Detection of HCV antigens in liver graft: Relevance to the management of recurrent post-liver transplant hepatitis C
Abstract
The aim of this study was to evaluate how the immunohistochemical detection of liver hepatitis C virus (HCV) antigens (HCV-Ag) could support the histologic diagnosis and influence the clinical management of post-liver transplantation (LT) liver disease. A total of 215 liver specimens from 152 HCV-positive patients with post-LT liver disease were studied. Histologic coding was: hepatitis (126), rejection (34), undefined (24; coexisting rejection grade I and hepatitis), or other (31). The percentage of HCV-Ag infected hepatocytes were evaluated, on frozen sections, by an immunoperoxidase technique. HCV-Ag were detectable early in 57% of cases within 30 days post-LT, 92% of cases between 31 and 180 days, and 74% of cases after more than 180 days. Overall, HCV-Ag were detected more frequently in histologic hepatitis as compared to rejection (P < 0.0001) with a higher percentage of positive hepatocytes (P < 0.00001). In 16 patients with a high number of HCV-Ag-positive hepatocytes (65%; range 40-90%) a clinical diagnosis of recurrent hepatitis (RHC) was made despite inconclusive histopathologic diagnosis. Multivariate analysis identified the percentage of HCV-Ag-positive hepatocytes and the time post-LT as independent predictors for RHC (P = 0.008 and P = 0.041, respectively) and the number of HCV-Ag-positive hepatocytes ≥50% as the only independent predictor for nonresponse (P < 0.001) in 26 patients treated with α-interferon plus ribavirin. In conclusion, HCV reinfection occurs early post-LT, reaching its peak within 6 months. Immunohistochemical detection of post-LT HCV reinfection support the diagnosis of hepatitis when the histologic features are not conclusive. A high number of infected cells, independently from the genotype, represents a negative predictive factor of response to antiviral treatment. Liver Transpl, 2006. © 2006 AASLD.
Hepatitis C virus (HCV)-related end-stage liver disease represents one of the most common indications for liver transplantation (LT) worldwide.1 Almost all patients undergoing LT for HCV-related liver disease show an early graft reinfection, with HCV-ribonucleic acid (RNA) viremia reaching and exceeding pre-LT levels.1-9 In this setting, histologic progression of chronic hepatitis C is more aggressive, resulting in a reduction of survival with respect to non-HCV transplanted patients and may often require retransplantation.10-13
Histopathologic assessment is considered essential for differentiating recurrent hepatitis C (RHC) from acute or chronic rejection. Although typical histologic features of hepatitis C are well defined in the immunocompetent patient, the distinction of the 2 pathologic patterns may be often very difficult in the post-LT immunosuppressed patient. Indeed, both RHC and rejection are associated with the presence of a lymphoid infiltrate and signs of biliary damage, and strong immunosuppressive therapy in the first months after LT can alter the typical histologic features of hepatitis C. Some authors suggest that the presence of an important eosinophil infiltrate14 or the presence of activated and blast lymphoid cells15 might better characterize rejection, but, considering that rejection and RHC may often coexist, the differential diagnosis remains difficult.4, 16, 17
A reliable histopathologic diagnosis is a crucial step in the therapeutic decision process. A wrong diagnosis of rejection may lead to pushing immunosuppression, thus worsening RHC and facilitating a more rapid progression to cirrhosis. Vice-versa, an improper diagnosis of RHC in patients with rejection may lead to tapering immunosuppressive therapy, consequently accelerating the rejection process.16-18 Unfortunately, the reliability of histopathologic assessment in differentiating between RHC and rejection is quite unsatisfactory given the relatively low inter- and intraobserver agreement rates.19-21 In conclusion, histopathology alone cannot be considered the gold standard in this context.
In order to validate the histopathologic diagnosis, virus-related parameters have been evaluated. Some investigators have shown that graft hepatitis is significantly related to a high post-LT viremia,3, 5, 7, 22 but others either have not confirmed those results23, 24 or have found a correlation with viremia but not with intrahepatic HCV replication.2 In addition, it has been reported that genotype 1 is more pathogenic as compared with other genotypes in the post-LT setting,5, 6, 25-27 but this has not been confirmed by other studies.1, 28, 29
Because patients usually remain persistently viremic after LT, the demonstration of the actual graft reinfection requires the direct identification of HCV within hepatocytes. The presence of viral RNA in liver samples has been demonstrated by in situ hybridization30 and reverse transcriptase in situ polymerase chain reaction.31, 32 These methods, however, suffer from technical difficulties and cannot be used routinely.
More data are available on the possibility of identifying HCV antigens (HCV-Ag) in the liver by immunohistochemistry.32-36 Few data, however, exist in the post-LT liver. Some authors suggest that a high expression of HCV-Ag seems to predict virus-mediated liver damage,5-7 but others disagree.2 Using this technique, we have previously demonstrated that the detection of liver HCV-Ag is sensitive enough to be used in clinical practice as a diagnostic tool to detect reinfection of the transplanted liver and that the number of infected hepatocytes correlates with the type of histologic damage.37
The aim of the present study was to evaluate in a large series of post-LT patients how the immunohistologic detection of HCV-Ag could support the histologic diagnosis and, consequently, guide the clinical management of post-LT liver disease.
Abbreviations
LT, liver transplantation; HCV, hepatitis C virus; HCV-Ag, hepatitis C virus antigens; RNA, ribonucleic acid; RHC, recurrent hepatitis C; PBS, phosphate buffered saline. Supported by research funds (ex 60%) of the University of Bologna.
Patients and Methods
Patients and Liver Specimens
We studied 215 consecutive frozen liver specimens from 152 patients (104/48 males/females) that were performed from 6 days to 4 years post-LT (from January 1996 to September 2005), due to the onset of increased serum alanine transaminase and/or bilirubin. All patients were HCV-RNA-positive before LT and all biopsies were obtained before starting any post-LT antiviral treatment. In 49 of these patients at least 2 biopsies were available: 2 in 37 patients, 3 in 10 patients, and 4 in 2 patients. In 118 cases the HCV genotyping was performed and detected genotype 1 in 79 patients, genotype 2 in 25 patients, genotype 3 in 11 patients, and genotype 4 in 3 patients.
The rate of end-of-treatment response was evaluated in 26 patients undergoing liver biopsy within 3 days before starting a 24/48 week antiviral treatment. An end-of-treatment biopsy (not included in the previous series) was available in 14 out of 26 patients.
All patients gave their written informed consent to liver biopsy, which was part of the diagnostic protocol. Liver biopsy specimens were performed percutaneously with 1.4- or 1.2-mm needles. Liver fragments were divided into 2 parts: the first (at least 20 mm) was fixed in formalin and embedded in paraffin for conventional histologic evaluation, the second (length of 7-15 mm) was snap-frozen in embedding media (Tissue Tek O.C.T.; Miles Inc. Elkhart, IN) and stored at −80°C. From each frozen liver sample serial 5-μm-thick sections were obtained. Sections were air dried, fixed in acetone for 5 minutes, wrapped with aluminum foil, and stored at −20°C until use.
Histology coding was made considering the prevalent pattern of liver damage, as follows: hepatitis, rejection, “other” (15 cholangitis/cholestasis, 4 reperfusion damage, 3 nonalcoholic steatohepatitis, 2 steatosis, 2 remodeling, 2 minimal lesions, 1 graft dysfunction, 1 lymphoma, and 1 toxic damage), or undefined (when rejection grade II-III was excluded but both hepatitis and rejection grade I patterns coexisted).
Hepatitis and rejection were defined according to accepted criteria.38, 39 In particular, recurrence of HCV was diagnosed when lobular or interface necroinflammatory activity was more prevalent and prominent than bile duct inflammation and damage. In this study we made no morphological distinction between “acute” and “chronic” hepatitis. In 4 cases out of 34, chronic rejection was identified, in 3 of 4 more than 6 months after LT.
Immunohistochemical detection of HCV-Ag
Before staining, sections were fixed for 5 minutes in chloroform at room temperature. HCV-Ag were detected using a 4-step immunoperoxidase technique with fluorescein isothiocyanate-conjugated human immunoglobulin G from an anti-HCV-positive patient, obtained as previously described.33, 35, 38 The reagent was able to recognize the 4 HCV antigens of RIBA3 (Ortho Diagnostic Systems, Raritan, NJ) strips.40
-
Fluorescein isothiocyanate-anti-HCV immunoglobulin G, diluted 1:15 with 30% normal AB human serum in phosphate-buffered saline (PBS), incubated 60 minutes in a damp chamber at 4°C, followed by a 30-minute PBS wash;
-
Anti-fluorescein mouse monoclonal antibody (DAK-FITC4; Dako A/S, Glostrup, Denmark) 1:50 in PBS, incubated 30 minutes at room temperature in a damp chamber, followed by a 15-minute PBS wash;
-
Anti-mouse, peroxidase-conjugated, rabbit immunoglobulins (Dako A/S, Glostrup, Denmark) 1:50 in PBS containing 5% normal AB blood group human serum, 30 minutes at room temperature followed by a 15-minute PBS wash;
-
Anti-rabbit peroxidase-conjugated swine immunoglobulins (Dako A/S, Glostrup, Denmark), diluted 1:100 in PBS containing 5% normal AB human serum, for 30 minutes at room temperature followed by a 15-minute PBS wash;
-
Tetrahydrochloride diaminobenzidine, 0.5 mg/mL (Sigma Chemical Co., St. Louis, MO) in Tris HCl buffer 0.05 M, pH 7.8, containing H2O2 0.003% for 7 minutes in the dark. Sections were then dehydrated in the series of alcohols and mounted with Eukit (O. Kindler GmbH, Freiburg, Germany).
Controls of specificity were conducted as previously reported and included both blocking experiments, using, in the first step of the procedure, high-titer anti-HCV-positive sera (which abolished the reaction) instead of the human AB blood group serum, and reproduction of the staining pattern using fluorescein isothiocyanate-conjugated immunoglobulins eluted from RIBA3 strips.35, 40
The presence of positive hepatocytes, the percentage of infected cells, and the intensity of staining (scored: 1, faint; 2, medium; and 3, strong) were evaluated by 2 observers (A.G. and G.B.) (Figs. 1 and 2). The data collected by the 2 observers were compared using Friedman analysis: no significant differences were found and mean values were used.
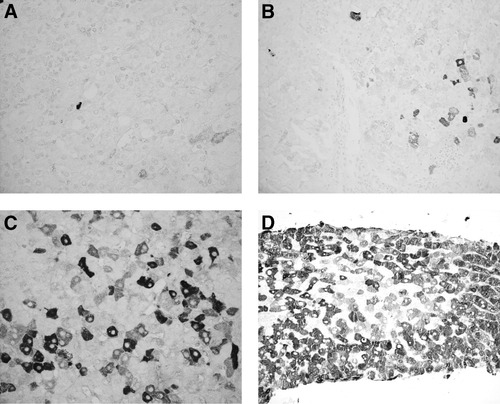
Lobular areas from different liver biopsies stained for HCV-Ag by immunohistochemistry. Cytoplasmic positivity of hepatocytes with different intensity of staining. Original magnification: (A,C) 40X and (B) D 20X. (A) Few hepatocytes faintly positive. (B) A small group of hepatocytes with different intensity of staining. (C) Widespread positivity with different intensity of staining. (D) Widespread positivity, with prevalent strong intensity of staining.
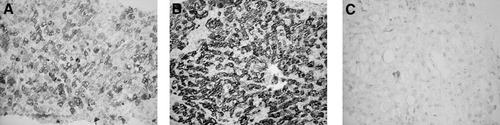
Lobular areas from serial biopsies of the same patient stained for HCV-Ag by immunohistochemistry. Original magnification: 20X. (A) At 24 days post-LT, widespread positivity, with medium intensity of staining. (B) At 47 days post-LT, widespread positivity, with strong intensity of staining. (C) At 146 days post-LT (after 90 days therapy with standard-interferon plus ribavirin), 1 residual hepatocyte faintly positive.
The pathologist (A.D.) who evaluated liver histology was blinded to the result of HCV-Ag immunohistochemical evaluation. Similarly A.G. and G.B. were blinded to the histopathological diagnosis.
Statistical Analysis
Prevalence and median values of different parameters have been compared with chi-squared, Fisher's exact, and Mann-Whitney tests, when appropriate. Linear correlation was evaluated when needed. After univariate analysis, multivariate logistic regression analysis was used to assess the accuracy of variables as predictors of recurrent hepatitis and response to therapy. Differences were considered significant for P less than 0.05. Statistical analysis was carried out with SPSS (SPSS Base 10.0, Application Guide; SPSS Inc., Chicago, IL, 1998).
Results
Data about HCV-Ag expression in 215 specimens are summarized in Table 1. Biopsies were grouped based on the timing after LT: within 30 days (“reinfection” phase), between 30 and 180 days (“acute” phase), and after more than 180 days (“chronic” phase) and were divided according to the histologic diagnosis.
| Histologic diagnosis | <30 days post-LT | 30-180 days post-LT | >180 days post-LT | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No.+ (%) | m % (range) | No. | No.+ (%) | m % (range) | No. | No.+ (%) | m % (range) | No. | No.+ (%) | m % (range) | |
| Hepatitis | 4 | 3 (75%) | 11 (0-70) | 54 | 54 (100%) | 60 (1-98) | 68 | 55 (81%) | 30 (0-90) | 126 | 112 (89%) | 40 (0-98) |
| Rejection | 10 | 6 (60%) | 1 (0-20) | 8 | 6 (75%) | 10 (0-90) | 16 | 8 (50%) | 0.5 (0-90) | 34 | 20 (59%) | 1 (0-90) |
| Undefined† | 12 | 6 (50%) | 2 (0-80) | 8 | 7 (88%) | 45 (0-90) | 4 | 2 (50%) | 27.5 (0-60) | 24 | 15 (63%) | 4 (0-80) |
| Other* | 9 | 5 (56%) | 1 (0-70) | 13 | 9 (69%) | 15 (0-70) | 9 | 7 (77%) | 4 (0-80) | 31 | 21 (68%) | 3 (0-80) |
| Total | 35 | 20 (57%) | 1 (0-80) | 83 | 76 (92%) | 50 (0-98) | 97 | 72 (74%) | 20 (0-90) | 215 | 168 (78%) | 20 (0-98) |
- Abbreviations: No., number of cases; No.+, number of HCV-Ag-positive cases; m %, median percentage of HCV-Ag-positive hepatocytes;
- † Undefined, rejection grade II-III was excluded but both hepatitis and rejection grade I patterns coexisted.
- * Other: 15 cholangitis/cholestasis, 4 reperfusion damage, 3 nonalcoholic steatohepatitis, 2 steatosis, 2 remodeling, 2 minimal lesions, 1 graft dysfunction, 1 lymphoma, and 1 toxic damage.
HCV-Ag were detected in 78% of all liver biopsies (57% and 92% of cases in “reinfection” and “acute” phases, respectively). HCV-Ag were always detectable during the “acute” phase, with a high median percentage of infected hepatocytes (60%, range 1-98%) when the histologic diagnosis was hepatitis. The median percentage of liver HCV-Ag-positive hepatocytes was lower when the histologic diagnosis was rejection, undefined or other (10%, 45%, and 15%, respectively) (Table 1).
In the “chronic” phase 74% of biopsies were found positive for HCV-Ag. In the case of hepatitis, this percentage was 81%, with a reduction in the median percentage of positive hepatocytes (30%; range 0-90%) with respect to the “acute” phase. The median percentage of liver HCV-Ag-positive hepatocytes was lower when the diagnosis was rejection, undefined, or other (0.5%, 27.5%, and 4%, respectively) as compared to the “acute” phase.
On the whole, histologic hepatitis was significantly more frequently HCV-Ag-positive when compared with rejection (89% vs. 59%; P < 0.0001), with a significantly higher median percentage of positive hepatocytes (40% vs. 1%; P < 0.00001).
The evaluation of follow-up clinical and biochemical parameters was concordant with the initial histopathological diagnosis in all but 16 patients (initial diagnosis: undefined in 9, other in 6, and rejection in 1 case), in which conclusive diagnosis was changed into recurrent hepatitis: in all these cases, a high number of HCV-Ag-positive hepatocytes (65%; range 40-90%) was recorded (Table 2).
| Patients | Days post-LT | Histological diagnosis | % HCV-Ag-positive hepatocytes | HCV-RNA (Meq/mL) |
|---|---|---|---|---|
| 1 | 12 | Undefined | 50 | N.A. |
| 2 | 15 | Undefined | 80 | >40 |
| 3 | 23 | Undefined | 70 | >40 |
| 4 | 24 | Other (remodeling) | 70 | >40 |
| 5 | 32 | Other (minimal lesions) | 60 | N.A |
| 6 | 39 | Undefined | 50 | 5.3 |
| 7 | 40 | Undefined | 70 | N.A |
| 8 | 49 | Other (cholestasis) | 70 | N.A |
| 9 | 51 | Undefined | 90 | 0.2 |
| 10 | 97 | Other (cholestasis) | 70 | 13.4 |
| 11 | 105 | Other (cholestasis) | 40 | N.A |
| 12 | 107 | Undefined | 60 | 5.2 |
| 13 | 111 | Undefined | 40 | 5.7 |
| 14 | 113 | Other (reperfusion damage) | 40 | N.A |
| 15 | 153 | Rejection | 90 | N.A |
| 16 | 249 | Undefined | 60 | 17.9 |
- Abbreviations: N.A., not available within 3 days from liver biopsy; Undefined: rejection grade II-III was excluded but both hepatitis and rejection grade I patterns coexisted.
The dynamics of HCV reinfection was similar in the different genotypes (Table 3), although a trend toward a higher median number of HCV-Ag-positive hepatocytes was found in genotype 1 (40%; range 0-95%) with respect to non-1 (10%; range 0-90%) (P = 0.088).
| Days post-LT | Genotype | Number | % of HCV-Ag-positive cases | Median % of HCV-Ag-positive hepatocytes (range) |
|---|---|---|---|---|
| <30 | 1 | 11 | 45% | 0 (0-80) |
| Non-1 | 9 | 44% | 0 (0-70) | |
| 30-180 | 1 | 37 | 97% | 50 (0-95) |
| Non-1 | 16 | 94% | 20 (0-80) | |
| >180 | 1 | 31 | 87% | 30 (0-90) |
| Non-1 | 14 | 64% | 17.5 (0-90) | |
| Total | 1 | 79 | 86% | 40 (0-95) |
| Non-1 | 39 | 72% | 10 (0-90) |
Comparing genotype 1 with non-1, no significant differences were found concerning the final diagnosis of hepatitis (76% genotype 1 and 62% other genotypes; P = 0.16). Moreover, when the diagnosis was hepatitis, the percentage of HCV-Ag-positive cases (95% and 88%, respectively; P = 0.46) and the median percentage of HCV-Ag-positive hepatocytes (50% and 27.5%, respectively; P = 0.06) were not significantly different in both groups. The frequency of a final diagnosis of rejection was similar in genotype 1 (11%) and non 1 patients (10%) (Table 4).
| Diagnosis | Genotype | Number (%) | % of HCV-Ag-positive cases | Median % of HCV-Ag-positive hepatocytes (range) |
|---|---|---|---|---|
| Hepatitis | 1 | 60 (76%) | 57 (95%) | 50 (0-98) |
| Non-1 | 24 (62%) | 21 (88%) | 27.5 (0-80) | |
| Rejection | 1 | 9 (11%) | 5 (56%) | 10 (0-70) |
| Non-1 | 4 (10%) | 3 (75%) | 2.5 (0-90) |
Quantitative HCV viremia at the time of biopsy was available in 124 cases. Qualitative and quantitative serum HCV-RNA was negative in 15 cases, which were also all negative for tissue HCV-Ag. A total of 100 (92%) out of 109 HCV-RNA-positive cases were positive for liver HCV-Ag by immunohistochemistry. A significant correlation between quantitative serum HCV-RNA and the percentage of HCV-Ag-positive hepatocytes was found (r = 0.41; P < 0.001) in the 109 HCV-RNA-positive cases. This correlation was high during the “reinfection” phase (r = 0.80; P < 0.001) and in the “chronic” phase (r = 0.44; P < 0.01), whereas no statistically significant correlation was found in the “acute” phase (r = 0.27; P > 0.05).
When specimens obtained within 180 days post-LT (“reinfection” and “acute” phase) were considered, univariate analysis indicated that recurrent hepatitis was significantly associated with serum HCV-RNA levels (P < 0.00001), percentage of HCV-Ag-positive hepatocytes (P < 0.00001), the intensity of immunostaining (P < 0.00001), the time post-LT (P < 0.00001), and serum bilirubin levels (P < 0.003). Multivariate analysis identified the percentage of HCV-Ag-positive hepatocytes and the time post-LT as the only significant independent predictors of recurrent hepatitis (P = 0.008; confidence interval 1.014-1.094 and P = 0.041, confidence interval 1.001-1.038, respectively). The cutoff percentage of ≥30% HCV-Ag-positive hepatocytes showed a sensitivity of 78%, a specificity of 98%, a negative predictive value of 73%, and a positive predictive value of 98% for the diagnosis of recurrent hepatitis. A number of HCV-Ag-positive hepatocytes (<30%) showed a sensitivity of 100%, a specificity of 57%, a negative predictive value of 100%, and a positive predictive value of 28% for the diagnosis of rejection.
Evaluating the dynamic modifications of HCV-Ag expression in patients who underwent more than 1 biopsy, we found an increase in the median percentage of positive hepatocytes between the first (1%; range 0-70%) and the second biopsy (50%; range 0-95%) in the 16 patients who obtained the first biopsy within 30 days after LT. On the contrary, a decrease of the median percentage of positive hepatocytes between the first (55.0%; range 0-90%) and the second biopsy (27.5%; range 0-85%) was observed in the 22 patients who underwent the first biopsy between 30 and 180 days after LT (Fig. 3).
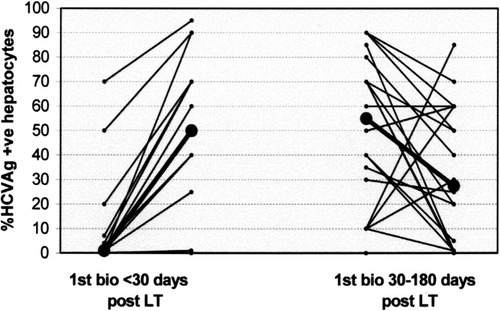
Temporal modifications of the number of HCV-Ag-positive hepatocytes in patients with 2 serial biopsies. On the left, 16 patients first biopsied within 30 days post-LT; on the right, 22 patients first biopsied between 30 and 180 days post-LT. Median values are reported in bold and black.
A total of 26 patients completed a 24/48 week course of therapy with standard or pegylated interferon plus ribavirin. Of these, 11 turned to be HCV-RNA-negative at the end of therapy (end-of-treatment response) (9 within 12 weeks of treatment), while 15 remained HCV-RNA-positive (nonresponders) (Table 5). Pretreatment histology showed hepatic damage of various degree without differences in the 2 groups; in particular, no patient with the severe cholestatic form was treated.
| End-of-treatment virological response to therapy | P | ||
|---|---|---|---|
| Responders | Nonresponders | ||
| Number | 11 | 15 | |
| Male/Female | 8/3 | 12/3 | 0.51 |
| Age; median (range) | 56 (44-67) | 51 (22-63) | 0.09 |
| Start-of-treatment: days post-LT; median (range) | 112 (23-1,187) | 124 (43-1,791) | 0.19 |
| Pretherapy HCV-Ag-positive hepatocytes ≥50% | 4 | 13 | 0.011 |
| Median % of pretherapy HCV-Ag-positive hepatocytes (range) | 30 (3-80) | 65 (3-95) | 0.05 |
| HCV-RNA pretherapy >4 Meq/mL | 7 | 15 | 0.022 |
| HCV-RNA pretherapy (Meq/mL): median (range) | 4.64 (0.41-36.22) | 15.29 (3.40->40) | 0.004 |
| Genotype 1 | 5 | 13 | 0.038 |
| Pegylated/standard interferon | 7/4 | 7/8 | 0.32 |
| Immunosuppressive therapy: tacrolimus/cyclosporine | 7/4 | 11/4 | 0.46 |
Univariate analysis indicated that nonresponse was significantly associated with HCV-Ag positivity ≥50% (P = 0.011), serum HCV-RNA >4 Meq/mL (P = 0.022), and genotype 1 (P = 0.038); multivariate analysis identified percentage of HCV-Ag ≥50% as the only significant independent predictor for nonresponse (P < 0.01; odds ratio, 11.11; 95% confidence interval, 1.79-68.89). When considering only the 18 genotype 1 patients, nonresponse was significantly associated with HCV-Ag positivity ≥50% (P = 0.044) but not with serum HCV-RNA >4 Meq/mL (P = 0.065).
In 4 end-of-treatment-response patients and 10 nonresponders, a biopsy performed at the end of therapy was available: liver HCV-Ag expression was negative in all the end-of-treatment-response patients, and positive in all the nonresponders (median 50%; range 5-70). The number of positive hepatocytes, however, decreased in 6 of the 10 nonresponder cases, whereas it was unmodified in 4 cases.
Discussion
It is widely accepted that graft reinfection after LT for HCV-related liver disease occurs in the vast majority of cases.1-9 Usually, the presence of reinfection is suspected mostly on the basis of histologic features (i.e., focal lobular necrosis)15 and it is considered infrequent during the first months after LT, when rejection seems to represent the main clinical problem. Qualitative determination of HCV-RNA is of little help in the diagnosis of reinfection, since patients become RNA-negative after LT only very rarely. Quantitative viremia, on the other hand, has so far produced conflicting results.3, 5, 7, 22-24 It is almost impossible to diagnose reinfection, when it is not associated to histologic liver damage, unless in situ HCV-RNA30-32 or HCV-Ag32-36 are determined.
We have previously demonstrated that the immunohistologic detection of liver HCV-Ag is sensitive and specific enough to be used in clinical practice as a diagnostic tool to detect reinfection of the transplanted liver.37
With the limitations intrinsic to a retrospective study performed in patients with altered serum parameters, we now have shown that liver reinfection does occur early after LT, as liver HCV-Ag expression is detectable as early as 8 days after LT and 57% of biopsies obtained within 30 days after LT are already HCV-Ag-positive. Reinfection appears to reach its peak (92% of cases) during the “acute” phase and decreases to 74% in the “chronic” phase. This trend in post-LT HCV reinfection has been confirmed by evaluating 38 patients with serial biopsies.
We confirm in a larger series our previous data,37 showing that HCV-Ag are more frequently found in patients with histologic hepatitis with respect to rejection.
We previously made the hypothesis of a possible direct viral cytotoxic mechanism in the presence of early massive reinfection, but this does not explain why massive infection was never found in a patient with severe rejection.
Nevertheless, our data appear to be in agreement with data reporting higher hepatic HCV-RNA levels in patients with recurrent HCV hepatitis with respect to patients with rejection,41 and a low serum HCV-RNA during moderate or severe acute and chronic rejection.39 From a pathogenic point of view, a significant acute and chronic rejection in liver allografts have been associated with a strong T-helper-1-type microenvironment and cytotoxic T-lymphocyte response, which cause rejection and, simultaneously, enhance HCV clearance,42 thus giving a possible physiopathological explanation to this finding. This hypothesis is in keeping with the observation of a detrimental role of steroid pulses and excessive immunosuppression on HCV recurrence.43
As a consequence, the impact of liver HCV-Ag evaluation in the clinical management of patients appears particularly important in the first 6 months after LT. In fact, in 15 out of 118 (13%) biopsies performed in this period, a final diagnosis of recurrent hepatitis was made during the follow-up despite previous inconclusive or discordant histopathologic diagnosis. In all these patients, a high number of infected hepatocytes was detected. Moreover, we showed that a number of HCV-Ag-positive hepatocytes greater than 30% “sensu stricto” confirmed the presence of recurrent hepatitis and “absolutely” excluded the presence of significant rejection. Urgent therapeutic decisions are usually not necessary in this phase, when mild rejection is found. However, we underline that immunohistologic evaluation proved to be useful to promptly confirm the presence of recurrent hepatitis and to exclude significant rejection, thus allowing to assume informed therapeutic decisions and possibly avoiding further biopsies to the patient.
On the contrary, in the “chronic” phase (after more than 180 days after LT) diagnosis was changed in only 1 out of 97 cases (1%) and all the patients have been treated either for rejection or for hepatitis independently of liver HCV-Ag expression. This is in keeping with our previous data in chronic HCV hepatitis, in which we found no relation between histologic activity and the number of infected cells.35
The number of HCV-Ag-positive hepatocytes significantly correlated with serum quantitative HCV-RNA, and this correlation was particularly high during the “reinfection” period, but not during the “acute” phase, when the need to differentiate between recurrent hepatitis and rejection is more frequently perceived. Moreover, only the number of HCV-Ag-positive hepatocytes was found to be an independent predictor for recurrent hepatitis at multivariate analysis. Thus, although quantitative HCV-RNA (a more standardized and readily-available technique than immunohistochemistry) can be used to suspect recurrent hepatitis in the very early phase post-LT, it represents only an indirect parameter. In fact, liver HCV antigens and not HCV-RNA play a role in inducing liver damage, either directly or as a target of immunologic reactions. Moreover, from a technical point of view, immunohistochemical evaluation of liver HCV-Ag expression can be available as early as 6 hours from the liver biopsy, thus allowing an earlier diagnosis and, eventually, an earlier treatment, whereas serum HVC-RNA determination takes at least 48 hours.
Multivariate analysis identified the number of HCV-Ag-positive hepatocytes as the only significant independent predictor for end-of-treatment virological nonresponse. Therefore, even if in clinical practice genotype and viremia represent appropriate tools to evaluate the “a priori” chance of response to treatment, our data suggest that a low chance of response should not be directly related to genotype 1 and/or high viremia, but rather to the number of infected hepatocytes (possibly resulting from complex virus and host interaction), usually higher in genotype 1, as previously reported in chronic hepatitis.35 The fact that response to treatment is more frequent in genotype 1 patients with a low number of infected hepatocytes supports this hypothesis.
In conclusion, we have demonstrated that HCV reinfection occurs early post-LT, reaching its acme within 6 months. During this period the inevitable uncertainty in distinguishing acute cellular rejection from recurrence of HCV has prompted the search for tools able to corroborate histological and clinical information. Immunohistochemical detection of HCV-Ag, demonstrated to be easy to handle and sensitive enough to be used in the clinical setting, might be relevant for the management of transplanted patients, as it supports a clinical diagnosis of hepatitis when histologic features are not concordant or inconclusive and allows us to undertake the adequate treatment earlier. Moreover, in the presence of recurrent hepatitis, a high number of infected hepatocytes represents an independent predictive factor of nonresponse to antiviral therapy.




