Immune Checkpoint Inhibitors Serve as the First-Line Treatment for Advanced Head and Neck Cancer
y.h. and h.z. contributed equally to this study.
This work was supported by National Natural Science Foundation of China (82073339), partly by Changzhou Sci&Tech Program (CJ20210166; CJ20220224), Scientific Program of Jiangsu Commission of Health (M2022051). The authors have no conflicts of interest to disclose.
Abstract
Objectives
Immune checkpoint inhibitor (ICI) therapy has demonstrated substantial benefits for certain patients. We try to evaluate the merits and demerits of each immunotherapy to aid clinical treatment.
Methods
We conducted a comprehensive search of the PubMed, Embase, and Cochrane databases for randomized clinical trials published as of June 10, 2023. Our study included published clinical trials of ICI monotherapy or combination therapy, along with data on treatment-related adverse events (TRAE). Data regarding survival efficacy and adverse events of each randomized controlled trial (RCT) were collected. The Bayesian random effects model was utilized for the network meta-analysis (NMA).
Results
This study incorporated 19 RCTs, involving 5900 patients. Among 14 treatment regimens, Pembrolizumab combined with chemotherapy emerged as the most promising primary treatment for overall survival (OS) and objective response rate (ORR). Toripalimab combined with chemotherapy exhibited the highest likelihood of becoming the primary treatment for extending progression-free survival (PFS). Durvalumab showed the lowest probability of adverse events, suggesting a safer profile compared with other drugs. Camrelizumab combined with chemotherapy demonstrated a heightened risk of adverse events. Dual ICI Nivolumab/Ipilimumab surpassed Durvalumab/Tremelimumab in terms of ORR and adverse events. The standard of care (SOC) regimen did not exhibit strong performance across the four outcome indicators.
Conclusion
Our analysis suggests that the integration of chemotherapy agents with ICIs enhances its efficacy as a first-line treatment for patients with advanced head and neck cancer (HNC).
Level of Evidence
1 Laryngoscope, 134:749–761, 2024
INTRODUCTION
Head and neck cancer (HNC) ranked as the seventh most prevalent cancer worldwide in 2018, comprising 3% of all new cases and slightly over 1.5% of all cancer-related fatalities in the United States.1 A majority of head and neck squamous cell carcinoma (HNSCC) cases are diagnosed at a locally advanced stage, and a conventional treatment strategy typically entails a combination of surgery, radiotherapy, chemotherapy, and targeted therapy.2 For patients with advanced HNC unsuitable for surgery or incapable of undergoing radiotherapy, systemic chemotherapy has emerged as the primary therapeutic method.3 Prior studies indicate that cisplatin-based chemotherapy can effectively treat HNC. Although the combination of cisplatin/5-fluorouracil (5-FU) is a popular therapy, it is associated with specific neurotoxic, ototoxic, and nephrotoxic effects.4
Epidermal growth factor receptor (EGFR) is frequently overexpressed in numerous HNC patients.5 Cetuximab, a monoclonal antibody directed against EGFR, has shown limited clinical benefits.5 The De-ESCALaTE trial, wherein patients were randomized to receive either cetuximab or cisplatin coupled with radiotherapy, did not show any significant differences in late severe toxicity. In addition, the cisplatin group demonstrated a markedly superior 2-year overall survival (OS) than the cetuximab group (97.5% vs. 89.4%).6
Years of rigorous research to harness the cytotoxic immune response against cancer have culminated in a revolutionary shift in cancer therapy that leverages the immune system's capacity to identify and eradicate tumors.7 In 2016, the FDA authorized the use of pembrolizumab for treating HNC, marking a significant stride in immunotherapy. Subsequently, the NCCN guidelines approved pembrolizumab alone or in combination with chemotherapy as the first-line treatment for HNC.8
In the wake of these extensive randomized trials, a high-level evidence landscape has been established, becoming the new standard of care (SOC) for advanced HNC. Nevertheless, the decision-making process between treatments remains challenging due to the absence of direct cross-comparison studies among the various options. The risk of inaccurate inter-trial comparisons is especially heightened due to variances in study patient populations, inclusion criteria, treatment strategies, and stratification factors. This study aims to systematically assimilate and compare all publicly accessible information to date on the efficacy of immune checkpoint inhibitors (ICIs), either as monotherapy, in combination, or along with chemotherapy, as the first-line treatment for patients with advanced/metastatic HNC using a network meta-analysis (NMA). The study was designed to evaluate safety and efficacy using parameters such as OS, progression-free survival (PFS), objective response rate (ORR), and ≥grade 3 treatment-related adverse events (TRAE). It seeks to inform clinical decisions on treatment options that deliver the best outcomes and controllable toxicity for HNC.
MATERIALS AND METHODS
This study was executed in compliance with the preferred reporting items for systematic review and meta-analyses (PRISMA) statement, as delineated in Table SI.9
Search Methods and Study Selection
To identify published clinical trials that were predicated on PD-1 inhibitor-based therapy, we conducted a comprehensive systematic review and meta-analysis. The trials in question reported post-treatment survival data in conjunction with the corresponding adverse events. An extensive search was executed in databases such as PubMed, Embase, and Cochrane Library, utilizing the following terminologies: nivolumab, pembrolizumab, camrelizumab, tislelizumab, toripalimab, sintilimab, durvalumab, ipilimumab, tremelimumab, PD-L1 inhibitors, CTLA-4 inhibitors, and PD-1 inhibitors. The search commenced with the inception of the database and concluded on June 10, 2023. In addition, the citations of pertinent reviews and articles were meticulously scrutinized to avoid overlooking any relevant articles. The precise retrieval strategy is delineated in Table SII.
Inclusion criteria encompassed published randomized controlled trials (RCTs) that satisfied the following conditions: 1. Histopathologically confirmed HNC patients; 2. RCT employing at least one type of ICI as the first-line treatment; 3. Provision of efficacy results in PFS, OS, ORR, or Grade 3 TRAE; 4. Clinical trials juxtaposing two or more HNC treatment regimens. Exclusion criteria comprised: 1. Non-randomized controlled trials or single-arm trials; 2. Trials involving fewer than 10 subjects; 3. Non-English publications. Upon discovery of multiple trials reporting the same population during screening, the publication furnishing the most comprehensive survival and adverse event data was chosen. Literature search, study selection, and data extraction were independently undertaken by two team members, with any discrepancies reviewed by another investigator (JL) on the team and resolved through consensus.10
Data Extraction
Excel spreadsheets were prepared by the research team members in advance for data extraction, defining the crucial information for extraction, including the first author's name, year of publication, trial registration number, disease stage, tumor location, ICIs used and dosage, patient count, mean age, ECOG score, PD-L1 expression, CPS score, median OS, median PFS, ORR, and ≥grade 3 TRAE.
Data Synthesis and Analysis
This mesh meta-analysis targeted outcome measures, such as OS, PFS, ORR, and ≥grade 3 TRAE. We evaluated the safety and efficacy of various treatment regimens by combining odds ratios (ORs) for the latter two outcome measures and hazard ratios (HRs) for the two survival measures along with their 95% confidence intervals (95% CI). The Bayesian NMA has superior classification power compared with conventional meta-analysis, as it can simultaneously summarize comparisons among multiple treatments, enabling more flexibility in utilizing complex models and providing relative scientific explanations in terms of causation.11 We implemented a Bayesian statistical model based on random effects, integrating a connecting network of direct and indirect evidence while comparing multiple immunotherapies. To assess publication bias, we developed a funnel plot using STATA.15.
NMA facilitates the comparison of different treatments in a single systematic review, including treatments that have not been directly compared head-to-head. NMA allows the effect of each treatment to be numerically ranked from the most effective to the least effective.12 In accordance with the National Institute for Health and Clinical Excellence (NICE) framework, parameter estimation was performed using the Markov Chain Monte Carlo method with 50,000 run-in iterations and 50,000 iterations.13, 14 The convergence of iterations was evaluated using convergence diagnostic graphs and locus density.15 We fitted a consistency model and assessed heterogeneity utilizing common variance.16 The treatment effect was estimated using a posterior mean with a corresponding 95% confidence interval, interpreted analogously to the 95%. I2 > 50% was considered indicative of statistically significant heterogeneity. We computed the surfaces under the cumulative ranking (SUCRA) to rank the preferences for each treatment. This probability was determined by calculating the proportion of iterations of the HR ordering for each process in the Markov chain. SUCRA values were utilized to evaluate the therapeutic effect of OS, PFS, ORR, and ≥grade 3 TRAE, with higher SUCRA scores implying superior therapeutic effects. Following the exclusion of studies with a moderate or high risk of bias, we performed a sensitivity analysis and recalculated the network results using ranking probabilities to estimate the robustness of the results. If no significant difference was found, the NMA results were deemed valid. Finally, we performed a head-to-head meta-analysis and forest mapping of clinical trials of the same treatment regimen.
All analyses were performed using R version 4.2.1 and Review Manager 5.2. Bias risk assessment was performed using the Cochrane framework.17
RESULTS
Baseline Characteristics and Quality of Included Studies
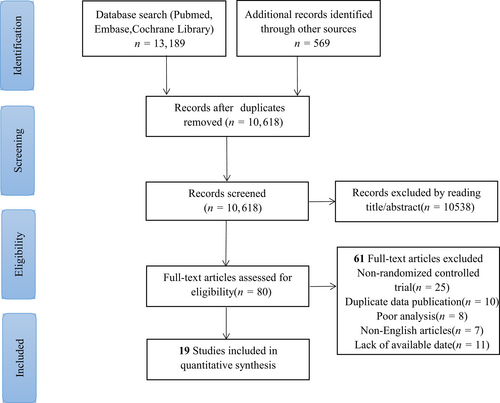
A total of 13,758 articles were screened during our search. Subsequent to the manual and automatic elimination of 3140 duplicate references, we reviewed the titles and abstracts of the remaining 10,618 articles. Upon careful examination of the full text of 80 references, we excluded 61 studies, which comprised single-arm studies and case reports. Finally, 19 randomized controlled studies were incorporated into the meta-analysis, encompassing a total of 5900 patients and 14 distinct treatment regimens (Fig. 1). The 14 treatment regimens included Camrelizumab, Camrelizumab/chemotherapy, Chemotherapy, Durvalumab, Durvalumab/Tremelimumab, EXTREME, Nivolumab, Nivolumab/chemotherapy, Nivolumab/Ipilimumab, Pembrolizumab, Pembrolizumab/chemotherapy, Standard of care, Toripalimab/chemotherapy, and Tremelimumab. The EXTREME regimen refers to a combination of platinum, 5-fluorouracil, and cetuximab. The “Standard of care” (SOC) mentioned in the study denotes a combination of standard doses of methotrexate, docetaxel, or cetuximab. The network diagram for the four indicators is displayed in Figure 2. Among the studies, nine RCTs originated from the United States, five from China, two from France, one from India, one from Greece, and one from the United Kingdom. The patients' ages ranged from 22 to 97 years. The trials encompassed tumors located in the oropharynx, tongue, oral cavity, nasal cavity, nasopharynx, larynx, hypopharynx, and so forth. The specific experimental design and population characteristics of the experimental arm and control arm are provided in Table I.
| First Author | Year | Country | Trial Type | Number Trial Name | Primary Site (%) | ICI Dose | Control Arm Treatment | Patients in Control Arm (n) | Age, Median (IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Barbara Burtness18 | 2019 | U.S.A | Prospective, Phase III Trial | KEYNOTE-048 | Hypopharynx (13) Larynx (25) Oral cavity (27) Oropharynx (38) |
Pembrolizumab 200 mg every 3 weeks | Pembrolizumab + chemotherapy | 281 | 61 (55–68) |
| Robert L. Ferris19 | 2016 | U.S.A | Prospective, Phase III Trial | CheckMate 141 | Larynx (14.2) Oral cavity (45) Pharynx (38.3) Other (2.5) |
Nivolumab 3 mg/kg every 2 weeks | Standard therapy | 121 | 61 (28–78) |
| Ezra E. W. Cohen20 | 2019 | U.S.A | Prospective, Phase III Trial | KEYNOTE-040 | NA | Pembrolizumab 200 mg every 3 weeks | Standard-of-care | 248 | 60 (54–66) |
| Hai-Qiang Mai21 | 2021 | China | Prospective, Phase III Trial | NCT03581786 | Nasopharyngeal carcinoma | Toripalimab 240 mg with GP Q3W for up to 6 cycles | Placebo + GP | 143 | 51 (21–72) |
| Yunpeng Yang22 | 2021 | China | Prospective, Phase III Trial | NCT03707509 | Nasopharyngeal carcinoma | Camrelizumab (200 mg on day 1) with GP Q3W for four to six cycles | Placebo + GP | 129 | 49 (40–56) |
| Jonathan D. Schoenfeld23 | 2020 | U.S.A | Prospective, Phase II Trial | NCT02919683 | Oral cavity squamous cell carcinoma | 2 cycles of Nivolumab at 3 mg/kg weeks 1 and 3 | Nivolumab | 14 | 64.4 (39.1–81.0) |
| Nancy Y. Lee24 | 2021 | U.S.A | Prospective, Phase III Trial | JAVELIN Head and Neck 100 | Oral cavity (13.5) Oropharynx (47) Larynx (18) Hypopharynx (21.5) |
Avelumab 10 mg/kg with IMRT | Placebo + chemoradiotherapy | 347 | 59 (54–65) |
| R. L. Ferris25 | 2020 | U.S.A | Prospective, Phase III Trial | EAGLE | Oral cavity (26.7) Oropharynx (38.3) Hypopharynx (17.1) Larynx (15.4) Other (2.5) |
Durvalumab 10 mg/kg every 2 weeks | Standard of care | 249 | 61.0 (22–82) |
| Sean McBride26 | 2021 | U.S.A | Prospective, Phase II Trial | NCT02684253 | Nasopharynx (16.1) Oropharynx (37.1) Other (46.8) |
Nivolumab 3 mg/kg Q2W and SBRT (9 Gy × 3 every other day) | Nivolumab | 30 | 60.5 (29–77) |
| A. Psyrri27 | 2023 | Greece | Prospective, Phase III Trial | KESTREL | Oral cavity (30.9) Oropharynx (31.9) Hypopharynx (15.7) Larynx (21.6) |
Durvalumab 1500 mg Q4W | EXTREME | 206 | 61.0 (22–84) |
| Robert I. Haddad28 | 2023 | U.S.A | Prospective, Phase III Trial | CheckMate 651 | Oral cavity (26.9) Oropharynx (42.8) Hypopharynx (9.5) Larynx (20.8) |
Nivolumab 3 mg/kg Q2W plus ipilimumab 1 mg/kg Q6W | EXTREME | 475 | 62 (29–86) |
| Caroline Even29 | 2021 | France | Prospective, Phase II Trial | NCT02605967 | Nasopharyngeal carcinoma | Spartalizumab 400 mg every 4 weeks | Chemotherapy | 40 | 50 (26–78) |
| Lillian L. Siu30 | 2019 | Canada | Prospective, Phase II Trial | CONDOR | Oropharynx (40.1) Oral cavity (20.2) Hypopharynx (16.1) Larynx (22.5) Other (1.1) |
Durvalumab 20 mg/kg Q4W and tremelimumab 1 mg/kg Q4W for 4 cycles | Durvalumab | 67 | 62 (23–82) |
| Wenfeng Fang31 | 2018 | China | Prospective, Phase I Trial | NCT02721589 NCT03121716 |
Nasopharyngeal carcinoma | Camrelizumab 200 mg, gemcitabine 1 g/m2, and cisplatin 80 mg/m2 Q3W | Camrelizumab | 93 | 45 (38–52) |
| Matthew H. Taylor32 | 2022 | U.S.A | Prospective, Phase II Trial | NCT02454179 | Head and neck squamous cell carcinoma | Pembrolizumab 200 mg Q3W plus acalabrutinib 100 mg twice daily | Pembrolizumab + acalabrutinib | 37 | 58.0 (45.0–97.0) |
| A. T. Chan33 | 2022 | China | Prospective, Phase III Trial | KEYNOTE-122 | Nasopharyngeal carcinoma |
Pembrolizumab 200 mg every 3 weeks | Chemotherapy | 116 | 53.0 (46.5–61.0) |
| Y. Tao34 | 2022 | France | Prospective, Phase II Trial | GORETEC 2015-01 | Oropharynx (59) Oral cavity (6) Hypopharynx (27) Larynx (8) |
Pembrolizumab 200 mg was given every 3 weeks, during RT | Cetuximab + RT | 65 | 67 (47–81) |
| Vijay Maruti Patil35 | 2023 | India | Prospective, Phase III Trial | CTRI/2020/11/028953 | Head and neck cancer | Nivolumab 20 mg once every 3 weeks | TMC | 75 | 46 (28–74) |
| Kevin J. Harrington36 | 2023 | UK | Prospective, Phase II Trial | CheckMate 714 | Oral cavity (33.3) Larynx (18.2) Oropharynx (34.0) Hypopharynx (10.1) |
Nivolumab 3 mg/kg Q2W plus ipilimumab 1 mg/kg Q6W | Nivolumab | 88 | 58.0 (36–77) |
| First Author | ECOG Status (%) | PD-L1 Tumor Score or Karnofsky Score (%) | Disease Status (%) | Experimental Arm Treatment | Patients in Experimental Arm (n) | Age, Median (IQR) | ECOG Status (%) | PD-L1 Tumor Score or Karnofsky Score (%) | Disease Status (%) | Median Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Barbara Burtness18 | =0 (39) =1 (61) |
<50% (77) ≥50% (23) |
Metastatic (68) Recurrent (31) |
Pembrolizumab | 301 | 62 (56–68) | =0 (39) =1 (61) |
<50% (78) ≥50% (22) |
Metastatic (72) Recurrent (27) |
23 mo |
| Robert L. Ferris19 | =0 (19.0) =1 (77.7) ≥2 (2.5) |
NA | NA | Nivolumab | 240 | 59 (29–83) | =0 (20.4) =1 (78.8) ≥2 (0.4) |
NA | NA | 5.1 (0–16.8) mo |
| Ezra E. W. Cohen20 | =0 (27) =1 (73) =2 (<1) |
<50% (72.6) ≥50% (26) |
II (3) III (7) IV (90) |
Pembrolizumab | 247 | 60 (55–66) | =0 (29) =1 (71) |
<50% (74) ≥50% (26) |
II (2) III (4) IV (94) |
8.4 (3.3–14.5) mo |
| Hai-Qiang Mai21 | =0 (57) =1 (43) |
Positive (76) Negative (17) |
Recurrent (61) Metastatic (39) |
Toripalimab + GP | 146 | 46 (19–72) | =0 (57) =1 (43) |
Positive (75) Negative (14) |
Recurrent (58) Metastatic (42) |
22.1 mo |
| Yunpeng Yang22 | =0 (34) =1 (66) |
NA | Metastatic (33) Recurrent (67) |
Camrelizumab + GP | 134 | 52 (40–58) | =0 (35) =1 (65) |
NA | Metastatic (35) Recurrent (65) |
24 mo |
| Jonathan D. Schoenfeld23 | NA | NA | II (28.6) III (21.4) IVA (50.0) |
Nivolumab + Ipilimumab | 15 | 65.2 (32.5–78.4) | NA | NA | II (26.7) IVA (73.3) |
NA |
| Nancy Y. Lee24 | =0 (62) =1 (38) |
NA | <T4 (56) T4 (44) |
Avelumab + chemoradiotherapy | 350 | 60 (54–65) | =0 (55) =1 (45) |
NA | <T4 (57) T4 (43) |
NA |
| R. L. Ferris25 | =0 (31.7) =1 (68.3) |
≥25% (28.9) < 25% (71.1) |
Recurrence ± distant metastases (85.9) Distant metastases (14.1) |
Durvalumab | 240 | 59.0 (24–84) | =0 (25.8) =1 (74.2) |
≥25% (28.3) <25% (71.7) |
Recurrence ± distant metastases (92.1) Distant metastases (7.9) |
7.8 mo |
| Sean McBride26 | NA | Negative (60) Positive (36.7) |
NA | Nivolumab + SBRT | 32 | 66 (35–83) | NA | Negative (56.2) Positive (34.4) |
NA | 20.2 mo |
| A. Psyrri27 | =0 (36.4) =1 (63.6) |
≥25% (31.6) < 25% (68.4) |
Metastatic (67) Recurrent (31.6) |
Durvalumab | 204 | 62.0 (26–89) | =0 (39.2) =1 (60.8) |
≥25% (30.9) <25% (69.1) |
Metastatic (64.2) Recurrent (35.3) |
NA |
| Robert I. Haddad28 | =0 (36.4) =1 (63.2) |
Positive (57.7) Negative (42.3) |
Recurrent (35.8) Recurrent + metastatic (24) Metastatic (40) |
Nivolumab + Ipilimumab | 472 | 61 (24–86) | =0 (32.2) =1 (67.8) |
Positive (57.2) Negative (42.6) |
Recurrent (28.2) Recurrent + metastatic (32.2) Metastatic (39.4) |
39.1 mo |
| Caroline Even29 | =0 (27.5) =1 (70) |
Positive (95) | NA | Spartalizumab | 82 | 51 (21–74) | =0 (42.7) =1 (54.9) =2 (2.4) |
Positive (95.1) | NA | NA |
| Lillian L. Siu30 | =1 (67.2) | NA | Advanced (32.8) Metastatic (67.2) |
Durvalumab + Tremelimumab | 133 | 62 (26–81) | =1 (69.9) | NA | Advanced (37.6) Metastatic (62.4) |
6.5 (0.2–20.0) mo |
| Wenfeng Fang31 | =0 (30) =1 (70) |
NA | Primary metastases (33) Recurrence with distant metastases (62) Local recurrence (4) |
Camrelizumab + GP | 23 | 44 (34–51) | =0 (35) =1 (65) |
NA | Primary metastases (22) Recurrence with distant metastases (78) |
10.2 (9.7–10.8) mo |
| Matthew H. Taylor32 | =0 (24.3) =1 (75.7) |
Positive (71%) Negative (4%) | I (2.7) II (2.7) III (2.7) IVA (29.7) IVB (2.7) IVC (59.5) |
Pembrolizumab | 39 | 61.0 (38.0–83.0) | =0 (23.1) =1 (76.9) |
Positive (57) Negative (24) | IVA (33.3) IVB (5.1) IVC (61.5) |
NA |
| A. T. Chan33 | =0 (33.6) =1 (66.4) |
CPS <10 (53.4) CPS ≥10 (39.7) |
Recurrent (8.6) Metastatic (25.0) Recurrent and metastatic (66.4) |
Pembrolizumab | 117 | 51.0 (42.0–59.0 | =0 (30.8) =1 (69.2) |
CPS <10 (46.2) CPS ≥10 (47) |
Recurrent (10.3) Metastatic (35.0) Recurrent and metastatic (54.7) |
NA |
| Y. Tao34 | =0 (37) =1 (63) |
NA | III (26) IVa (62) IVb (12) |
Pembrolizumab + RT | 66 | 65 (48–79) | =0 (41) =1 (59) |
NA | III (24) IVa (50) IVb (26) |
25.8 mo |
| Vijay Maruti Patil35 | =0 (8) =1 (92) |
1%–50% (42.7) >50% (29.3) |
NA | TMC + Nivolumab | 76 | 50 (28–77) | =0 (3.9) =1 (96.1) |
1%–50% (57.9) >50% (23.7) |
NA | 10.9 mo |
| Kevin J. Harrington36 | =0 (22) =1 (75.6) =2 (2.4) |
Negative (43.9) Positive (56.1) |
NA | Nivolumab + Ipilimumab | 159 | 59.0 (24–82) | =0 (23.9) =1 (75.5) =2 (0.6) |
Negative (42.1) Positive (57.9) |
NA | NA |
- ECOG = Eastern Cooperative Oncology Group; IMRT = intensity-modulated radiotherapy; IQR = interquartile range; NA, not applicable; GP = gemcitabine-cisplatin; Q3W, every 3 weeks; standard therapy, methotrexate 40–60 mg/m2 weekly, docetaxel 30–40 mg/m2 weekly, or cetuximab 400 mg/m2 once, then 250 mg/m2 weekly; SBRT = stereotactic body radiotherapy; EXTREME regimen, platinum, 5-fluorouracil, and cetuximab; standard-of-care, standard doses of methotrexate, docetaxel, or cetuximab intravenously; TMC, methotrexate 9 mg/m2 once a week, celecoxib 200 mg twice daily, and erlotinib 150 mg once daily.
Quality Assessment
The overall quality level of the studies is depicted in Figure SI. Among the 19 trials included, 17 reported on the generation of random sequences, while 18 studies detailed their allocation concealment methodologies. Two trial presented a high risk of selection bias, and one trial indicated a high risk of data attrition bias. The quality of the remaining trials did not pose a significant risk of bias.
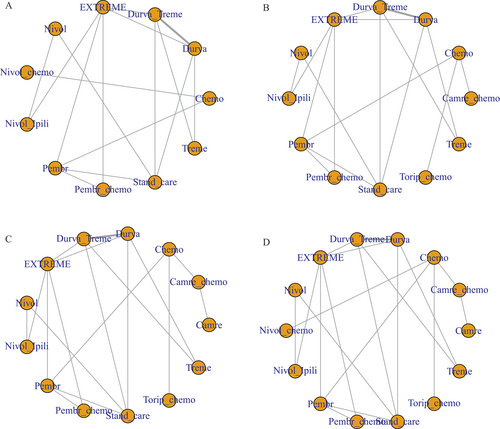
Network Meta-Analysis
OS and PFS
The network meta-analysis (NMA) for OS incorporated 10 RCTs that provided OS data across the entire population or specific patient subsets and examined 11 treatment modalities that incorporated ICI. In the comprehensive NMA, Pembrolizumab in combination with chemotherapy demonstrated a notable OS advantage compared to Pembrolizumab monotherapy (1.03, −2.18–4.53), the EXTREME regimen (2.42, −0.78–5.70), Durvalumab/Tremelimumab (3.12, −0.96–6.74), and chemotherapy alone (3.16, −4.68–10.93) (Fig. 3A). No such advantage was discernible for Nivolumab combined with chemotherapy as opposed to Nivolumab alone (−0.44, −9.42–7.92). Pembrolizumab demonstrated a longer OS (1.29, −1.44–3.92) than the EXTREME regimen, as compared with conventional chemotherapy (1.96, −4.78–8.91). The SUCRA values for each treatment were calculated to rank the OS survival effect in HNC patients (Table SIII). Relative to the other 10 interventions, SUCRA projected an 80.17% probability that Pembrolizumab/chemotherapy was the optimal treatment for extending OS, followed by Nivolumab (SUCRA = 75.96%), Nivolumab/chemotherapy (SUCRA = 71.94%), and Pembrolizumab (SUCRA = 65.81%). The lowest likelihood of survival improvement was associated with Tremelimumab monotherapy (SUCRA =15.25%).
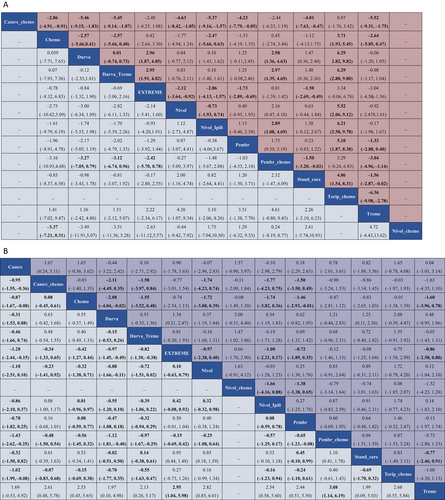
Eleven RCTs reported 12 therapeutic interventions, including ICI. Toliperizumab combined with chemotherapy significantly extended PFS in comparison to chemotherapy alone (3.71, 1.93–5.45), SOC (4.86, 1.54–8.31), Pembrolizumab (5.10, 1.87–8.38), Nivolumab (5.52, 2.06–9.12), and Durvalumab (6.29, 2.82–9.82) (Fig. 3A). The EXTREME regimen conferred a higher PFS advantage over SOC (1.50, 0.49–2.69) and Tremelimumab (3.04, 1.56–4.50). According to the SUCRA ranking, Toripalimab/chemotherapy and Camrelizumab/chemotherapy had a 96.86% and 91.31% likelihood, respectively, of being the preferred drugs for extending PFS, followed by EXTREME (SUCRA = 74.41%) and Pembrolizumab/chemotherapy (SUCRA = 74.27%). Tremelimumab had a 13.04% probability of being the drug of choice.
ORR and ≥grade 3 TRAE
ORR was reported in 12 RCTs, encompassing 13 regimens, and ≥grade 3 TRAE were reported in 13 RCTs, including 14 regimens. Pembrolizumab/chemotherapy demonstrated a significant ORR advantage compared to Camrelizumab (1.43, 0.25–2.62), Durvalumab monotherapy (1.12, 0.40–1.81), and Tremelimumab monotherapy (3.08, 1.14–6.19) (Fig. 3B). Likewise, the EXTREME regimen displayed superior ORR compared to Camrelizumab (1.28, 0.15–2.44), and Tremelimumab monotherapy (2.95, 1.04–5.98). Nivolumab had a superior ORR compared to Camrelizumab (1.18, –0.10–2.51), and Tremelimumab monotherapy (2.82, 0.85–6.01). The SUCRA value of each treatment was calculated to rank the ORR control effect in HNC patients (Table SIII), indicating that Pembrolizumab/chemotherapy (SUCRA = 91.79%) had the highest likelihood of cancer remission compared to the other 12 interventions, followed by EXTREME (SUCRA = 84.81%), Nivolumab (SUCRA = 77.72%), and Toripalimab/chemotherapy (SUCRA = 70.19%). Tremelimumab monotherapy had the lowest likelihood of achieving remission in cancer patients (SUCRA = 1.35%).
Durvalumab showed a significant benefit in terms of grade 3 and above adverse events compared to the EXTREME regimen (1.34, 0.21–2.47). It also exhibited a reduced risk of adverse events in comparison to several combination treatments, namely Nivolumab/chemotherapy (2.00, −0.51–4.40), Pembrolizumab/chemotherapy (1.21, −0.44-2.83), Toripalimab/chemotherapy (2.08, −0.39–4.47), and SOC (1.25, 0.11–2.36) (Fig. 3B). Nivolumab possessed an advantage in terms of adverse events over Nivolumab/chemotherapy (1.63, −0.91–4.12) and Toripalimab/chemotherapy (1.72, −0.79–4.20). The OR values of Camrelizumab compared to Camrelizumab/chemotherapy were (1.67, 0.24–3.11). The findings suggested that ICI monotherapy had a lower incidence of grade 3 and above adverse risks than ICI combined with chemotherapy. The SUCRA value for each treatment was calculated to rank the ≥grade 3 TRAE toxic effect in HNC patients (Table SIII), which showed that Durvalumab (SUCRA = 87.97%) had the lowest likelihood of adverse events relative to the other 13 interventions, followed by Nivolumab/Ipilimumab (SUCRA = 75.30%), Nivolumab (SUCRA = 74.12%), and Camrelizumab (SUCRA = 69.73%). Conventional chemotherapy had the highest likelihood of adverse events in HNC patients (SUCRA = 16.96%).
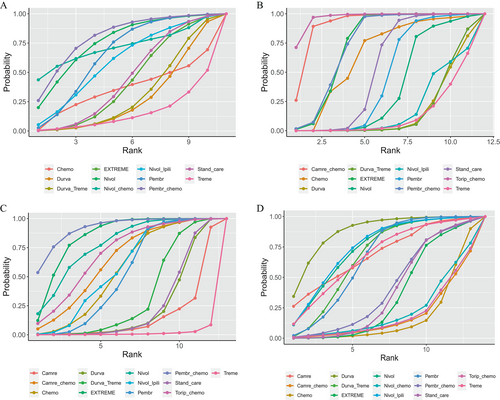
Rank probabilities
Figure 4 presents the Bayesian sequences of different measures for various treatment regimens. Table SIII details the comprehensive ranking results. A holistic analysis was conducted using SUCRA values to compare the likelihood of different treatment options being the optimal choice (Fig. 4).
Heterogeneity and inconsistency assessment
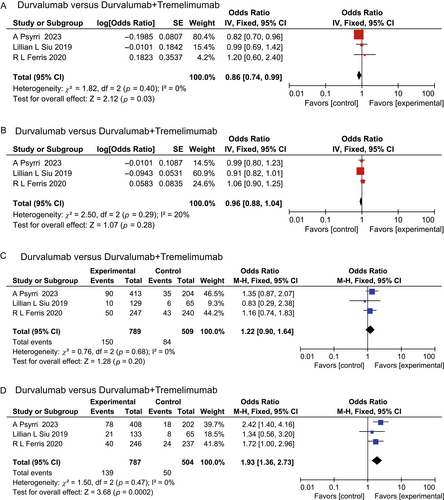
Among the incorporated studies, only the comparison of Durvalumab versus Durvalumab/Tremelimumab included three RCTs. Consequently, these four outcomes were subjected to a direct comparison (Fig. 5). The heterogeneity among these four outcomes was minimal. The results derived from the inconsistency test (p < 0.05) underscored a high degree of consistency.
Assessment of publication bias
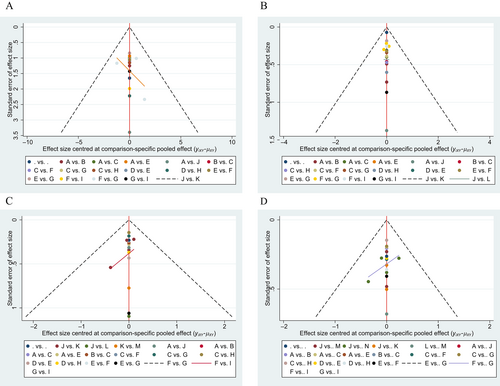
The funnel plots, derived from the included studies, did not exhibit any substantial publication bias (Fig. 6).
DISCUSSION
- Pembrolizumab in combination with chemotherapy exhibits significant efficacy benefits over conventional monotherapy chemotherapy or the combined therapy of cetuximab, 5-FU, and platinum chemotherapy. Furthermore, this combination therapy manifests a slightly lower incidence of adverse events of grade 3 or higher than chemotherapy alone. Out of the 14 treatment regimens, Pembrolizumab in combination with chemotherapy has the highest likelihood of being the primary choice for OS and ORR.
- Toripalimab in combination with chemotherapy demonstrates the highest likelihood of being the primary treatment for extending PFS. Camrelizumab combined with chemotherapy shares a similar likelihood.
- Durvalumab has the lowest incidence of adverse events, making it safer than other comparable drugs. However, Camrelizumab combined with chemotherapy poses a higher risk of adverse events.
- The dual ICI treatment of Nivolumab/Ipilimumab exhibits better benefits than Durvalumab/Tremelimumab in terms of ORR and adverse events (AE). The EXTREME regimen performs well in controlling disease progression and extending PFS, but it also carries a high risk of adverse events. The SOC regimen did not exhibit strong performance across the four outcome indicators.
This systematic review and meta-analysis incorporated 19 RCTs exploring the survival benefit profiles and incidence of related adverse events in head and neck tumors based on monotherapy or combination therapy with PD-1 inhibitors. We concluded that ICIs in head and neck tumors are primarily employed in conjunction with chemotherapy, targeted therapy, or radiation therapy. Most patients experienced at least one adverse event across all three categories, and the occurrence of toxicity of grade 3 or higher was not uncommon. High incidences of serious adverse events were particularly notable in patients receiving concurrent PD-1 inhibitors and chemotherapy. Thus, the balance between survival benefits and drug safety should be carefully considered.
We conducted a network meta-analysis to evaluate the effects of various monotherapies and combination therapies in patients with HNC. Apart from the network meta-analysis, we also performed direct comparisons, yielding evidence of low heterogeneity and reliable results. Given that the network meta-analysis amalgamates direct and indirect evidence from RCTs, its results can be deemed more reliable than those of single RCTs.
The profile of adverse events serves as a vital factor in garnering informed patient consent prior to drug administration. Close monitoring and prompt identification of associated symptoms and signs can facilitate proper management. Accordingly, we have comprehensively outlined the toxicity characteristics of several major combination regimens to inform clinical application. Our meta-analysis reveals that combination therapy exhibits a higher toxicity profile than ICI monotherapy. Common adverse events encompass nausea, reduced white blood cell count, anemia, skin toxicity, and elevated liver enzymes.22 This outcome may arise due to the cumulative effect of PD-1 inhibitors and chemotherapy drugs. Hematological events were the primary adverse events, largely attributable to the cytotoxicity of chemotherapeutic agents.37 VEGF induces hypertension and proteinuria in patients, presumably due to an anti-angiogenic mechanism. In the case of combined radiation therapy, the prevalent adverse events included dysphagia, oral mucositis, and sore throat),38 potentially linked to increased local radiation dosage rather than systemic toxicity.
Strengths and Limitations
This study presents a number of strengths and limitations. Its major strength lies in its comprehensive search strategy and extensive inclusion of numerous studies. In addition, the considerable number of patients in both the experimental and control groups enhances the robustness of the results. Apart from the network analysis of direct and indirect evidence, the study incorporated head-to-head analyses of individual treatment regimens, bolstering the credibility of the evidence.
On the downside, despite reviewing the full text and supplementary documents, there remains a dearth of valuable information, including unpublished official data. Therefore, the possibility of publication bias cannot be disregarded. In addition, our discussion did not group according to baseline population characteristics such as PD-L1 expression and HPV status. Moreover, the limited number of trials identified in the evidence bank resulted in a sparse network, with most treatment comparisons being informed by a single RCT. Furthermore, while the NMA allows for simultaneous comparisons of multiple treatments, a substantial number of comparisons are indirect, introducing a degree of uncertainty in outcome assessment.
CONCLUSION
This systematic review and NMA affirm the significance of ICI treatment as a first-line therapy, thereby providing evidence-based substantiation for the new care standards for advanced HNC. First, chemotherapy is evidently inferior to any combination of ICI and chemotherapy. Second, among the ICI treatment combinations, Pembrolizumab in combination with chemotherapy demonstrated superior survival benefits and manageable toxicity. The addition of chemotherapy to ICIs improves the efficacy of first-line treatment in patients with advanced HNC. The EXTREME regimen proves more beneficial than SOC or single-agent chemotherapy. Furthermore, Nivolumab/Ipilimumab appears to offer more benefits than Durvalumab/Tremelimumab.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (82073339), partly by Changzhou Sci&Tech Program (CJ20210166; CJ20220224), Scientific Program of Jiangsu Commission of Health (M2022051).




