The Use of a Dehydrated Cellularized Collagen Matrix to Replace Fibrotic Vocal Fold Mucosa
There are no disclosures or conflicts of interest to declare.
Abstract
Objectives
Fibrosis of the vocal fold lamina propria reduces vocal cord vibration resulting in a chronically hoarse voice. We describe a novel approach using umbilical cord-derived mesenchymal stem cells in a dehydrated collagen matrix (cellogen) to reconstruct the delicate balance of extracellular matrix within the vocal fold lamina propria whilst limiting the host inflammatory response to the implant.
Methods
Human umbilical cord-derived mesenchymal stem-cells were embedded in bovine type I collagen hydrogel and dehydrated using the RAFT™ 3D culture system. The extracellular matrix, cellular viability and composition, paracrine profile, and genomic profile were assessed and the scaffold engrafted onto the hind muscle of NUDE mice.
Results
The cells retained stem-cell markers following fabrication and secreted collagen III, fibronectin, and glycosaminoglycans within the scaffold. Electron microscopy showed the scaffold consisted of single strands of protein with interspersed bundles of a similar size to native vocal fold lamina propria. The use of the dehydration step improved cell viability and upregulated the expression of genes important in wound healing and matrix organization compared with unmodified collagen hydrogel carriers. The cells were shown to secrete exosomes and cytokines and, following engraftment within an immunocompromised mouse model, appeared to modulate the host inflammatory response compared with controls.
Conclusion
This article provides a scalable cell-protein scaffold that with further modifications could provide a replacement for lost or damaged vocal fold mucosa. Further investigations are required to assess the mechanical properties of the scaffold and inhibit the differentiation of the umbilical cord-derived stem-cells following implantation. Laryngoscope, 134:882–893, 2024
INTRODUCTION
The vocal cord mucosa is made up of a stratified squamous epithelial layer overlying a lamina propria that is subdivided into superficial, intermediate, and deep layers.1 Each of these layers has a unique arrangement of extracellular matrix that enables the vocal cord to vibrate during phonation for the production of voice (Fig. 1). The superficial layer is the most viscoelastic and contains small interwoven fibrils of collagen amongst bundles of elastin and collagen III.2 In this protein network, the superficial layer is rich in proteoglycans such as decorin, the glycosaminoglycan hyaluronic acid, and to a lesser extent, glycoproteins such as fibronectin, which contribute to viscosity.3-5 The intermediate and deep layers are referred to as the vocal ligament and are made up of more densely packed fibers of collagen III and elastin.6
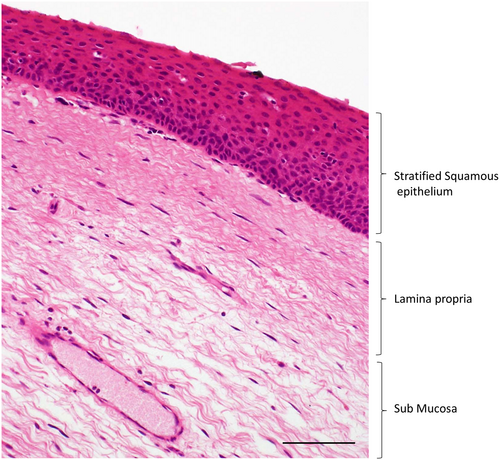
In adults, there is minimal regenerative potential within the lamina propria following injury. Instead, the mucosa heals via a reparative pathway resulting in the deposition of fibrous protein that increases the stiffness of the vocal cord and reduces its ability to vibrate.7, 8 Fibrotic change, particularly if extensive or within the superficial layer, reduces the quality and strength of the voice leading to difficulties with communication, employment related issues, social isolation and associated mental health disorders.9, 10
Experimental therapies using biomaterials with or without growth factors or cells have attempted to replace fibrotic segments of vocal fold lamina propria, although significant challenges exist in successful clinical translation.11-13 These center around the host's inflammatory response to implanted material resulting in recurrent fibrosis and difficulties in enabling cell survival following engraftment.14-18 Challenges also remain in translating preclinical therapies into successful clinical products due to the high costs involved in GMP manufacture. This is particularly relevant in therapies that utilize autologous cells that require ex vivo expansion from a recipient. The approach described in this article moves toward addressing these challenges by providing a collagen sheet with embedded human umbilical cord-derived mesenchymal stem cells (CD-MSCs) that aims to act as a lamina propria replacement product, termed cellogen, to replace fibrotic vocal fold lamina propria and restore function. We use the Real Architecture for 3D Tissue (RAFT™) method to deliver a dehydrated collagen scaffold with embedded cells within a three-dimensional protein environment.19 A similar approach was found to be advantageous by the lead author when delivering respiratory progenitor cells to the upper airway as the encasement of cells avoided cell death from shear during implantation, and the three-dimensional protein matrix appeared to support cell survival following implantation.20 Other groups have demonstrated how the RAFT™ method enables protein density to be predetermined with an advantageous effect on embedded cell behaviour in bone and corneal applications.21, 22 Biological material is also preferred over synthetic constructs given the advantages in sustaining cell function by providing a more biomimetic extracellular matrix environment.23 The method has been formulated without the use of feeder layers and only with materials that can be scaled up into a clinical product. We believe this is a crucial part of pre-clinical testing to improve the efficiency of the translational process. This article will investigate the potential of this method to deliver a bioactive collagen layer with embedded CD-MSCs and examine the application of this technique to replace fibrotic vocal fold lamina propria.
MATERIALS AND METHODS
A full scientific description of the methods used is available in Supplementary Information 1.
Preparation of Cellogen Scaffolds
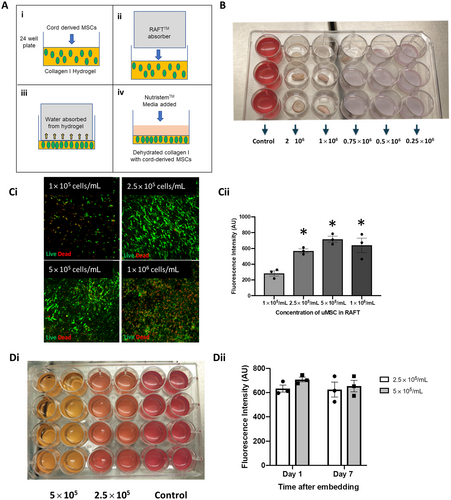
To prepare the scaffolds, bovine collagen type I (Koken 3 mg/mL, 2BScientific, Upper Heyford, UK) was mixed with Minimal Eagle's Medium 10× (Gibco, Hemel Hempstead, UK) in a ratio of 8:1 over ice. The mix was neutralized with 5 M NaOH (Lonza, Slough, UK) until it turned straw yellow in color (neutral pH). Umbilical cord-derived MSCs were mixed with the solution and incubated at 37°C for 30 min to allow the gel to set. RAFT™ absorbers (Lonza, Slough, UK) were inserted over the gels within the 24-well plates (Fig. 2A). After 30 min, the absorbers were removed and MSC Nutristem with 2.5% stemulate was added to the cell/collagen scaffold (Cellogen) on the bottom of the wells.
Cell Viability
To assess the viability of the MSCs within the 3D collagen matrix, a cell viability assay (AlamarBlue, Life Technologies, Hemel Hempstead, UK) was used. After 2 h, fluorescence (excitation 560 nm, emission 590 nm) was recorded, and a final value was calculated by subtracting values generated from negative control wells containing scaffolds but no MSCs.
Live Dead Stain
To assess MSC viability within collagen scaffolds, a Live/Dead™ cell viability assay (Life technologies, Hemel Hempstead, UK) was used. Then, 1 mL of assay consisting of calcein (4 μm/mL) and ethidium homodimer-1 (3.4 um/mL) was added to each of the scaffold containing wells and then incubated at 37°C for 45 min. Scaffolds were analyzed using a Zeiss 700 confocal microscope.
Human Cytokine Antibody Array
A Human Cytokine Antibody Array (Abcam, Cambridge, UK; ab133998) was used to assess for cytokine release from the scaffolds. MSC Nutristem without supplement was added to the well and the supernatant removed after 48 h. Densitometry was analyzed using Image J software and background subtracted to measure integrated density. Integrated densities from control scaffolds were subtracted from cellogen scaffolds to assess final integrated density value for each cytokine.
RNA Extraction for RNA Sequencing
Differential gene expression was assessed between CD-MSCs in unmodified type I collagen hydrogel and CD-MSCs within cellogen scaffolds. Scaffolds were prepared as previously described and incubated in MSC Nutristem media (Cellseco, Salisbury, UK). After 2 days, the culture media was removed and the RNA extracted from the scaffolds using 500 μL of TRIzol® reagent (Thermo Fisher, Hemel Hempstead, UK) followed by RNeasy Plus Mini kit (Qiagen, Manchester, UK). Sample quality was assessed using Nanodrop (ND-1000, Thermofisher, Hemel Hempstead, UK) and an RNA concentration of >50 ng/μL per sample was sent to GENEWIZ Ltd for RNA sequencing.
Isolation and Analysis of Nanoparticles
Nanoparticles were prepared from scaffolds using ultracentrifugation of the scaffold supernate. Using nanoparticle track analysis (LM10. Malvern Panalytical, Malvern, UK), the size and concentration of the nanoparticles were recorded. Concentrations derived from acellular scaffolds were subtracted from the cellogen scaffold concentration value as means of controlling for background values.
Flow cytometry was used to identify the cell surface glycoprotein found on exosomes. The Antibodies CD9 FITC (BD Biosciences, Wokingham, UK; 555371) and CD63 PE (BD Biosciences, Wokingham, UK; 556020) and PBS were used to label potential exosomes and run on a flow cytometer (Novocyte Advanteon, Agilent, UK).
Scaffold Engraftment in Nude Mice
Nude mice (Charles River, Huntingdon, UK) were anaesthetized and maintained with sevoflurane (National Veterinary Services, Stoke-on-Trent, UK). A 5 cm transverse incision was made on the back of the mouse and a subcutaneous pocket made. A fascial flap overlying the flank muscle was raised, and a 4 mm punch biopsy was used to wound the muscle. Scaffolds (4 mm diameter) were grafted onto the muscle wound and the fascial layer repositioned and secured with a suture to protect against shear. Cellogen scaffolds were prepared using the described method and cultured for 7 days before implantation. A control scaffold lacking cells was cultured at the same time using the same method and engrafted onto the contralateral flank. After 7 days (n4), the mice were sacrificed and the wound was reopened and the graft and underlying mouse muscle explanted for histological processing. All animal experiments were carried out under a UK Home Office Project license in accordance with all animal care regulations.
RESULTS
Optimization of Cell Number Within Cellogen
At 24 h following graft manufacture, scaffolds with cell concentrations of 1 × 106 cells per milliliter of collagen or greater had all contracted (Fig. 2B). At 24 h, live/dead viability assay demonstrated a greater number of dead cells at seeding concentrations of 1 × 106 cells per milliliter of collagen and no increase in the number of live cells when compared with seeding concentrations of 2.5 × 105 and 5 × 105 cells per milliliter of collagen (Fig. 2C). Alamar Blue assay was used to measure cell viability and was significantly greater in scaffolds with a cell concentration of 2.5 × 105, 5 × 105, and 1 × 106 per milliliter of collagen compared with a cell concentration of 1 × 105 per milliliter of collagen (one-way ANOVA with Sidak's test for multiple comparisons, *p = 0.002) (Fig. 2C). There was no significant difference in viability between cell concentrations of 2.5 × 105, 5 × 105, and 10 × 105 per milliliter of collagen (one-way ANOVA with Sidak's test for multiple comparisons, p = 0.42).
Alamar Blue assay was used to measure cell viability of scaffolds with 2.5 × 105 and 5 × 105 cells per milliliter of collagen at one and 7 days following fabrication and showed no significant difference (two-way ANOVA with Sidak's test for multiple comparisons, p = 0.61) (Fig. 2D). At 7 days, contraction was observed in five out of eight scaffolds seeded at a concentration of 5 × 105 cells per milliliter of collagen compared with acellular control scaffolds. Cells seeded at 2.5 × 105 cells per milliliter of collagen did not show any signs of contraction compared with acellular controls and this concentration was taken forward as the optimized concentration for later experiments.
Characterization of Cells and Extracellular Matrix Protein
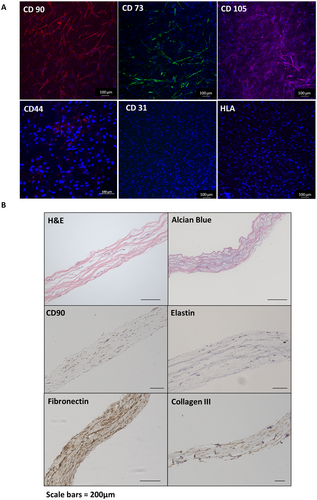
Top-down immunofluorescence confirmed the retention of the mesenchymal stem-cell markers CD73, CD90, and CD105 and the absence of differentiated mesenchymal cell markers, CD31, human leukocyte antigen (Fig. 3A), and CD34 (Supplementary) in cellogen scaffolds at 7 days. Immunohistochemistry of paraffin sections of the scaffold at 7 days showed CD90 positive cells throughout the scaffold matrix (Fig. 3B). Positivity to fibronectin and collagen III was seen within scaffold compared with acellular control scaffolds. Alcian Blue staining showed evidence of glycosaminoglycan deposition between the protein networks. Minimal elastin staining was observed throughout the scaffolds.
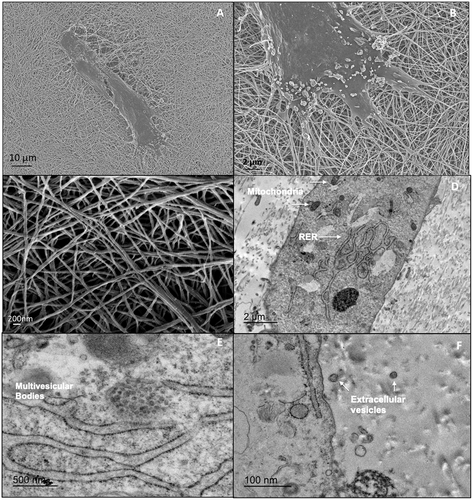
Surface electron microscopy showed the filopodia of CD-MSCs interacting with ECM fibers (Fig. 4). The fibers themselves were a combination of single strands measuring 50–100 nm in diameter and fiber bundles measuring approximately 200 nm in diameter. Transmission electron microscopy showed the cells to be rich with intracellular organelles important in protein synthesis such as mitochondria and rough endoplasmic reticulum. Within the cells, multivesicular bodies could be seen and apparent extracellular vesicles were noted adjacent to cells within the ECM.
Assessment of Cytokine and Exosome Secretion
To examine the paracrine effect of the cellogen scaffold, a cytokine antibody array was used to detect cytokines released into culture media collected after 24 h of incubation with the scaffolds (Fig. 5A). An acellular scaffold was used as a negative control and values subtracted. Integrated density increased for a range of cytokines with the largest increases being found with cytokines ena-78, gro, gro-a, il-6, il-8, mcp-1, rantes, igfbp-4, nap-2, osteopontin, timp-1, and timp-2.
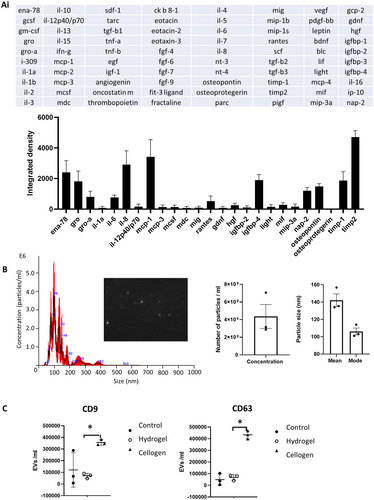
The release of exosomes was assessed using nanosight imaging of scaffold exosome-free supernatant and compared against control scaffolds (Fig. 5B). Analysis showed a particle concentration of 4.4 × 109 particles per milliliter with a mean particle size of 141 nm (mode 105 nm). Flow cytometry was used to compare the surface receptor exosome markers CD63 and CD9 on supernatant taken from cellogen scaffolds, acellular control scaffolds, and unmodified collagen I hydrogel scaffolds with the same concentration of CD-MSCs (Fig. 5C). The number of labeled extracellular vesicles was significantly greater in cellogen scaffolds compared with collagen hydrogel scaffolds with embedded CD-MSCs, based on the abundance of CD9 and CD63 (one-way ANOVA with Tukey's test for multiple comparisons, *p = <0.01). The wide data spread in the CD9 control group is likely due to a technical failure of one of the readings.
Comparison of Cellogen with Hydrogel Carrier
The advantage of using a dehydrated collagen scaffold to more conventional cell carrier methods was examined by comparing cell density and global transcriptional change between cells within the cellogen scaffold and cells within unmodified collagen I hydrogel (Fig. 6). Top-down immunofluorescence and assessment of the nuclear DNA stain (DAPI) demonstrated a greater cell density within the cellogen scaffold at 24 h after fabrication compared with the hydrogel scaffold.
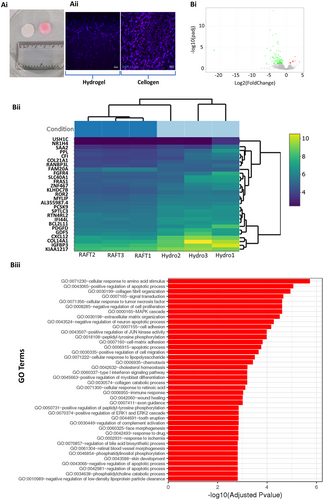
Global transcriptional changes were compared between CD-MSCs within an unmodified collagen I hydrogel and CD-MSCs within the cellogen scaffolds using RNA sequencing (Fig. 7B). A total of 12 genes were upregulated and 108 genes were downregulated in cellogen CD-MSCs compared with CD-MSCs within the hydrogel. The top five differentially expressed genes sorted by their adjusted p-value were CXCL12, COL14A1, PDGFD, KLHDC7B, and PCSK9. All of these genes were downregulated in the cellogen scaffolds. Gene ontology terms were used to classify the function of the differentially expressed genes. The top five significantly overrepresented (adjusted p value <0.05) gene ontology terms were cellular response to amino acid stimulus (GO: 0071230), positive regulation of apoptotic process (GO: 0043065), collagen fibril organization (GO: 0030199), signal transduction (GO: 0007165), and cellular response to tumor necrosis factor (GO 0071356). Overrepresentation was also found for the gene ontology terms cell-matrix adhesion (GO: 0007160), cell adhesion (GO: 0007155), wound healing (GO: 0042060), and skin development (GO: 0043588).
Engraftment of Cellogen in Nude Mice
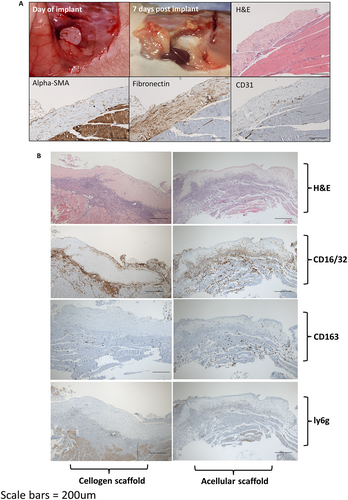
To assess cell survival and host response following engraftment in-vivo, cellogen scaffolds were grafted onto hind muscle in nude mice and compared with acellular control scaffolds (Fig. 7). Digital photography at 1 week following engraftment showed persistence of the scaffold without contraction and with signs of new vessel formation over the surface. Immunohistochemistry indicated spindle shaped cells throughout the scaffold that stained positivity to alpha-SMA. The scaffold was rich in fibronectin. Early signs of neovascularisation were indicated by positivity to the vascular marker CD31 at the base. At the one-week time point, there was a greater infiltration of CD16/32 positive inflammatory cells within the control scaffolds (three out of four mice) compared with cellogen scaffolds (one out of four mice). The M2 macrophage marker CD163 demonstrated infiltration of one of the four control scaffolds but none of the cellogen scaffolds. There was minimal staining of the neutrophil marker ly6g across all scaffolds.
DISCUSSION
The Cellogen scaffold provides a platform that may enable an eventual replacement product to treat fibrotic segments of vocal cord mucosa. The scaffold can be rapidly assembled with CD-MSCs from a biobank. As CD-MSCs lack expression of human leucocyte antigens (HLAs), they can be given as an “off the shelf” treatment,24 without the need for patient-specific autologous cell expansion. This greatly increases the scalability of this approach by avoiding lengthily and costly manufacture within a GMP facility.
The embedded cells retained the expression of undifferentiated mesenchymal stem-cell markers without expression of differentiated markers. This indicates the embedded cells retain their undifferentiated state following fabrication although further assessment using flow cytometry to quantify expression will be performed in future studies. Embedded cells also expressed CD44 which whilst widely expressed by other cell types has been used to identify mesenchymal cells in the macular flava of the vocal fold that exhibit stem-cell properties.25, 26 Like the superficial layer of the vocal cord lamina propria, electron microscopy demonstrated single strands of collagen fibres measuring 50 nm or less in diameter with interspersed bundles of protein.2 The CD-MSCs were found to retain the capacity to secrete new ECM within the scaffold giving it the potential to reconstruct the delicate balance of ECM seen in healthy vocal fold lamina propria. Importantly, like the superficial layer of the human vocal fold lamina propria, collagen III was detected with glycosaminoglycans and glycoprotein between the protein networks. Elastin is a key component of human vocal fold ECM and was not shown to be extensively secreted by the embedded cells. It may be possible to manipulate the cells in-vitro with the addition of growth factors, such as platelet derived growth factor, to stimulate elastogenicity.27 Alternatively, elastogenicity could be stimulated through the mechanotransduction after the cells have been implanted and exposed to the vibratory forces of the vocal fold. A similar paradigm has been observed in vascular smooth muscle cells derived from human pluripotent stem cells, whereby gene expression of elastin is upregulated following long-term uniaxial strain.28 Given elastin is relatively sparse in the superficial layer of the vocal fold lamina propria, a scaffold that aims to replace this layer only may not require a high elastin content.
Compared with unmodified collagen I hydrogel scaffolds, the use of a dehydration step upregulated transcriptomes important in wound healing and matrix reorganization. The dehydration step also increases collagen fiber and cell density resulting in a scaffold that is more comparable with native lamina propria. Genes important in cell-to-cell and cell-matrix contact were also differentially expressed in cellogen scaffolds compared with hydrogel scaffolds. This may explain the enhanced cell survival given the importance of cell-to-cell and cell-matrix interactions in cell functioning and survival.29, 30 Enhancing cell survival is a crucial aspect of cell transfer, not only to enable the cells to exert an effect on the local tissue environment following implantation, but also, as nonviable cells, stimulate the host inflammatory immune response and recurrent fibrosis.31 The encasement of cells within the scaffold also provides protection from shear during implantation and further improves cell survival. Previous work has demonstrated that shear of cells cultured on the surface of scaffolds is a key limitation when implanting onto complex three-dimensional structures such as the stroma of the upper airway.32
One week following engraftment, the cells embedded within the scaffold expressed alpha-SMA indicating differentiation of the CD-MSCs to myofibroblasts.33 The hypoxic environment of a wound is known to stimulate differentiation of mesenchymal cells to myofibroblasts through a TGFβ-dependent pathway.34, 35 Myofibroblasts are involved in the deposition of fibrotic matrix protein and matrix contraction resulting in fibrosis. Whether the differentiation to myofibroblasts can be blocked by inhibiting cytokines such as TGFβ, or downstream signaling pathways such as p38 mitogen-activated protein kinases,36 will be explored further in later studies.
Following engraftment, the cellogen scaffold showed less inflammatory cell infiltrate compared with acellular controls. The avoidance of inflammatory cell infiltrate is an important step in avoiding the deposition of new fibrose protein that leads to recurrent scarring after implantation. The cellogen scaffolds excreted anti-inflammatory cytokines such as TIMP-1 and TIMP-2 although pro-inflammatory cytokines such as IL-6 were also detected. A more comprehensive assessment of cytokine secretion under hypoxic culture conditions would help establish how the anti-inflammatory effect may be mediated. The extracellular vesicles secreted by the cellogen scaffolds were compatible with exosomes in terms of size and surface markers. MSC-derived exosomes are believed to exert a range of effects on the surrounding microenvironment through the delivery of cargo from one cell to another.37 Cargo can include micro-RNA, nucleic acids, RNA, proteins or lipids, and certain cargos, such as MiR-181c, have been shown to exert an anti-inflammatory response.38 The content of the MSC-derived exosome cargo will be examined further in future studies.
Limitations and Future Directions
Assessment of the biomechanical properties is clearly important for scaffolds that aim to restore vocal fold vibration. Given the anticipated remodeling of any scaffold implanted within the vocal fold, a meaningful assessment of biomechanics should take place following orthotopic implantation in an in vivo study and will be the focus of future studies. The collagen used in this study was derived from bovine dermis. Experience with bovine collagen as an injectable in vocal cord medialization has shown approximately 3% of recipients exhibit a hypersensitivity reaction.39 This can be avoided to some extent by skin hypersensitivity testing, but future studies should investigate for comparability with a human collagen-based hydrogel.
CONCLUSIONS
The combination of umbilical CD-MSCs within a collagen I scaffold that undergoes a dehydration step forms a protein matrix with densely packed cells that retain an undifferentiated mesenchymal phenotype. The cells secrete collagen III, fibronectin, and glycosaminoglycans to form a layer with a similar extraceullar matrix composition as native vocal fold lamina propria in terms of protein fiber size and content. The use of a dehydration step improves cell viability and upregulates transcriptomes important in matrix organization and wound healing. The cells secrete cytokines and exosomes following embedding within the scaffold, and there is modulation of the host inflammatory immune response once implanted in vivo. Future studies should build upon this platform and explore implantation to replace fibrotic vocal fold lamina propria.
ACKNOWLEDGEMENTS
This research was supported by the Academy of Medical Sciences (SGL021\1046) and the Royal College of Surgeons of England Pump Priming award. NJIH was supported by the National Institute for Health and Research through the Clinical Lecturer scheme.




