“Hepar divisum”– as a rare donor complication after intraoperative mortality of the recipient of an intended living donor liver transplantation†
See Editorials on Pages 358 and 361
Abstract
In this study, we present our experience with 4 healthy donors having the rare condition of “hepar divisum” after the intraoperative death of the recipient of an intended right adult living donor liver transplantation (LDLT). The study included 4 donors and 4 intended right LDLT recipients affected by neuroendocrine tumor (n = 2), hepatocellular carcinoma (n = 1), and cryptogenic cirrhosis (n = 1). All 4 recipients died intraoperatively. At the time of recipient death, the dissection of the donor liver parenchyma was complete, the vessels intact, and the hepatic duct(s) already divided. In each case, reconstruction of the donor biliary tract was performed: hepaticojejunostomy (HJ) in 2 cases, each with 3 ducts, and duct-to-duct anastomosis in 2. Of the donors receiving the HJ, 1 had a cut surface bile leak and the other experienced an anastomotic leak, treated by percutaneous drainage and reoperation, respectively. The latter patient experienced recurrent HJ stenosis at 44 months and 50 months after the operation and was treated by percutaneous balloon dilatation. One duct-to-duct reconstruction was complicated by early stenosis (postoperative day 6) and treated with endoscopic stenting. In conclusion, in the case of intraoperative death of the recipient of an intended LDLT, when the parenchyma and the hepatic duct of the donor have already been divided, the options are completion of the donor hepatectomy or the status of “fegatum divisum” with reconstruction of biliary tract. The high incidence of biliary complication, however, is of concern. When more than 1 hepatic duct is present, the donor hepatectomy should be completed and the graft reallocated according to the policy of the transplant institution. Liver Transpl 12:428–434, 2006. © 2006 AASLD.
The most serious ethical concerns in living donor liver transplantation (LDLT) focus on the risks to the donor and relate to the principle of “do no harm.” There is extensive literature focused on the incidence and type of complications after living liver donation, although a clear definition of what should be considered a “complication” is lacking.1, 2
Typically reported complications are biliary fistula,3 gastric stasis/dyspepsia,3, 4 wound infection,3 abdominal wall hernia,3 pleural effusion,3 pulmonary embo-lism,5 bleeding,6 psychosocial problems,7 postoperative liver dysfunction,3 and donor need for liver transplantation.1, 8, 9 The right hepatectomy has been associated with higher rate of complications in comparison to left and left lateral hepatectomies.3
We herein report on the outcome of 4 donors who ended up with the status of “hepar divisum” (“divided liver”) after attempted LDLT (intraoperative death of a recipient of an intended right adult LDLT). To the best of our knowledge the status of iatrogenic hepar divisum as consequence of resection of liver parenchyma in a healthy individual donor for a planned living donor liver transplant has never been reported. There are no set rules for how to proceed in these situations, and the main goal of our report is to present this unique experience and the complications that occurred in the attempt to reestablish biliary drainage. Surgeons facing this most unusual situation must consider the ethical issues presented by the scenario as well as the pros and cons of biliary reconstruction vs. the alternative of removing the resected hemiliver.
Abbreviations
LDLT, living donor liver transplantation; HJ, hepaticojejunostomy.
PATIENTS AND METHODS
Recipients
Four male recipients with a median age of 55 years (range, 48–60 years) affected by neuroendocrine tumor metastasized to the liver (n = 2, Child-Turcotte-Pugh Class A), hepatitis C virus–cirrhosis with multifocal hepatocellular carcinoma exceeding Milan criteria (n = 1, Child-Turcotte-Pugh Class A) and cryptogenic cirrhosis (n = 1, Child-Turcotte-Pugh Class C) were evaluated for a right LDLT according to our standardized protocol.10, 11 The standard pretransplant cardiopulmonary evaluation was completed in all 4 recipients with electrocardiogram, transesophageal echocardiography, stress electrocardiogram, myocardial scintigraphy, pulmonary function tests, and cardiology consult. No pathological findings were detected, and the recipients were found to be suitable candidates for liver transplantation.
Donors
The 4 donors (3 male and 1 female; median age, 27 years [range, 20–52 years]) underwent our standardized multistep evaluation protocol for right lobe liver donation.12
All donors were properly informed of the risks of both surgical procedures (donor and recipient) and their potential complications. These discussions included information regarding potentially suboptimal results in cases of extended indications for transplant and, specifically, the risk of the recipient's death. As German law allows only for transplantation of the donated organ into the intended recipient, none of the donors was asked about the possibility of allocating the graft to a different recipient in case of the intraoperative death of the intended recipient.
Methods
The donor's and recipient's operations were performed according to the techniques already described by our group.10, 13 The operations were usually timed so as to minimize long graft ischemia and to avoid longer operative time for the recipient. For this reason the donor hepatectomy and the recipient total hepatectomy were completed almost simultaneously by 2 different surgical teams. When the recipient was affected by a malignant process, an exploratory laparotomy to exclude extrahepatic disease preceded the donor operation. Once this was accomplished, the actual recipient hepatectomy was started according to the standard protocol to partially overlap with the donor surgery. When a prolonged anhepatic phase occurred a temporary porto-caval shunt was performed.13
In the 4 cases reported, 3 recipients underwent preliminary exploratory laparotomy. A porto-caval shunt was performed in 2 cases and venovenous bypass utilized in one. The recipient operation was started an average of 4 hours after the initiation of the donor surgery. At the time of recipient's death the hepatic duct(s) had already been transected in all 4 donors.
Intraoperative and postoperative data regarding donors and recipients were collected prospectively and analyzed, with particular attention to the anatomy of the hepatic duct, the methods of biliary reconstruction, the incidence of early and late donor complications, and the last available clinical information regarding each donor.
RESULTS
From April 1998 to June 2005, we performed 150 LDLTs, 116 adult right LDLTs, 9 left adult LDLTs, and 25 left lateral paediatric LDLTs. In 4 cases of adult-to-adult right LDLT, the donor's operation was aborted because of intraoperative death of the recipients. Two recipients died before the anhepatic phase because of unexplained vascular collapse during the liver mobilization (recipients 1 and 3) and 2 during the anhepatic phase, one because of lung embolism and the other because of myocardial infarction (recipients 2 and 4). In the latter recipient a systemic porto-caval shunt (venovenous bypass) had already been constructed.
Our standard pretransplant evaluation was completed in all 4 recipients. No significant cardiopulmonary pathology was detected, and all 4 recipients were found to be suitable candidates for liver transplantation. Review of the preoperative evaluation and of the intraoperative anaesthesia records for each recipient showed nothing abnormal until the time of cardiac arrest. Table 1 summarizes the recipients' results.
| Recipient | Age | Sex | Liver disease | Child-Turcotte-Pugh Class | UNOS | Risk Factors | Cause of Death | OP Phase |
|---|---|---|---|---|---|---|---|---|
| 1 | 55 | Male | NET-metastasis | A | 3 | Neuroendocrine heart | Acute heart failure | Liver mobilization. |
| 2 | 48 | Male | NET-metastasis | A | 3 | Thrombus portal vein | Lung embolism | Anhepatic |
| 3 | 60 | Male | Cryptogenic. | C | 2B | Pulmonary hypertension | Acute heart failure | Liver mobilization |
| 4 | 55 | Male | HCV + HCC | A | 3 | None | Myocardial infarction | Anhepatic |
- Abbreviations: UNOS, United Network for Organ Sharing; OP, operation; NET, neuroendocrine tumor; HCV, hepatitis C virus; HCC, hepatocellular carcinoma.
The demographics and volumetric data of the 4 donors are summarized in Table 2. In all 4 cases the donor operation was well under way at the time of the recipient death with the hepatic duct(s) divided and the liver parenchyma already fully or almost completely resected. In all cases, the vascular pedicles were still intact. In each donor, we decided not to complete the donor hepatectomy but instead to maintain the status of hepar divisum with reconstruction of biliary tree (Table 3).
| Donor | Age | Sex | Relation | % of Resection | RLVBWR |
|---|---|---|---|---|---|
| 1 | 52 | Male | Brother | 61 | 0.8 |
| 2 | 21 | Male | Son | 63 | 0.6 |
| 3 | 33 | Female | Daughter | 68 | 0.7 |
| 4 | 20 | Male | Son | 60 | 0.7 |
- Abbreviation: RLVBWR, remnant liver volume body weight ratio.
| Donor | Number of Transected Right Ducts | Reconstruction | Stent | Early Complications | Treatment | Late Complications | Treatment | Follow-up (months) | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | Roux-en-Y HJ | Silicon* | Bile leak (from resection surface and anastomosis | Stitch on resection surface and Re-do of HJ | 44 and 51 months cholangitis and stenosis of BD anastomosis | Percutaneous dilatation of HJ (twice) | 53 | Fine |
| 2 | 1 | DDA | T-drain | Stenosis | ERCP and Stenting | No | - | 47 | Fine |
| 3 | 1 | DDA | Silicon* | No | - | No | - | 19 | Fine |
| 4 | 3 | Roux-en-Y HJ | Silicon* | Bile leak from resection surface | Percutaneous drain | No | - | 16 | Fine |
- Abbreviations: BD, bile duct; DDA, duct-to-duct anastomosis; ERCP, endoscopic retrograde cholangiopancreatography.
- * Silicon tube (Marquat GBM, Boissy St. Leger, France).
In 2 donors who presented with a single right hepatic duct (donors 2 and 3), a direct duct-to-duct anastomosis was performed and stented with a T-drain in donor 2 and with a silicon tube (Marquat GBM, Boissy St. Leger, France) in donor 3, respectively. The anastomoses were performed with 6-0 PDS in interrupted fashion. In donors 1 and 4, because of the presence of multiple hepatic ducts, a Roux-en-Y hepaticojejunostomy (HJ) had to be performed. In these cases, the anastomoses were performed with the use of 6-0 polydioxanone suture in interrupted fashion. An externalized transanastomotic silicon drain (Marquat GBM) was placed in both patients and removed 6 weeks later.
All donors recovered immediately after the operation. None of them experienced any postoperative liver dysfunction.
One patient with duct-to-duct anastomosis (donor 2) developed an early stenosis at the bifurcation of the main bile duct, which was successfully treated by endoscopic stenting of both right and left bile duct. The stents were removed after 6 weeks, and no further late complications occurred at a follow-up of 47 months.
Both donors in whom HJ was performed suffered a major early complication. In donor 1 an anastomotic leak from HJ was detected 5 days after the surgery. The patient underwent reoperation, and a dehiscence of HJ was repaired by refashioning of the anastomosis. The other patient with HJ (donor 4) experienced a bile leak from the cut surface with consequent biloma. The complication was treated conservatively by percutaneous computed tomography–guided external drain placement. Both donors recovered within a few days.
At present follow-up, only 1 donor experienced a late complication. Forty-four months after the operation, donor 1 developed cholestasis and cholangitis due to a stenosis of the HJ. The stenosis resolved with percutaneous balloon dilation, and the donor was free of symptoms for 3 more months when he developed a recurrent stricture of the HJ. Another percutaneous balloon dilatation of the anastomosis with stenting was performed. The clinical examination and cholestatic parameters normalized within a short time after the latter treatment.
Our normal postoperative follow-up of liver donors includes imaging solely intended to monitor graft regeneration. In light of the deaths of the 4 donors' intended recipients, we restricted postoperative studies in these donors to only those that were clinically indicated. Based upon computed tomography and magnetic resonance imaging of the 1 donor with a late complication, we did observe a scar-like plane connecting the 2 hemilivers, giving it the appearance of a normal liver. Additionally, no volumetric increase or regeneration was observed in this 1 patient with late postoperative imaging.
All 4 donors have had a full physical and psychological recovery and have fully returned to their presurgery quality of life (observation period, 14–53 months).
DISCUSSION
Intra-operative mortality of an intended LDLT recipient prior to implantation of the graft is presumably a very rare event. Compounding the tragedy of this event would be a scenario in which the donor resection has already been completed, potentially rendering the graft “in limbo.” In this situation, the donor would be emotionally and potentially physically devastated by having undergone the donation without a positive end result for the recipient.
In this, some major issues should be discussed: recipient selection and evaluation of risk factors, timing of bile duct division during the donor's operation (point of no return), when hepatectomy should and should not be completed, and what to do in the case of an “orphan graft.”
Recipient Selection and Evaluation of Risk Factors
The initial evaluation of the LDLT recipient is identical to that for conventional deceased donor liver transplantation. The further selection of appropriate recipients for LDLT is an evolving process and differs among centers according to the indication and clinical status of the patient selection.14
Medical urgency and the severity of the underlying liver disease are predictors of perioperative mortality.
Cirrhotic cardiomyopathy is the term used to describe the impaired cardiac contractility under stress conditions in patients with cirrhosis.15 Its cause is not yet known and to date there is no single diagnostic test that can identify a patient in this condition and predict who will develop perioperative complications.
Neuroendocrine tumors represent an additional cardiological risk factor. In fact, by releasing vasoactive substances into the circulation (e.g., serotonin), carcinoid tumors can cause right-sided valvular heart disease (so-called carcinoid heart). The risk of progressive heart disease is higher in patients who receive chemotherapy than in those who do not.16 Neither of the 2 transplanted patients with metastatic neuroendocrine tumors had preoperative signs of carcinoid heart. Notwithstanding, we can speculate only that the basal disease could have caused a rapid decompensation of heart function during the critical phase of the operation.
Because of the aforementioned reasons, an accurate selection and preoperative evaluation of the recipient is essential.17 In particular, the preoperative cardiovascular status of the patients is important in determining how they will cope with the stress imposed by liver transplantation. Updated guidelines have been recently reported by Therapondos et al.18
All 4 recipients underwent a detailed standardized preoperative evaluation with special regard to cardiopulmonary situation (see ”Patients and Methods”). Additionally, anaesthesia record showed nothing abnormal until cardiac arrest occurred.
Timing of Bile Duct Division and Related Problems
The division of the right hepatic duct is one of the most important steps of the donor hepatectomy, potentially influencing both the outcome of the anastomosis in the recipient and the safety of the donor. The primary objective should then be to make the division of the right hepatic duct as easy and accurate as possible, avoiding any mechanical and ischemic injury to the graft and to the donor and maintaining high the chances of obtaining a single orifice when the anatomical conditions are favorable.
The early, sharp division of the hepatic duct prior to liver parenchyma resection prevents thermal injury to the duct. Our group first described the division of the hepatic duct prior to the parenchyma.10 In our experience, this technique offers better visualization of the transection plane and yields perfectly vascularized stumps for both the graft and the donor side. Since the introduction of this technique, we have observed a reduction of biliary complications in both donors and recipients.
The donor scenario of “healthy” patients with “inadvertent” division of the bile duct(s) without associated vascular injury is very similar to that of bile duct injuries after cholecystectomy.19 In such cases the Roux-en-Y procedure for biliodigestive drainage is most widely adopted. In this regard, different long-term complication rates ranging between 5% and 43% have been reported and are mainly represented by chronic cholangitis, stricture, fibrosis, and cirrhosis, as well as blind loop syndrome.20-24 Temporary stenting and bile diversion from the HJ remains controversial: It has to be selective according to the individual characteristics of each patient and the experience of each surgeon.25-28.
Stenosis of HJ is usually treated by percutaneous transhepatic balloon dilation, by percutaneous transhepatic biliary drainage, or by both. If either of these percutaneous approaches fails to improve the biliary complications, patients then undergo surgery with refashioning of the HJ.21
In the 2 cases with a single duct, we opted for a direct reconstruction with a duct-to-duct anastomosis. In fact, the anatomical conditions and the excellent perfusion of both stumps made the direct anastomosis relatively easy and avoided the construction of a Roux-en-Y loop with the potentially additional complications. The general rule in hepatobiliary surgery has been to protect the direct anastomosis with a stent, and although our first donor had a T-tube placed, in the second donor, a Silastic transanastomotic drain (Marquat GBM, Boissy St. Leger, France) was used. The preference for the transanastomotic drain derives from the vast experience with duct-to-duct anastomosis in the recipient biliary reconstruction.
When Hepatectomy Should and Should Not Be Completed
The high incidence of biliary complications suffered by these donors has raised the question of whether the donors would have been better off by having the hemiliver removed.
Based on our own experience, one would be inclined to entertain the possibility of completing the donor hepatectomy especially when the donor liver presents with 2 or more right hepatic ducts and a biliodigestive anastomosis must be fashioned. In this case, the construction of an HJ is technically challenging and the donor is exposed to the additional complication of the Roux-en-Y loop. On the other hand, the risks of a right hepatectomy and the usually longer recovery needed must be considered and weighed against 1 or 2 anastomoses, the biliary alone or in combination with the jejuno/jenunostomy of the Roux-en-Y loop. One would probably not hesitate to face the potential complications of a biliary reconstruction in the presence of a marginal rest volume, a moderate liver steatosis, or an older donor—all factors that could render the postoperative recovery potentially more prone to complications. As a counter argument and taking into account the exceptionality of the cases presented here, it could be said that with the great experience accumulated in right living donor hepatectomy the incidence of complications is definitely lower when the liver is removed. Consequently, in the case of a perfect donor, the decision of removing the liver could rationally be considered.
What to Do in the Case of an “Orphan Graft”?
In the case of completion of the hepatectomy, the situation is even further complicated by the ethical issue of either discarding the harvested liver or donating it to another recipient. Many donors are willing to take risks because they know they are helping a loved one, somebody they have a close relationship with. In the case of death of the loved one, the emotional gain for the donor disappears, and in theory the donor has no gain by having someone unknown receiving his or her liver. Therefore, at any point in time when the graft can be left safely in the donor, it should be,unless it becomes a greater risk to the donor to leave it than to remove it. In this latter case the question becomes how to now allocate the graft to “next-best” maximize the benefit for the donor.
Different options have been proposed regarding the graft's allocation in the case of an “orphan graft”29 after completed donor hepatectomy and intraoperative death of the recipient: discard it, or give it to somebody else, either the next recipient on the waiting list of the local organ procurement organization, or an alternate recipient selected preoperatively by the transplant team according to an in-b-up living donor liver recipient policy,30 or an alternate recipient selected preoperatively by the donor/recipient pair (so-called ”Cross-donation to friends”). Each of these reallocation scenarios has advantages and disadvantages that must be considered (Table 4). Different scenarios according to the operative timing strategy (donor's and recipient's operations parallel or one after the other) and to the time of recipient's death have been summarized in Fig. 1. Since at the time we had no defined policy of graft allocation in such cases of recipient intraoperative death, we opted for the condition of hepar divisum.
| Policy | Advantages | Disadvantages |
|---|---|---|
| Local organ procurement organization | Ensures justice with allocation according to waitlistNo conflict of interest for transplant team | No emotional benefit to donorLast-minute allocation with longer cold ischemic timeUnfamiliarity of accepting transplant team with details of procurement and graft anatomy |
| Center-selected alternate | Known to be suitable recipient for graft | Potential for conflict of interest |
| Prepared for transplant (in-house) | No emotional benefit to donor | |
| Surgeons familiar with graft | ||
| Donor/recipient-selected alternate | May ensure some emotional benefit to donor | Donor/recipient may be unable to identify suitable alternate |
| Known to be suitable recipient for graft | ||
| Prepared for transplant (in-house) | ||
| Surgeons familiar with graft | ||
| Potentially eliminates conflict of interest concerns |
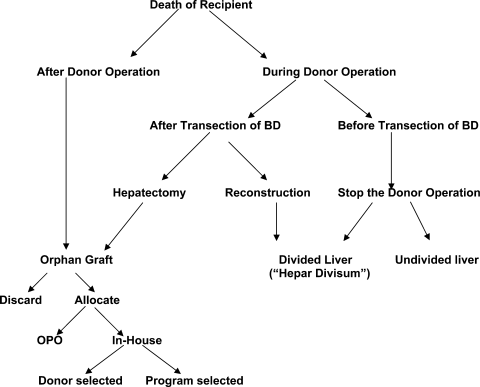
Different scenarios according to the operative timing strategy and to the time of intraoperative recipient's death. BD, bile duct; OPO, organ procurement organization.
Although the main purpose of our paper is to describe this rare situation, it raises important questions. There is, at least in our experience, an increased risk of harming the donor by reconstructing biliary continuity. Though a comparison with the postoperative course of the donors who have completed the donation can be considered only from a speculative point of view, it is clear that the difference in complications is real. In facing such rare and dramatic situations, we conclude that maintaining both options, biliary reconstruction and liver graft removal, particularly when an alternative allocation policy is present, is probably the better approach.
In conclusion, the condition of hepar divisum after right hepatectomy for living donation must be seen as a major donor complication, since the planned operation is not brought to its end and the aim of the surgery is not achieved. Moreover, the surgery to reconstruct the biliary continuity exposes the donor to risks that should be considered in addition to those of the donor hepatectomy. On the other hand, the reconstruction of the hepatic duct(s) in the situation described herein is not technically easy and becomes definitely challenging when more than 1 hepatic duct is present.
In these cases, the alternative of completing the donor hepatectomy and offering the graft to a different recipient according to the internal policy of the transplant institution should be considered.