Mediators of rat ischemic hepatic preconditioning after cold preservation identified by microarray analysis
Abstract
Hepatic ischemia-reperfusion injury associated with liver transplantation is an as yet unresolved problem in clinical practice. Preconditioning protects the liver against the deleterious effects of ischemia, although the mechanism underlying this preconditioning is still unclear. To profile gene expression patterns involved in hepatic ischemic preconditioning, we analyzed the changes in gene expression in rat livers by DNA microarray analysis. Approximately 116 genes were found to have altered gene expression after 8 hours of cold ischemia. Moreover, the expression of 218 genes was modified by classic preconditioning followed by the same ischemia process. Given the importance of the effects of ischemic preconditioning (IP) in minimizing the liver damage induced by sustained ischemia before reperfusion, this study analyzed the putative genes involved in the beneficial role of IP in liver grafts undergoing cold ischemia before its implantation in the recipient (IP+I). Great differences were found in the gene expression pattern of ischemic preconditioning + long cold ischemia (IP+I) group when compared with the long cold ischemia alone condition (I), which could explain the protective regulatory mechanisms that take place after preconditioning. Twenty-six genes that were downregulated in cold ischemia were found upregulated after preconditioning preceding a long cold ischemia period. These would be genes activated or maintained by preconditioning. Heat shock protein genes and 3-hydroxy-3-methylglutaryl-coenzyme A reductase are among the most markedly induced transcripts. Liver Transpl. 12:1615–1625, 2006. © 2006 AASLD.
Ischemia-reperfusion (I/R) is an unavoidable process in liver transplantation, and protection of the liver against injuries resulting from I/R remains a major problem.1-3 Brief episodes of ischemia and reperfusion elicit organ tolerance to longer subsequent periods of ischemia. This phenomenon, known as ischemic preconditioning, was first described in the heart,4 and it has been also observed in the liver.3, 5, 6
In spite of intensive research, the exact biochemical mechanisms of preconditioning are still a subject of debate because of the complexity of the cellular mechanisms. A variety of substances, including adenosine, nitric oxide, reactive oxygen species, and tumor necrosis factor-α, have been shown so far to play a role both in I/R injury and in the development of the protective effects of liver preconditioning.5, 7-11 However, the traditional biochemical and pharmacological approaches have been insufficient so far to explore the key cellular events in I/R injury and preconditioning.3
DNA microarray analysis followed by powerful data analysis has the potential to uncover previously undescribed genes participating in IP. High-density DNA microarrays that examine expression of many genes simultaneously can not only provide new information about gene function, but also generate insights into transcriptional processes and identify new mechanisms of pathogenesis.12 Overall, the pattern of genes expressed in a cell can provide detailed information about its state.
To profile gene expression patterns associated with long cold ischemia and classic preconditioning, we used the Affymetrix Genechip probe array to monitor transcript levels in rat livers in the hope of identifying new cellular pathways involved in hepatic cold ischemia and ischemic adaptation. In the present study, we provide the first report of large-scale changes in gene expression induced by IP followed by a long cold ischemia period. The focus of this study was to discover the effects of IP on long-term cold ischemia in order to understand IP effects on gene expression that confer protection against preservation injury. Our model may be relevant for the identification of genes whose expression could be the key to preserve livers before its implantation in the recipient.
Abbreviations
IP, ischemic preconditioning; IP+I, ischemic preconditioning + long cold ischemia; ATP, adenosine triphosphate; TGF, transforming growth factor; HSP, heat shock protein.
MATERIALS AND METHODS
Experimental Animals
Male Sprague-Dawley rats weighing 200-250 g were used as a donors and recipients. All animals were anesthetized with isofluorane. Studies were performed following the European Union regulations for animal experiments (EC-guideline 86/609/CEE).
Experimental Design
To evaluate whether IP could induce the activation or inactivation of specific gene expression in a liver graft before and during a long cold ischemia period, animals were distributed into the following 4 experimental groups (Fig. 1): Control (C) (n = 4): Normal livers were flushed with University of Wisconsin (UW) solution. Ischemic preconditioning (IP) (n = 4): To induce IP, the blood flow of the donor liver was interrupted by placement of a bulldog clamp at the portal vein and hepatic artery for 10 minutes, followed by reflow during another 10 minutes.5, 9 Finally, livers were flushed with UW solution. Long cold ischemia (I) (n = 4): Livers were flushed with UW solution and then stored in cold (4°C) UW solution for 8 hours. (4) Ischemic preconditioning + long cold ischemia (IP+I) (n = 4): To induce IP, the blood flow of the donor liver was interrupted by placement of a bulldog clamp at the portal vein and hepatic artery for 10 minutes, followed by reflow during another 10 minutes.5, 9 Then were flushed with UW solution and then stored in cold (4°C) UW solution for 8 hours.
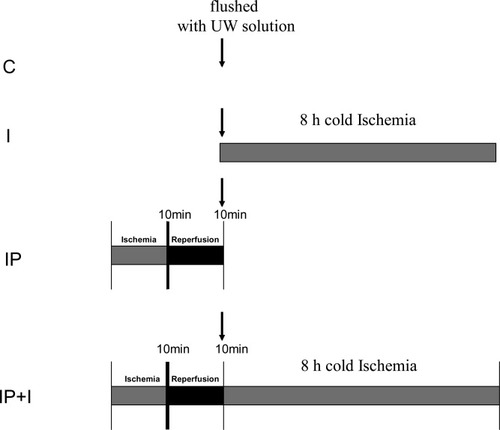
Experimental groups design. C, control; IP, ischemic preconditioning; I, long cold ischemia; IP+I, ischemic preconditioning + long cold ischemia.
RNA Isolation
After the periods indicated in the experimental design for each group, liver samples were collected and stored a −80°C until RNA was isolated. Total RNA was extracted from rat liver tissue using an Rneasy mini kit (Qiagen) following the manufacturer's RNA cleanup protocol. RNA samples of the 4 different groups (C, IP, I, and IP+I) were subjected to microarray study.
Microarray Analysis
Yield of total RNA was determined by obtaining A260 measurement on a spectrophotometer. Sample quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). All samples had 18S and 28S rRNA peaks with no degradation. The total RNA used for fragmentation and labeling was 8 μg. Double-stranded cDNA was synthesized from total RNA. An in vitro transcription was performed to produce biotin-labeled cRNA from cDNA. The Genechip Test 3 Array was used with all different samples. Finally, once prepared, cRNA was hybridized to Affymetrix U34A rat arrays (Affymetrix), which contain more than 7,000 genes and 1,000 expressed sequence tags. The whole microarray process was accomplished at the Microarray Facility “Centro de Investigación del Cáncer” (CIC, Salamanca, Spain).
GeneChip rat U34A Array Set (Affymetrix, Santa Clara, CA) allowed us to monitor the abundance of 8,784 mRNA transcripts representing known genes and expressed sequence tags; 38.7% were found to be present in rat liver.
Data Analysis
Data analysis was performed using Affymetrix GeneChip 3.1 software. All chip data were normalized to a target intensity of 100. We decided to use conservative criteria to differentially identify expression transcripts. Accordingly, 4-fold or greater change in average difference values for all the genes present in rat liver on the microarrays was defined as signifying differentially expressed genes, as stated in the manufacturer's instructions. For each gene, the “absolute calls” for significant fold changes were determined according to Affymetrix algorithms and procedures.
Real-Time PCR
Real-time PCR (QRT-PCR) was performed with the ABI PRISM 7700 sequence detection system (Applied Biosystems). First, 5 μg of total RNA of each sample were reverse transcribed in the presence of random hexamers (Amersham Biosciences) in a total volume of 33 μL by the use of a Ready-To-Go You-Prime First-Strand Beads kit from Amersham Biosciences. After 1/10 dilution of the mix, 0.33 μL were used as template in the QRT-PCR reaction driven by the Taqman Universal PCR Master Mix (Applied Biosystems). Selected genes were specifically amplified by using the following Assay-on-Demand probe/primer sets from Applied Biosystems: Rn00583013 (Hspa1a), Rn00710306 (Igf1), Rn00565598 (Hmgcr), Rn00565555 (Madh1). The relative quantity of the specific RNA in each sample was calculated by the comparative threshold cycle (CT) method, normalized to the endogenous reference β-actin (Rn00667869≪m1, Actb) and expressed as increase or decrease relative to a calibrator (control condition). The method was previously validated by assessing whether the efficiency of the target amplification and the efficiency of the endogenous reference were approximately equal.
RESULTS
Identification of Differentially Expressed Genes
In order to analyze gene expression patterns to elucidate the mechanisms responsible for the useful role of the IP step preceding a long cold ischemia period in the rat liver model, we carried out comparative analyses of global liver gene expression using the Affymetrix GeneChip probe array technology. In our model design, samples were grouped in 4 different experimental conditions: control (C), long cold ischemia (I), ischemic preconditioning (IP), and long cold ischemia preceded by ischemic preconditioning (IP+I) (Fig. 1). Determining which genes are highly or poorly expressed in these different conditions can reveal the extent to which the transcriptional programs of the livers are shared. The importance of sample collection in our study is worth noting. Tissue samples were carefully handled and snap-frozen in order to assure mRNA stability. Otherwise, expression measurements and microarray analysis results would be doubtful.
All samples were hybridized to U34A rat genome GeneChip probe array, and then a comparison analysis was done. Each pairwise analysis consisted of baseline and experimental assays that were compared in order to detect and quantify changes in gene expression. Out of 8,784 total probe sets analyzed, 38.7%, 34.5%, 38.1%, and 42% showed marked intensity for each control (C) and the 3 experimental conditions (I, IP, and IP+I), respectively. Consequently, more than 50% of genes forming part of the analysis were not found in any of the samples. It is of interest to note that the lowest gene expression profile was obtained after long cold ischemia condition. Furthermore, several clones encoding hypothetical proteins or expressed sequence tags having no homology to known proteins exhibited significant up- or downregulation, respectively, vs. the control sample—that is, 5 upregulated and 13 downregulated in ischemia condition, and 13 and 12 in IP condition; and 13 and 19 in IP+I vs. I pairwise comparison. The cellular function of most of these genes and their relationship with hepatic ischemia and preconditioning needs to be elucidated.
Genes displaying differential expression encoding proteins with known functions were grouped into the following: (1) cell organism defense and homeostasis; (2) cell division; (3) cell signaling; (4) cell structure and extracellular matrix; (5) gene expression; (6) metabolism; and (7) transport. Up- and downregulation of interesting pairwise comparisons are described in detail below.
Gene Expression Alteration After Prolonged Cold Ischemia
Relative expression changes in response to long cold ischemia were determined by using the expression profiles of control livers as baseline. The transcriptional response to injury by damaging cold ischemia produced upregulation of 28 genes (Fig. 2, Table 1A). Out of 28 upregulated genes in the ischemic condition, 93% showed increments over 3-fold, being cell signaling (Akt2, Grin2a, Grm4, TRH) and metabolism and transport (Acly, Cyp3A3, Galnt1, Gck, GSTA2, Lox), the 2 categories involving the largest number of genes. In contrast, 44 genes were downregulated. These would be explained considering that some mRNAs could be very sensitive to degradation after 8 hours of cold ischemia (Table 1B). The functions for this group of genes involve mainly stress (Psmb6, 8), signaling (Fkbp1a, IGF-1, Kdr, SRBC), gene expression (Gef2, Hnf-6, Jun, Madh1, Tieg), and metabolic genes (Atp50, Atp5g2, Bckdha, cyb5, Odc1, Pgcp, Sds and different Cyp family members).
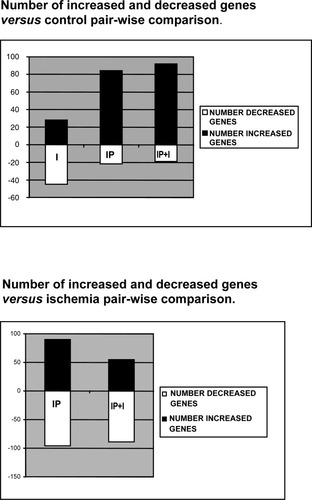
Effect of preconditioning on genetic response to ischemia. Data shown do not include genes for which no functional information was available.
 |
Gene Expression Alteration Due to Preconditioning.
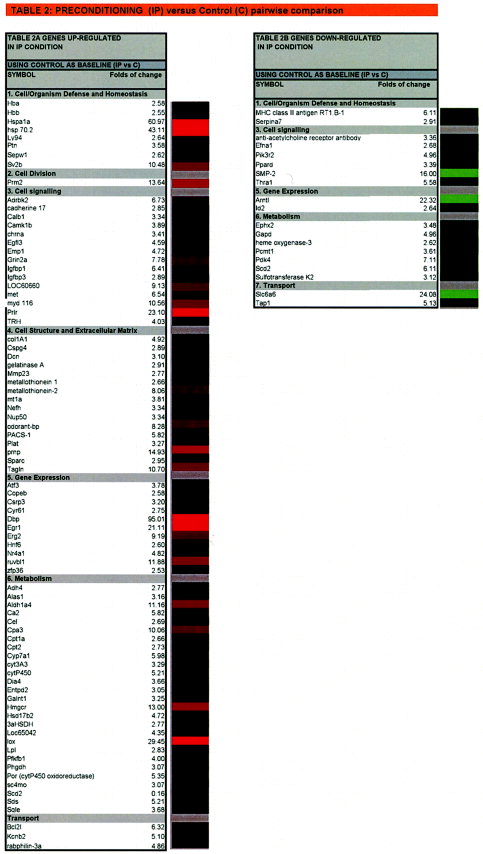
IP caused pronounced changes in gene expression. In response to preconditioning, 84 genes exhibited significant upregulation (Fig. 2, Table 2A). Extensive changes occurred in gene groups that are important for maintaining cell organism defense and homeostasis against stress (Sv2b, Hsp-70), in cell division (Prm2), signaling (Adrbk2, Igfbp1,3, LOC60660, Met, Prlr, Trh), calcium binding (calb1, camk1b, Sparc), cell structure and extracellular matrix (tagh, mt1a, mt2, plat), gene expression (Atf3, Mt1a, Dbp; Egr1,2, HNF6, Nr4a1), and metabolism and transport (Aldh1a4, Ca2, Cpa3, cpt, cyt, Hmgcr, Lox, Lpl, pfkfb1, Sds, Bcl2l, Kcnb2). Out of 21 genes downregulated in response to IP, 77% show more than 3-fold decrease (Table 2B). The most remarkable downregulated genes are from cell signaling (Smp-2, Pik3r2, PPard, Thra1), gene expression (Arntl, Id2), and metabolism and transport (Ephx2, Gapd, Hemox3, Pdk4, Scd2, Slc6a6, Tap1).
 |
Gene Expression Profile in Preconditioning Before Cold Ischemia
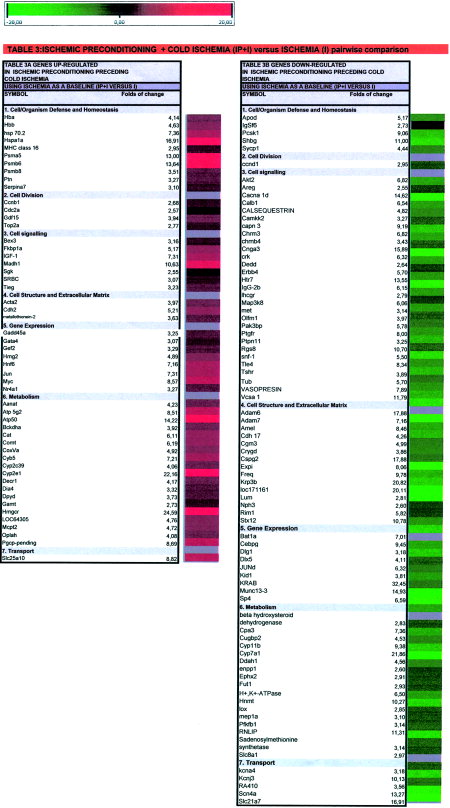
When the effects of preceding IP on cold ischemia (IP+I) samples were compared with cold ischemia alone (I), when this condition was used as baseline, we found 53 genes upregulated and 88 downregulated (Table 3 and Fig. 2). As expected, the main up- and downregulated genes in (IP+I) condition vs. I, appear also in IP vs. I in pairwise comparison (data not shown). There are some remarkable differences in the gene expression pattern of IP+I samples as compared with I condition that are of special interest because they could be involved in the regulation mechanisms and processes that take place during IP to sustain long cold ischemia. Among them, there are 26 genes upregulated in IP+I samples that appeared as downregulated in cold ischemia (I). These would be genes with mRNA transcripts degraded during cold ischemia and activated or maintained by preconditioning previous to cold ischemia. Among these, the most significant upregulated genes are from cell organism defense, stress and homeostasis (HB, Hsp-70, Psma5,6 and 8), cell division (Ccnb1,Cdc2a, Gdf15, Top2a), signaling (Fkbp1a, Igf-1, Madh1, Srbc, Tieg), gene expression (Myc Gata4, Gef2, Hmg2, Hnf6) and metabolism and transport (Atp50, Atp5g2, Cat, Comt, CoxVa, Cyp2c39, Cyp5, Decr1, Hmgcr, Oplah, Metallothionein, Pgcp, Slc25a10). Out of 88 genes downregulated in response to IP+I vs. I, nearly 90% of them showed more than a 3-fold decrease (Table 3B). The most remarkable downregulated genes are from cell organism defense, stress, and homeostasis (Pcsk1, Shbg), signaling (Akt2, Cacna1d, Calb1, Calsequestrin, Capn3, Cnga3, Erbb4, Htr7, IgG-2b, Map3k8, Met, Pak3bp, Ptgfr, Ptpn11, Rgs8, Tshr, Tle4, V7-3, Vasopresin, Vcsa1), cell structure and extracellular matrix (Krp3b, Loc171161, Adam6,7, Amel, Cspg2), gene expression (Bat1a, Cebpg, Munc13-3, Sp4, KRAB, LOC54193), and metabolism and transport (Cpa3, Cyp11b, Cyp7a1, Ddah1, H+,K+-ATPase, Hnmt, Kcnj3, Lox, Pfkfb1, Rnlip, Scn4a, Slc21a7).
 |
Confirmation Gene Chip Array by Real-Time PCR
In order to confirm the differential expression of genes revealed by microarray analysis of rat liver after ischemia with and without preconditioning, several genes were analyzed by QRT-PCR. We selected Hspa1a, Hmgcr, Madh1, and Igf1, whose expression was significantly altered in preconditioned livers, for real-time RT-PCR analysis. Figure 3 shows that Hspa1a, Hmgcr and Igf1 genes were increased after IP and IP+I, and Madh1 in IP+I in concordance with the microarray data.
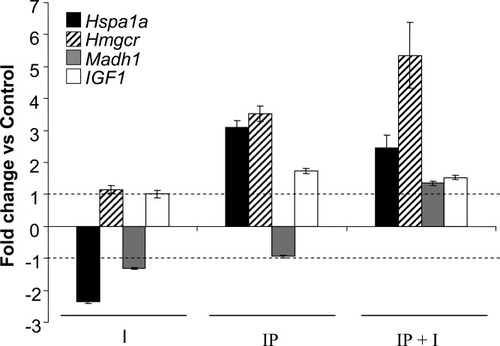
Quantitative determination of transcript levels by real-time PCR. Changes in transcript levels in rat livers during ischemia (I), preconditioning (IP), and ischemia after preconditioning (IP+I) were confirmed by real-time PCR. Results were normalized to the endogenous reference gene β-actin and expressed as fold change vs. control value. The expression of the following genes was determined: Hspa1a, Hmgcr, Madh1, and IGF1. Dashed lines indicate the interval in which changes in expression were not considered significant in relation to control. Average of at least 3 experiments is shown.
DISCUSSION
Hybridization to cDNA microarrays permits the simultaneous parallel expression analysis of multiple genes. Such information is useful for the identification of genes that might have key roles in cold ischemia and/or preconditioning.
When a broad range of genes is examined, it becomes clear that the changes in their expression patterns are strongly dependent on the duration and/or the severity of the ischemic insult. After long cold ischemia, major biochemical changes take place, decreasing the metabolic rate and shifting from predominantly aerobic metabolism to anaerobic glycolysis. The data summarize genes that appear to be important in cold ischemia. Of particular note, 44 genes were downregulated, probably representing proteins that are unnecessary during hypoxic conditions. Different genes encoding proteasome proteins (psmb6, psmb8) and protease inhibitors (spink1) were decreased, probably protecting specific proteins from degradation. Decreased expression of these genes could prolong the half-life of various proteins and lower energy requirements via decrease in adenosine triphosphate (ATP) demands. The evolution of these genes as well as those related with cell cycle (CDK10, a Cdc2-related kinase that may play a role in regulating the G2/M phase of the cell cycle) revealed a pattern of growth arrest. Many genes involved in signaling and gene expression were downregulated (IGF-1, insulin growth factor, inducer of PI3K survival pathway through activation of alpha-serine/threonine-protein kinase (protein kinase B) (AKT); Madh 1 and Tieg, related to transforming growth factor beta (TGF-β) signaling; Fkbp1a, identified as a cytosolic receptor for the immunosuppressant drugs FK506 and rapamycin, and later shown to be involved in cell cycle regulation and intracellular calcium homeostasis; SRBC gene encoding protein kinase Cδ-binding protein, substrate and/or receptor for PK-C, induced by serum starvation) whereas others were increased (Akt2, serine/threonine kinase, mediator of cell survival, induced by IGF-1 and activated by PI3K; Grin2a and Grm4, family of metabolotropic G-protein-coupled glutamate receptors; TRH, thyrotropin; Egr1, early growth response gene family that is induced by ischemia and upregulates chemokine, adhesion receptor, procoagulant and permeability-related genes that participate in the physiological response to various kinds of stress13; and Cugbp2, CUG triplet repeat binding protein that is induced in cells when subjected to stress-mediated apoptosis. The gene product of Cugbp2 is a multifunctional protein that controls various aspects of posttranscriptional regulation of gene expression, including mRNA editing, splicing, stability, and translation.14
Genes involved in mitochondrial metabolism and transport were decreased (cyp2E1, cyp2c39, ATP50, ATP5g2, cyb5, Decr1, cox8A, sic25A10, Bckdha), slowing down oxidative metabolism and depleting cellular ATP, which would be a signal for the increased expression of anaerobic metabolism, redox and conjugating enzymes (Gck, glucokinase; Acly, ATP-citrate-lyase; GSTA 2, glutathione S-transferase, and other transferases such as Galnt1, Siat8b). Cytochromes p450 2c23, 2c39, 2e1, CYP3, all of which are involved in oxidation, were suppressed. When these enzymes are active, they lead to consumption of oxygen. Hence, when tissue is subjected to severe ischemic injury, these enzymes are likely to be suppressed in an attempt to minimize oxidation injury. ATP depletion will also lead to an increase in the cytosolic calcium concentration in cells,15 thus leading to an increase in the expression of Ca2+ binding proteins (Ocm, sparc).
It is well known that hepatic ischemia leads to cell death, either by necrosis or apoptosis, and that the survival of a postischemic liver depends on factors that limit necrosis and/or apoptosis. Previous work on the liver16, 17 and on other tissues18-20 has demonstrated that preconditioning induces a gene program of cell survival. This means hepatic ischemia and reperfusion elicit antagonistic forces, directing the hepatocytes toward either death or survival. It would be important to understand factors that may tilt this balance, which would potentially provide novel therapeutic directions. Our results demonstrate that IP is accompanied by the expression of a genetic program of cell survival that potentially counteracts apoptosis.
The transcriptional response to preconditioning induced genes of defense and homeostasis, such as hsp70, a heat shock protein (HSP) that prevents the denaturation of various intracellular proteins and participates in cell survival.21 Recently, it has been shown the involvement of HSPs in the benefits of IP, demonstrating the induction of HSP70 by preconditioning22 in nonstetatotic and steatotic livers and in an experimental model of reduced-size liver transplantation23 The HSP induction could also be produced by geranylgeranylacetone (an HSP activator), which protected both type of livers at 6 hours of reperfusion, being these effects blocked by quercetin (an inhibitor of HSP70).23
In addition, Sv2b, a gene encoding a protein abundant in synaptic vesicles that resembles transporter proteins, is also upregulated. Genes coding for metabolic enzymes, particularly peptidases (Cpa3), alcohol, aldehyde and glycerate dehydrogenases (Aldh4, Aldh1a4, Phgdh, Hsd17b2), glycolysis (pfkfb1), and redox proteins and enzymes (Lox,Cyp7a1, cyt3a3, cytp450, Dia4, sc4mo) were also increased. These enzymes could reduce intracellular reactive oxygen species, as has been shown for ADH4.24
IP also induced genes that encode proteins related to carbohydrate and lipid metabolism (Lpl, lipoprotein lipase), particularly glycolysis (pfkfb1, responsible for the synthesis of Fructose 2,6-P2, the most potent allosteric effector of PFK-1,25), favoring mitochondrial pyruvate and lipid oxidation (decreasing pdk4, pyruvate dehydrogenase kinase, which is inhibited by phosphorylation26; increasing Cpt1a, Cpt2, carnitine palmitoyltransferases) and, also, microsomal oxidation/reduction of different substrates (cytochromes P450, HMGCoA reductase). We also observed increased expression of hepatoprotective genes, of which HSPs (hsp70.2 and hspa1a) were the most amplified. There was also increased expression of genes that encode insulin growth factor binding proteins (igfbp1, igfbp39), Bcl-2 (which suppresses apoptosis in a variety of cell systems and prevents liver damage after I/R27), and MYD116 (an apoptosis and DNA damage inducible gene that is upregulated in response to ischemia and interacts with Smad 7, recruiting the catalytic subunit of protein phosphatase 1 to dephosphorylate TGF-β receptor I28). Furthermore, genes involved in hepatocyte growth and differentiation were overexpressed: hepatocyte growth factor and prolactin receptors (Met, Prlr), transcription factors such as Egr1 (early growth response protein), hepatocyte nuclear factor 6 (Hnf6), Nr4a1 (steroid nuclear receptor), Dbd (albumin promoter binding protein) and ruvbl1 (TATA-binding protein-interacting protein). As described,29 hepatocyte growth factor may induce the expression of antiapoptotic proteins such as Bcl-2 and Bcl-xl and may also inactivate the proapoptotic Bad protein. Hepatocyte growth factor can also decrease the expression of tumor necrosis factor alpha and interferon gamma, leading to a reduction of inflammation.30 Interestingly, metallothionein 1 and 2 genes, the proteins of which have a high content of cysteine residues that bind various heavy metals, are also increased. Preconditioning has been shown to induce both genes and both molecules have been proposed as detoxifying agents for reactive metals and free radicals, both of which contribute to ischemia-induced cell damage.31, 32
At the same time, the genes from the signaling group were decreased: SMP-2, a critical factor in coordinating phospholipid biosynthesis at the nuclear/ER membrane with nuclear growth during cell cycle. Recent studies have shown that a mouse homologue of Smp2, Lipin1, is involved in the regulation of key transcription factors controlling adipogenesis including the peroxisome proliferator activated receptor (PPARγ) and the CCAAT enhancer binding protein (C/EBPα).33 In agreement with this, PPARγ (PPARd) was also repressed. PPARγ is a nuclear receptor that serves as a transcription factor that regulates several pathways, particularly those involved in lipid metabolism.34 It has been found to be decreased after renal I/R35 and in the preconditioned heart.18 In contrast, pharmacological activation of PPARγ protects the ischemic heart.36 These observations suggest that the role of this factor in ischemic injury and ischemic adaptation is still unclear. Pik3r2, PI3K regulatory subunit; Thra1, thyroid hormone receptor α; and gene expression (Amtl, Id2), metabolism (Gapd, Pdk4, heme oxygenase-3, Scd2), and transport (Slc6a6, Tap1) were further downregulated.
As previously reported,11 IP is able to confer protection against the preservation injury in a liver undergoing 8 hours of cold ischemia. Great differences were found in the gene expression pattern of IP+I samples as compared with the I condition, which could explain the protective regulatory mechanisms that take place after IP. Twenty-six genes downregulated in cold ischemia were found upregulated after IP+I. These would be genes activated or maintained by IP. Among these, we found changes in genes from cell cycle group (cyclinB1, cell division cycle control protein; growth differentiation factor 15, topoisomerase II), in improving cell viability (heat shock protein 70, hemoglobin, pleiotrophin and IGF-1), in protein processing (20S proteasome α5 and α6, and β8 subunits, serpina7 proteinase inhibitor), and in signaling (FK506 binding protein, involved in cell cycle regulation and calcium homeostasis; SRBC, protein kinase C δ binding protein, and SGK, serum corticoid regulated kinase, which plays an important role in cellular stress response). Genes encoding HSPs were the most upregulated. Overexpression of these proteins protects from ischemic insult.21 Most importantly, HSP are directly involved in antiapoptotic mechanisms, inhibiting the activation of caspase-9 and preventing the activation of the JNK pathway.37, 38
Insulin growth factor 1 (IGF-1) is a 70 amino acid growth factor that is the ligand for 2 receptor tyrosine kinases, although it preferentially binds to the widely expressed IGF-1 receptor.39 IGF-1 has several functions, including inhibition of apoptosis, promotion of cell growth, and proliferation of different cell types.40 Exogenous administration of IGF-1 has been shown to be protective in several different experimental conditions, even when given after the ischemic insult.41
Cholesterol appears to be an “acute phase reactant” with tissue levels increasing with either systemic stress (such as oxidative stress, heat shock, sepsis) or direct tissue damage.42 Increased HMGCR protein expression has been observed after sublethal ATP depletion and seems critical to cellular resistance to injury.42 The upregulated values observed for hmgcr gene after IP and IP+I, can contribute to this result. The subcellular mechanisms by which cell injury evokes cholesterol accumulation remain largely undefined.
It is remarkable to find, in these conditions, GDF15 (belonging to the TGF family), Madh1 (Smad1) and Tieg (TGF inducible early growth response) genes upregulated. This means that the TGF-β pathway could play a significant role in this particular situation. GDF15 is a member of the TGF-β superfamily of potent growth inhibitors that initiate apoptosis and suppress tumorigenesis in many cell types. GDF15 is an immediate-early gene that contributes to the initiation of the process of stimulus-induced adaptive change that is induced in hepatocytes by surgical or chemical injury and heat shock.43, 44
Furthermore, genes encoding transcription factors such as Myc, Jun and Gadd45a, which are induced by DNA damage and the hepatocyte nuclear factor 6 (Hnf6), which is important for liver gene transcription, inhibiting pfkfb1 and pepck gene transcription, are also increased. From a metabolic point of view, IP+I-induced genes that encode proteins related to microsomal oxidation/reduction of different substrates, such as cytochrome P450 or HMGCoA reductase; peroxisomal, such as catalase; cytosolic (Diaphorase 4, dihydropyrimidine dehydrogenase) or mitochondrial (Atp 5g2, Bckdha, CoxVa, Decr1). Interestingly, metallothionein 1 and 2 also increased, as with preconditioning.
In contrast, IP+I downregulates a great number of genes, mainly from the following gene groups: cell division (ccnd1), signaling (Met, hepatocyte growth factor receptor; Ptgfr, prostaglandin F receptor; Lhcgr, luteinizing receptor; Chrm3, cholinergic receptor muscarinic 3; Chrnb4, acetyl choline receptor β4; Erbb4, receptor tyrosine kinase; Erbb4, erb B4 receptor tyrosine kinase; Htr7, high affinity serotonin receptor; Tshr, thyroid stimulating hormone receptor; Akt2, serine/threonine kinase activated by PI3K; camkk2, calcium calmodulin kinase 2; Map3k8, mitogen-activated protein kinase kinase kinase 8; Snf-1, Snf-1/AMP-activated protein kinase, metabolic sensor of the eukaryotic cell.45; Ptpn11, protein tyrosine phosphatase; Rgs8, regulator of G-protein signaling; Crk, (protooncogen c-crk); calcium modulation (calcium channel 1d, Ca-binding protein, calsequestrin, calcium-activated neutral protease), cell structure and extracellular matrix and gene expression (C-EBPγ, junD, Sp4), metabolism (pfkfb1, cytochrome P450, histamine-N-methyl transferase, lipase, S-adenosylmethionine synthetase, H+/K+ ATPase) and transport (potassium voltage gated channel, potassium channel, sodium channel, organic anion transporting polypeptide or sodium-calcium exchanger). The decrease in signaling and ion transport induced by IP during cold stress would likely enhance hepatocyte survival.
Most of the mRNA changes caused by IP appear to have potentially beneficial effects. Preconditioning genetically reprograms the response to ischemia and may underlie the basis of tolerance. The genetic profile of ischemic tolerance is characterized by dampened expression of numerous genes involved in metabolism, cell cycle regulation and ion channel function. Decreased transcription of these genes could have functional consequences, restricting ATP turnover and conferring protection during prolonged periods of ischemia. These results suggest suppression of cellular energy use and attenuation of ion channel activity. Similar changes occur when oxygen availability is limited.46 In these situations, controlled arrest of cellular functions preserves cellular homeostasis.47 Specifically, metabolic suppression leads to reduced glucose oxidation, reduced protein turnover and channel arrest. Collectively, these features mimic hypoxia tolerance and suggest that conserved endogenous adaptations to oxygen limitation enhance survival. Understanding the molecular mechanisms involved in these pathways may yield therapeutic strategies for liver preservation.
In short, here we present a number of genes whose expression is markedly altered as a result of long cold ischemia and that could be preserved by preconditioning. Although some of the genes have previously been shown to play a role in I/R injury in other tissues, this is the first demonstration that most of the genes presented here are involved in the model described. The newly identified genes might contribute to elucidate the mechanisms involved and optimize the protection elicited by IP in liver grafts undergoing cold ischemia before its implantation in the recipient.
Acknowledgements
We are grateful to Dr. F. Ventura and J.L. Rosa for reading the manuscript and providing helpful comments, Dr. E. Castaño for helpful technical advice and E. Adanero for skilful technical assistance. We thank Robin Rycroft at the language advisory service of the University of Barcelona for revising the English text.




