Cell-Based Outer Vocal Fold Replacement Both Treats and Prevents Vocal Fold Scarring in Rabbits
This article was presented at the American Laryngological Association Annual Meeting at the Combined Otolaryngological Spring Meetings (COSM), Boston, MA, U.S.A., May 7, 2023.
This work was supported by the National Institutes of Health, NIDCD R01 DC016959 (J. Long). Additional support was provided by Department of Veteran Affairs, Greater Los Angeles VA Healthcare System; UCLA Jonsson Comprehensive Cancer Center; and NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objective
Numerous pharmacological and cell-based treatments have shown promise in preventing vocal fold (VF) scarring when applied at the time of injury. A common clinical scenario, however, is the finding of mature scar impeding voicing. Many treatments are less effective in remodeling existing scar tissue. This objective of this study is to determine if a cell-based outer vocal fold replacement (COVR) effectively restores VF function when applied to existing scar.
Methods
Eighteen rabbits were allocated to three groups: unilateral COVR implant at the time of cordectomy (acute COVR); unilateral cordectomy followed by COVR implant 2 months later (chronic COVR); and unilateral cordectomy followed by sham implant surgery 2 months later (chronic scar). Larynges were harvested 2 months after implant or sham surgery.
Results
All larynges in the COVR groups demonstrated human leukocyte antigen labeling on immunohistochemistry (IHC). COVR groups had increased hyaluronic acid content compared with normal. VF stiffness as measured by elastic moduli in acute COVR and chronic COVR were similar to their contralateral unoperated VF.
Conclusion
COVR implantation in both acutely injured and chronically scarred VF demonstrate persistence of implanted cells, restored tissue biomechanics, and increased hyaluronic acid content.
Level of Evidence
NA Laryngoscope, 134:764–772, 2024
INTRODUCTION
Scarring of the vocal fold (VF) after trauma or lesion excision can cause dysphonia and increased effort of voicing that can negatively impact quality of life. Animal models have shown that scarred VF undergo increased collagen deposition, decreased collagen organization, decreased elastin content, and decreased elastin organization. Such changes are thought to increase stiffness, impairing VF biomechanics and thus phonation.1, 2 Previous efforts have attempted to prevent scar formation after acute injury. However, clinical experience has shown that it is more difficult to reverse existing scar.3, 4
Cell-based outer vocal fold replacement (COVR) consists of human adipose-derived mesenchymal stromal cells (hASCs) embedded within a fibrin hydrogel. After exposure to epidermal growth factor, the superficial layer differentiates to epithelium, whereas the deeper mesenchymal cells remain. This design aims to replace VF epithelium and lamina propria and has been shown to confer wound-healing benefits not seen with implantation of cells or scaffold alone.5, 6 In a rabbit model, COVR has been shown to produce better functional outcomes than reimplanted VF mucosa after mucosal resection.7 This assessed implantation after acute injury, a scenario that best represents reconstruction after cancer resection. However, COVR is yet to demonstrate its efficacy in chronic scar, which is more commonly seen in practice. This study directly compares COVR implantation at the time of acute mucosal resection with COVR implantation after scar has already formed. The purpose is to determine if the beneficial wound-healing effects of the COVR can extend to a previously scarred environment. Functional outcomes of VF vibration and mechanical behavior are also assessed. Finally, cell persistence, tissue microstructure, and hyaluronic acid content were also analyzed.
MATERIALS AND METHODS
Cell Culture and COVR Synthesis
Cells and culture conditions are as previously described.6 hASCs, from ATCC, were expanded in DMEM culture medium with 10% fetal bovine serum (FBS) added. COVR implants were formed using rabbit fibrinogen (Innovative Research) at 5 mg/mL in HEPES-buffered saline, hASCs concentrated to 1.2E6 cells/mL, and bovine thrombin (Sigma–Aldrich) at 2 U/mL in HEPES-buffered saline with 7.5 mM calcium chloride, all mixed at a 4:1:1 ratio. After gelation, differentiation medium containing 10 ng/mL human recombinant epidermal growth factor (EGF, Promega Corp) in DMEM + FBS was supplied. Media were changed every 2-3 days until the COVR was harvested for implantation after 2 weeks.
Animal Treatments
The Institutional Animal Care and Use Committee approved all activities, which were performed in accordance with USDA guidelines. Eighteen male New Zealand white rabbits were randomly assigned to treatment groups: unilateral cordectomy with immediate COVR implantation (acute COVR); unilateral cordectomy with COVR implantation 2 months later (chronic COVR); unilateral cordectomy with sham implant surgery 2 months later (chronic scar) (Fig. 1A).
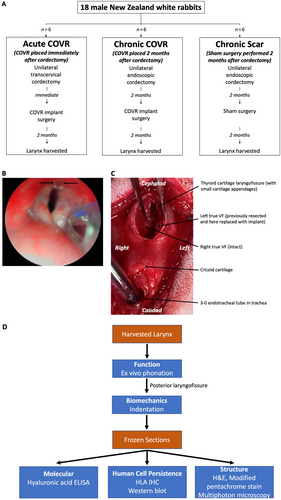
Chronic COVR and chronic scar groups first underwent unilateral endoscopic cordectomy. Under direct visualization of the larynx, one membranous VF was removed with cup forceps (Fig. 1B). All biopsies were performed by an experienced surgeon and received a European Laryngological Society type 3 cordectomy, removing some but not all of the thyroarytenoid muscle.8 scar was allowed to mature for 2 months before either COVR implant or sham surgery.
Initial steps for the COVR implant or sham surgery were performed identically in all groups, as previously described.9 Midline laryngofissure opened the thyroid cartilage, exposing the VFs. For the acute and chronic COVR implants, vibratory mucosa from one VF (the previously injured side in the chronic group) was resected by sharp dissection, exposing thyroarytenoid muscle. One-half of a COVR gel was then applied to the defect and sutured in place with anterior and posterior sutures of 5–0 fast gut (Fig. 1C). For the chronic scar group, the endolarynx was only visualized by laryngofissure, without manipulating the VFs (sham surgery). Animals were euthanized for larynx harvest after 2 months. Excised larynges were frozen at −80°C until use for phonation and indentation studies and then subsequently processed into frozen sections for molecular, structural, and human cell persistence analysis (Fig. 1D).
Ex Vivo Phonation
As described previously, vocal processes of larynges were sutured together in adduction to hold the larynges in phonation position while mounted on a pipe that provided controlled, humidified airflow.7 A high-speed video camera (Vision Research Inc., Wayne, NJ) filmed the larynx from above at 512 × 512 pixels and 6000 frames per second. The larynges were excited to oscillation at airflow of 500, 700, and 1000 cc/s. Manual pressure was applied as needed to the thyroid cartilage ala to produce phonatory adduction. Afterward, larynges were bisected posteriorly and frozen at −80°C until indentation.7 Glottal area segmentation and glottal area waveform (GAW) describing glottal area over time were assessed using Glottis Analysis Tools (version 2020, FAU Erlangen, Erlangen, Germany).10 Glottal area was segmented and the glottal area waveform (GAW) describing glottal area over time was calculated. GAW-based fundamental frequency was calculated as this is one of the parameters most robust to influencing factors.11
Elastic Modulus Measurement by Indentation
Elastic modulus was measured by microindentation testing as described by Chhetri et al.12 A cylindrical indenter was loaded into the VF tissue until 0.5 mm indentation depth was achieved and then unloaded to its original position. The slope of the initial portion of the unloading force-indentation depth curve was then used to estimate Young's modulus.
Microscopic Analyses
Right and left hemilarynges were separated by reopening the anterior laryngofissure suture. Frozen sections from each VF demonstrating the entire antero–postero length were fixed in ethanol and then stained with hematoxylin and eosin (H&E) and a modified pentachrome stain. Frozen sections were then prepared and analyzed on a Leica SP8 DIVE Fluorescence Lifetime Imaging Microscope (Wetzlar, Germany). Two-photon laser excitation was carried out at a wavelength of 830 nm and power setting of 500 mW. Collagen fibers were detected by reflected second harmonic generation imaging at wavelengths of 405–425 nm; elastic fibers were detected by transmitted autofluorescence at wavelengths of 522–532 nm. Images were collected using a 40× objective lens with water immersion.
Human Cell Detection
Frozen sections were used for immunohistochemistry (IHC) to detect implanted cells via human leukocyte antigen (HLA). The monoclonal primary antibody was formed against a fusion protein containing human HLA-A, B, and C epitopes (Monoclonal HLA-66013, Thermofisher Scientific). After application of 3% hydrogen peroxide and 5% goat serum, primary antibody (1:1000 in 1% bovine serum albumin [BSA]) and negative control slides (1% BSA alone) were incubated. After washing, secondary antibody (goat anti-mouse IgG-633) was applied at 1:400 dilution. Slides were washed and coverslipped with VECTASHIELD aqueous mounting agent with 4′,6-diamidino-2-phenylindole (DAPI).
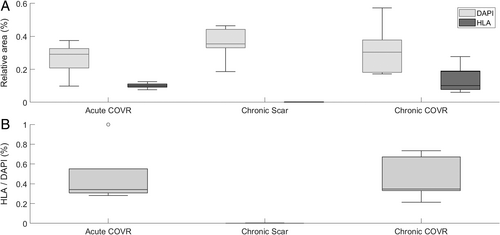
Digital photographs of DAPI and HLA staining were analyzed using ImageJ FIJI (version 1.51). Single-channel images were exported, and a static threshold of 20 was applied to exclude dark noise. Relative area above threshold (area above threshold/total image area) was calculated for each single-channel image. Higher value represents more human cells for HLA and rabbit or human nuclei for DAPI. The ratio of HLA-relative area to DAPI-relative area was calculated for each section. Relative areas and HLA/DAPI area ratios were averaged within each of the three experimental groups.
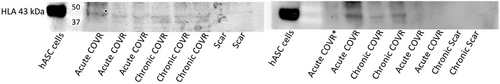
Western blot was performed as a secondary method to detect HLA. Frozen tissue specimens were homogenized in buffer containing sodium dodecyl sulfate (SDS) and 6% beta mercaptoethanol (BME), boiled, electrophoresed on polyacrylamide gel, and then transferred onto blotting membrane. Membranes were incubated with 1:2000 mouse anti-HLA antibody (Proteintech HLA goat anti-mouse 66013-1-Ig) followed by 1:3000 goat anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Sino Biological CAT# SSA007). Chemiluminescence was finally exposed to film.
Hyaluronic Acid Quantification
Frozen tissue samples were processed as described above then underwent enzyme-linked immunosorbent assay (ELISA) specific for hyaluronan (R&D Systems, Inc. CAT # DHYAL0). Fluorescence was measured with a plate reader at 450 nm.
RESULTS
Experimental Course
Of the 18 rabbits, 17 completed the experimental course. One animal in the acute COVR group suffered hematoma that obstructed the trachea, causing perioperative death in the operating suite, and was excluded from analysis.
Ex Vivo Phonation
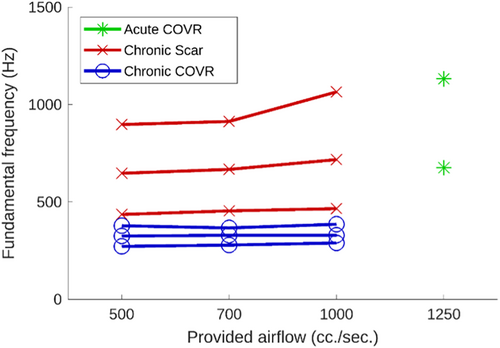
Phonation was achieved in over half of the larynges. High-speed videos from some larynges did not adequately visualize the glottal area to enable segmentation, leaving two acute COVR and three chronic scar and chronic COVR larynges for analysis. Figure 2 shows example images from one larynx. As illustrated in Figure 3, oscillation fundamental frequency only increased very slightly with airflow in most larynges that achieved phonation. Larynges in the acute COVR group only achieved phonation at very high airflows (1250 cc/s) and with slight pressure asserted on the thyroid cartilage to bring the VFs into adduction. Fundamental frequencies were lower in the chronic COVR group compared with either acute COVR or scar groups. Statistical significance was not evaluated due to the small sample size.

Mechanical Testing
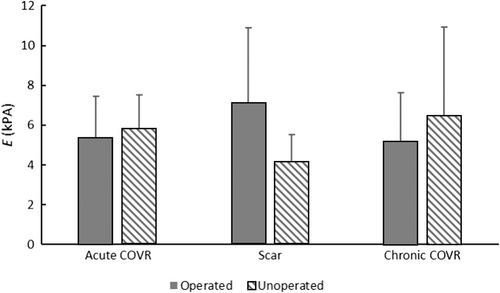
Group means and standard deviations of elastic moduli are shown in Figure 4. The operated VFs differed from the contralateral unoperated VFs only in the chronic scar group (p < 0.1 by one-tailed t-test), in which the injured VFs were stiffer than the normals. Both groups receiving COVR implants (either acutely at the time of injury or delayed after chronic scar maturation) were indistinguishable from normals. One-way ANOVA demonstrated no difference among the three groups for operated or unoperated VFs.
Microscopy
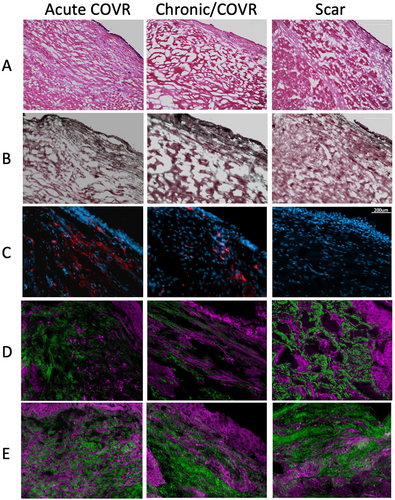
Histologic appearance was evaluated using H&E, elastic stain, and multiphoton microscopy 8 weeks after surgery (Fig. 5). Freezing artifact was evident in the vacuolated appearance in the extracellular matrix. Elastin and collagen fibers were successfully visualized with multiphoton analysis, and elastic fibers were apparent in all groups.
Human Leukocyte Antigen Expression
Presence of HLA was assessed with immunohistochemistry labeling of frozen VF sections (Fig. 5C). All larynges in the COVR groups demonstrated HLA labeling, whereas none of the scar larynges had labeling. Negative control for each larynx demonstrated no such labeling.
Image analysis confirmed these findings quantitatively (Fig. 6). The ratio of HLA IHC labeling to nuclear DAPI staining was comparable in the acute COVR and chronic COVR groups (p > 0.05). All animals in the chronic scar group demonstrated only insignificant HLA labeling, as expected with no human cells implanted. Relative area of nuclei (as measured by DAPI labeling) did not differ statistically among the three groups.
Select samples from each group were run on Western blot (Fig. 7). HLA protein was present in chronic COVR samples and variably present in acute COVR samples. No HLA was seen in chronic scar samples.
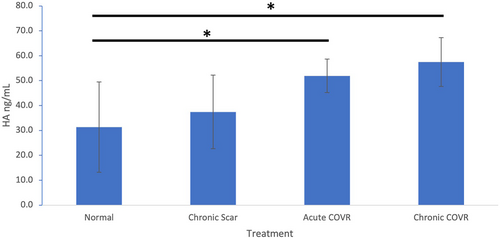
Hyaluronic Acid Content
As assessed via ELISA, there were increased hyaluronic acid levels in both acute and chronic COVR groups compared with both normal (unoperated) and chronic scar groups (Fig. 8). One-way ANOVA demonstrated significance between both COVR VFs and normal VF.
DISCUSSION
Although prevention of VF scarring is favored over treatment, therapy is typically performed at a later stage after scarring has matured. Yet, most preclinical work involving VF regeneration has addressed acute injuries rather than established scars.3, 4 Therefore, this study compares a COVR in a chronic scar model versus an acute injury scenario. This chronic scar model involves COVR implantation onto a previously injured VF that has had time to develop mature scar, whereas previous work has only studied COVR implantation immediately after injury. We aim to determine the feasibility of COVR for its intended clinical use of treating mature scars, which are often considered more difficult to reverse than a fresh injury. Results show that the COVR's wound-healing benefits extend to mature scars similarly to acutely injured VFs.
Persistence and Function of Implanted Human Cells
The three-dimensional implant studied here uses hASCs implanted into immunocompetent rabbits. In the normal VF, mesenchymal cells (fibroblasts) work to maintain and restore the extracellular matrix (ECM) structure. Orderly ECM restoration after major injury is the key action of the COVR implant, by which it prevents subsequent VF scarring. Our previous work showed that the presence of cells early in the postoperative period was essential to achieve the desired wound-healing outcome, and we hypothesize that multiple actions of the mesenchymal stromal cells—including immune modulation, prevention of fibroblast transformation to a contractile myofibroblast phenotype, and suppression of collagen deposition—contribute to this.
This laboratory has previously published two studies assessing implanted cell persistence. In the first, allogeneic male rabbit DNA was detected by in situ hybridization 4 weeks after implantation in all four implanted female rabbits.13 In contrast, a subsequent study implanting human ASC demonstrated HLA by IHC in about half of rabbits after 6 weeks, when tissues were formalin-fixed paraffin-embedded (FFPE).6 This discordance can be attributed to the harsh antigen retrieval method required for FFPE IHC, which can damage epitopes. Findings in this current study with human ASC in rabbits demonstrated clearly detectable HLA by IHC in all implanted animals. We attribute the difference from our previous report in the same model to the use of frozen sections rather than FFPE tissue sections. Western blot supported IHC results.
The field of tissue engineering in general has de-emphasized the need for cell persistence, as evidence has accumulated that many implanted cells do not engraft long-term and that implanted cells can cause long-lasting phenotype shifts in native cells that counteract scar-forming profibrotic shifts brought about by VF injury.14, 15 Regardless, the universal cell persistence identified in this study is not the norm in many tissue engineering models. Possible reasons for better engraftment include an altered immune microenvironment in the larynx relative to other body sites, improved cell niche within the fibrin-based COVR, or optimization of cell nutrition requirements with a thin, highly aqueous implant that maintains contact with the vascular bed.
Importantly, cell persistence as measured by IHC in this study was the same in both the acute COVR and chronic COVR group. Having a previously established scar did not decrease the success of the implant as indicated by cell engraftment. However, some acute COVR VFs did not express HLA in the Western blot. This could be due to the inability of the assay to detect dimerized protein. Alternatively, if cell engraftment is slightly improved in the chronic COVR group versus acute COVR, this could indicate that the immune microenvironment of acutely injured tissue may be more hostile to implantation compared with the immune microenvironment of already-scarred tissue. The altered ECM and vascularity of the latter scenario may hinder immune response and therefore allow persistence of implanted cells. Additionally, although the chronic COVR group addressed the common clinical scenario of mature scar due to iatrogenic injury or phonotrauma, radiation-induced scarring was not assessed here but warrants further investigation.
Molecular Environment
Hyaluronic acid (HA), a GAG polymer of the ECM, has been shown to decrease collagen deposition and contributes to the viscoelastic properties of the VF.16 Previous work showed that, early after injury, scarred rabbit VF exhibits decreased HA compared to normal, but HA later recovers to normal levels.17, 18 This current study affirms this finding as normal, and 4-month scar groups had no significant difference in HA. Both COVR groups here showed increased HA content relative to scar and normal. This could be due to active synthesis of HA from the persistent implanted cells, improved incorporation of HA into the COVR ECM rather than the native VF, and/or a result of the downstream phenotypic shifts of macrophages, immune cells, or fibroblasts induced by the implanted cells. Increased HA content in the COVR groups may improve their viscoelastic properties and therefore contribute to restored biomechanics as described below.
Remaining work includes quantitative and qualitative measurement of elastin and collagen. We attempted assessment of these key ECM molecules on histology, but were greatly limited by freezing artifact. Although ideal in preserving HLA isotopes for IHC, frozen sections were not optimal for ECM analysis. Despite these challenges, we have demonstrated that multiphoton microscopy is able to highlight elastin and collagen fibers in VFs. Subjectively, fiber length and straightness were greatest in the chronic COVR specimens, with scar VFs showing short clumps of both elastin and collagen. Future studies with FFPE sections and other microstructure-preserving methods may allow for quantification of these findings.
Restoration of VF Biomechanics
The purpose of the implanted COVR is to restore a functional, pliable ECM after acute or chronic injury and to prevent subsequent tissue scarring. A previous study demonstrated markedly increased GAGs, reduced collagen deposition, and restoration of lamina propria width 6 weeks after acute COVR implantation.6 This work compared that acute implant scenario with the common clinical case of implantation after mature scar. The elastic modulus of the untreated scar VF was greater than the contralateral uninjured VF, consistent with the stiffening effect of VF scarring. In contrast, both acute COVR implant and chronic COVR implant groups were indistinguishable from their contralateral unoperated VF. These data support that the COVR implant effectively restored tissue biomechanical properties both in an acute and chronic injury model. However, the data did not detect a difference across groups (i.e., scarred VF moduli did not differ statistically from COVR-implanted moduli). We attribute this discrepancy to limited statistical power (6 animals per group). Alternatively, biological variation between animals may make the inter-animal comparison less valid than the intra-animal comparison of operated versus unoperated VF. Finally, the microindentation technique is highly sensitive to tissue hydration, which may contribute to error.
Functional Outcomes
Ex vivo phonation was performed to assess vibratory function of all three groups. Methodological difficulties produced usable phonation in only about half of the larynges, distributed across all groups. Although laryngeal vocal processes were sutured together into phonation position, this failed to achieve adduction along the entire membranous VF, resulting in inconsistent achievement of phonation. This may have also contributed to the limited effect of airflow had on fundamental frequency. Because the VFs may have been adducted at or just above the positional threshold to phonate, much of the airflow may have passed the larynx without inducing oscillation. Additionally, the difference in fundamental oscillatory frequency between the chronic scar and chronic COVR groups is distinct, but its significance is not clear until this shortcoming of our phonation approach is resolved. Previous studies have shown effective vibratory assessment in implanted rabbit VF.7, 9 Methodological refinements are planned to improve the robustness of this technique in future experiments.19
CONCLUSIONS
COVR was effectively able to restore VF mucosa in a scarred model. This is a significant advance over previous work, which assessed the wound healing only after acute cordectomy. This study demonstrates the feasibility of COVR for its intended clinical use of treating mature scars. Notably, 8 weeks after implantation, both acute COVR and chronic COVR groups had persistence of implanted cells with detectable HLA by IHC. Results also demonstrated increased hyaluronic acid content in COVR VFs. Moreover, acute and chronic COVR implantation resulted in recovery of VF stiffness to normal levels as measured by elastic moduli compared with unoperated VF, suggesting that COVR implantation restored tissue biomechanics in both acute and chronic injury models. With refinements in methodology and technique, further work will continue to elucidate the functional phonatory outcomes and characterize ECM components between acute and chronic COVR.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical assistance of Feng Schrader and Carlos Castillo-Galdamez and the Division of Laboratory Animal Medicine.




