, 11 female | 19 male, 12 female |
| Aetiology of liver disease | 7 HCV-related cirrhosis | 9 HCV-related cirrhosis |
| 9 alcoholic cirrhosis | 11 alcoholic cirrhosis | |
| 3 HCV + alcoholic cirrhosis | 3 HCV + alcoholic cirrhosis | |
| 2 HCC + HCV-related cirrhosis | 1 HCC + HCV-related cirrhosis | |
| 2 primary sclerosing cholangitis | 2 HBV-related cirrhosis | |
| 4 others | 5 others | |
| Dose of glucocorticoids (mg/day) | 5.1 ± 2.4 | 3.7 ± 2.8 |
| Dose of calcineurin inhibitor (mg/day) | 215 ± 72 | 4.8 ± 2.9 |
| Use of antimetabolite | MMF: 8 | MMF: 7 |
| Mean dose: 1.5 ± 1.0 g/d | Mean dose: 1.5 ± 0.7 g/d | |
| Antihypertensive drugs | 1-drug: 10; 2-drug: | 1-drug: 9; 2-drug: |
| 3; 3 and 4-drug: 1 | 4; 3 and 4-drug: 3 |
- Abbreviations: HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; MMF, mycophenolate mofetil.
All serum samples were stored frozen at −20°C until use. The study protocol complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Ethics Committee for Paris-Cochin.
The “Epo ELISA” enzyme-linked immunoassay kit from Roche Diagnostics (Neuilly-sur-Seine, France) was used, as recommended by the manufacturer. Briefly, 30 μL of a serum sample were mixed with 50 μL immunoreagent solution (anti-hEpo-horseradish peroxidase) on anti-Epo-coated microtiter plates and incubated for 3 hours at room temperature under constant agitation. The plates were washed 3 times, 100 μL of the tetramethylbenzidine substrate solution was added, and the mixture was incubated for 20 minutes at room temperature under constant agitation. The reaction was stopped by adding 25 μL of the stop solution. Photometric measurements were performed at 450 nm, with a 690-nm reference wavelength. A 6-point calibration curve was used to correlate each absorbance value to an Epo concentration. All Epo assays were performed in duplicate. The measurement range of the method was between 3 and 200 mIU/mL Epo. The threshold of Epo detection using this test was 0.24 mIU/mL Epo. The operative was not informed of any immunosuppressive therapy when performing Epo determinations.
Statistical Analysis
The course of biological parameters was described using means and standard deviations. Due to the non-normal distribution of some variables and to the small number of subjects in groups of interest, a Wilcoxon paired test was used to examine changes to these parameters. Two-tailed P values were used. P values below 0.05 were considered to indicate significance.
The precise relationship between Epo and Hte was assessed using multiple linear models. These models made it possible to provide estimated mean parameters of a polynomial (intercepts, slopes, etc.) for each group of patients (tacrolimus, cyclosporine, and controls) and to test null hypotheses that these parameters were equal among the 3 groups. For each model tested, residuals were checked for normality, and the goodness-of-fit was assessed by the R2 statistic. In transplanted patients, we also examined the influence of renal function (assessed in terms of creatinine), any past history of biopsy-proven rejection and significant infections (requiring antibiotherapy), and the use of drugs (mycophenolate mofetil, antihypertensive drugs) on the relationship between Epo and Hte by introducing these variables (and interaction terms) into the multiple linear models constructed as described above.
RESULTS
First Part
Mean serum Epo levels were compared in 35 transplanted patients before and 1 year after liver transplantation. Mean Hte values were higher after (37.3%) than before (33.5%) transplantation (P = 0.0027). Creatinine levels rose significantly after transplantation (114.40 vs. 83.05 μmol/L) (Table 3). Mean Epo values were significantly lower after (15.8 mIU/mL) than before transplantation (29.07 mIU/mL) (P = 0.0145). Moreover, Epo levels appeared to be lower in transplant recipients treated with cyclosporine than in those receiving tacrolimus, although this difference was not statistically significant.
| Epo level before LT (mU/mL) | Epo level after LT (mU/mL) | Hte before LT (%) | Hte after LT (%) | Creatinine before LT (uM/L) | Creatinine after LT (uM/L) | |
|---|---|---|---|---|---|---|
| Cyclosporine, n = 14 | 29.2 ± 27.8 | 12.0* ± 6.5 | 32.4 ± 7.6 | 37.6* ± 3.5 | 83.6 ± 24.6 | 108.2* ± 26.8 |
| Tacrolimus, n = 21 | 29.0 ± 39.8 | 18.4 ± 21.2 | 34.2 ± 6.4 | 36.1 ± 5.2 | 74.6 ± 18.8 | 119.2* ± 34.6 |
| Total, n = 35 | 29.1 ± 22.7 | 15.8 ± 10.1 | 33.5 ± 5.9 | 37.3* ± 4.2 | 83.0 ± 17.8 | 114.4* ± 28.1 |
- Abbreviation: LT, liver transplant; Hte, hematocrit.
- NOTE: Values are expressed as mean ± SD.
- * Wilcoxon paired test, posttransplant vs. pretransplant value, P < 0.05.
Second Part
Because there was a trend toward lower Epo levels in transplant recipients treated with cyclosporine, we sought to compare the effect of the immunosuppressive drugs cyclosporine and tacrolimus on Epo production in a larger series of grafted patients with stable renal function. Epo measurements were performed in 58 liver transplant recipients (27 treated with cyclosporine and 31 with tacrolimus). Serum Epo levels in this patient cohort were compared with those of a reference group composed of 22 blood donors and 21 subjects with iron-deficiency anemia. The mean Epo level was 7.1 mIU/mL in normal subjects, with a mean Hte value in this group of 44.7%. The mean Epo level was 40.1 mIU/mL in patients with iron-deficiency anemia, with a mean Hte value of 28.1% (Table 4). In liver transplant recipients, the mean serum Epo level was lower in patients under cyclosporine (8.4 mIU/mL) than in those receiving tacrolimus therapy (17.3 mIU/mL)(P = 0.016) (Table 4).
| Epo level (mU/mL) | Hte (%) | Ferritin (μg/L) | Creatinine after LT (uM/L) | |
|---|---|---|---|---|
| Cyclosporine, n = 27 | 8.4 ± 7.7*† | 33.6 ± 5.3‡ | 86.7 ± 12.3† | 105.7 ± 21.4 |
| Tacrolimus, n = 31 | 17.3 ± 19.6† | 34.1 ± 5.3‡ | 101.2 ± 9.1† | 109.8 ± 27.8 |
| Controls with anemia, n = 21 | 40.1 ± 39.6 | 28.1 ±4.1‡ | 7.8 ± 5.1 | ND |
| Blood donors n = 22 | 7.1 ± 4.2† | 44.7 ± 3.5 | ND | ND |
- Abbreviations: LT, liver transplant; ND, not done; Hte, hematocrit.
- NOTE: Values are expressed as mean ± SD.
- * Wilcoxon paired test, vs. tacrolimus value, P < 0.05.
- † Wilcoxon paired test, vs. controls with anemia, P < 0.05.
- ‡ Wilcoxon paired test, vs. blood donors, P < 0.05.
As shown in Figure 1, the third-order polynomial relationship between serum Epo levels and Hte values was similar in control subjects and in patients under tacrolimus therapy, whereas the relationship differed in patients under cyclosporine, in whom Epo levels were lower in those with low Hte values.
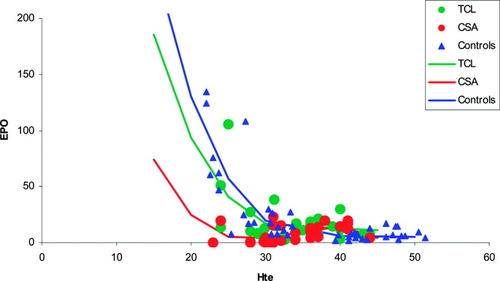
Epo levels as a function of Hte values in stable liver recipients under tacrolimus (TCL; green line and circles) or cyclosporine (CSA; red line and circles), or in control subjects with anemia and low ferritin or in normal blood donors (Controls; blue line and triangles). The polynomial curves were fitted simultaneously in a linear regression model and were as follows: Epo = 809 · 511-63 · 213 × Hte+1,659 × Hte2 − 0,01441 × Hte3 in the TCL group; Epo = 505 · 732 − 47,344 × Hte+1,453 × Hte2 − 0,01441 × Hte3 in the CSA group; and Epo = 994 · 66-73 · 218 × Hte+1,789 × Hte2-0,01441 × Hte3 in the control group. The R2 of the model was 0.73.
In transplanted patients, creatinine had an independent negative impact on Epo production (P = 0.03) but did not interact with the Epo/Hte relationship (neither for patients treated with cyclosporine nor for those treated with tacrolimus). Because antihypertensive drugs can impair cytokine or growth factor production and antimetabolite can increase anemia, we also investigated whether additional treatments could modify serum Epo levels. However, antihypertensive therapy as a whole or treatment with any type of antihypertensive drugs or antimetabolites did not have any effect on Epo values in these subjects. It was also verified that a past history of rejection episodes or significant infections had no influence on the relationship between Epo and Hte.
DISCUSSION
Although Epo levels, and not Epo production itself, were measured, our results suggest that Epo production was reduced in patients receiving cyclosporine. Indeed, the relationship between serum Epo levels and Hte values was similar in control subjects and in patients under tacrolimus therapy, whereas it differed in patients under cyclosporine, in whom Epo levels were lower, especially in patients with low Hte values.
Mean Epo levels in our patients fell significantly after liver transplantation, a result that could be correlated with an improvement in Hte values. Indeed, Epo levels were clearly well correlated with Hte values. However, Hte remained lower in liver graft recipients than in blood donors (Table 4) 1 year after transplantation. Other causes, such as hypersplenism, renal failure, iron deficiency, or metabolic or vitamin deficiencies, may play a role in this phenomenon.10 Some patients harbored a significant degree of renal insufficiency at the time of post–liver transplantation assessment. However, mean creatinine levels were within the normal range in patients receiving both cyclosporine and tacrolimus. These data probably explain why creatinine had a minimal independent influence on serum Epo values in the present work. Folic acid and vitamin B12 levels were not measured; no significant iron deficiency was observed in these patients (Table 4).
We agree that our results may have been biased, since they were not obtained in the setting of a randomized study. However, during both parts of this work, patients were included consecutively, were studied postoperatively after similar intervals (1 year), and were similar in terms of demographic data and associated treatments (Tables 1 and 2).
Cyclosporine has potent immunosuppressive properties, reflecting its ability to block the transcription of cytokine genes in activated T cells. This activity is mediated through the inhibition of the phosphatase activity of calcineurin, which regulates nuclear translocation and subsequent activation of NFAT transcription factor. However, cyclosporine also blocks the NFkB, JNK, and p38 signaling pathways,11 and it induces the transcription of another set of genes, including the gene of TGF beta and genes controlling extracellular matrixdeposition.12, 13 Tacrolimus was introduced more recently and has proved to constitute a more potent immunosuppressive drug.
Cyclosporine and tacrolimus have several common effects on the production of cytokines and growth factors: Both drugs impair the production of interleukin 2 interleukin 3, and IFN gamma, and both have little or no effect on that of interleukin 4 and interleukin 10.14, 15 However, tacrolimus has less or no significant effect on the transcription of the gene of TGF beta and on those coding for extracellular matrix deposition.16 The 2 drugs may thus exert different effects on the signaling pathways and on the transcription of the target genes.
The effects of anticalcineurin drugs on hematopoietic growth factors remain elusive.17 Our data suggest that cyclosporine, but not tacrolimus, may have significantly impaired Epo production. The mechanisms involved in this inhibition are currently under investigation. Impaired Epo production under cyclosporine therapy was not found in the papers by Peter et al.18 or Vasilopoulos et al.19 This discrepancy with our results may be related to technical differences. Indeed, one of the strengths of our study is that Epo levels in liver transplant recipients were compared with those in control patients (with or without anemia), using the same measurement techniques. Moreover, the relationship between Epo and Hte was assessed using multiple linear regression models, thus allowing a comparison of the different groups, whereas this had not been the case in previous papers.
This result may have important consequences if organ transplant recipients suffer from anemia, and particularly in patients receiving antiviral therapy involving ribavirin. Therapy combining pegylated interferon and ribavirin achieved a 21 to 35% sustained virological response.6, 20 However, in those series, a substantial percentage of transplant patients withdrew from treatment because of symptomatic anemia.6, 20 We show here that cyclosporine may increase the degree of anemia by impairing the production of Epo. In these patients, early treatment with high doses of Epo may prevent ribavirin dose reduction and withdrawal and consequently increase the viral response.
In conclusion, our data suggest that Epo production remains dependent upon Hte values in liver transplant recipients, and that cyclosporine, but not tacrolimus, may inhibit Epo production.




