Prognostic Role of Tumor-Infiltrating Lymphocytes and Tumor Budding in Early Oral Tongue Carcinoma
Editor's Note: This Manuscript was accepted for publication on April 14, 2021.
This study was supported by a grant from Kanagawa Prefectural Hospitals' Cancer Fund.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives/Hypothesis
Occult lymph metastasis is an important prognosticator for the treatment of early oral tongue squamous cell carcinoma (SCC). The objective of this study was to evaluate the prognostic significance of tumor-infiltrating lymphocytes (TILs) in early oral tongue SCC. The combination of the TIL subtype and intermediate- or high-grade budding scores was investigated as a prognostic marker for occult neck metastases.
Study Design
Retrospective study.
Methods
Specimens from 62 patients with early oral tongue SCC treated with only primary surgery were analyzed by immunohistochemistry for CD4+, CD8+, FoxP3+, and CD45RO+ T cells and CD163+ macrophages. The highest number of each TIL subtype was counted in two areas of parenchyma and stroma in the tumor (Tumor) and peripheral stroma of the invasion margin.
Results
Based on multivariate analysis, a high density of Tumor CD163+ macrophages served as the poorest prognostic factor for regional control (RC) and disease-free survival (DFS). Patients with both a high density of Tumor CD163+ macrophages and an intermediate- or a high-grade budding score had a poor prognosis for RC according to the log-rank test.
Conclusions
In summary, each TIL subtype may use different mechanisms during early and advanced stages of oral tongue SCC. A high density of Tumor CD163+ macrophages was determined to be a risk factor for RC and DFS as well as an additional stratification factor for RC in patients with intermediate- or high-grade budding scores. Therefore, identifying TIL subtypes in daily clinical practice can help determine a more successful and individualized therapeutic approach for early oral tongue SCC.
Level of Evidence
Step 4 (Level 4) Laryngoscope, 131:2512–2518, 2021
INTRODUCTION
Management of the diagnosis and treatment of early oral tongue squamous cell carcinoma (SCC) is controversial. Depth of invasion (DOI) has been added to the T classification in the eighth edition of the American Joint Commission on Cancer/Union for International Cancer Control (AJCC/UICC) TNM classification. These changes were made because occult lymph node metastases are reported in 20% to 40% of early oral tongue SCC, which are major prognostic factors of the disease.1-6
Tumor budding (TB) is defined as the invasion of a single cell or small clusters of tumor cells (<5 cells) at the tumor invasive front.7 According to the scoring system of the International Tumor Budding Consensus Conference, the budding score is defined as the highest amount of TB viewed at 20×, and a score < 5 is defined as low grade, <10 as intermediate grade, and ≥10 as high grade.8 Previously, we found few late neck metastases in patients with low-grade budding score in early oral tongue SCC. Moreover, the BD model, which is a combination of TB and DOI, has been shown as a useful pathological prognostic factor for lymph node metastases.9, 10 Recent studies reported the prognostic significance of TB for oral SCC (OSCC).11-15
The recent elucidation of the mechanisms of the tumor microenvironment in tumor progression led to remarkable breakthrough in the field of cancer immunotherapy. Determination of immune mechanisms and identification of novel prognostic biomarkers are useful for selecting the best cancer therapy to improve survival rates.16
In the current study, we focused on inflammatory cells in the early stages of oral tongue SCC, which are thought to define the states before and after microscopic metastasis. It has been reported that combinations of pathological characteristics such as tumor invasion patterns, TB and DOI, and immune cells are useful predictors of occult neck metastases.17, 18 Thus, we investigated tumor-infiltrating lymphocytes (TILs) as an additional stratification factor that may be associated with occult neck metastases in patients with intermediate- or high-grade budding scores.
MATERIALS AND METHODS
Patients
Examination by computed tomography (CT), magnetic resonance imaging, ultrasonography, and positron emission tomography/CT (since 2004) was performed prior to surgery. All patients underwent excision of the primary tumor with adequate margins (≥10 mm). Patients were followed by examination with ultrasonography or CT once every 1 to 3 months for the first 2 years. For neck recurrence, selected neck dissection (ND) was performed. Postoperative radiotherapy or concurrent chemoradiotherapy was recommended for cases with adverse features.9, 10 A total of 189 patients were diagnosed with clinical early oral tongue SCC between September 2000 and March 2017 at the Kanagawa Cancer Center. Excluding patients with carcinoma in situ, recurrence of SCC, history of preoperative chemotherapy, or high-dose-rate brachytherapy, 62 patients were enrolled in the current study. The staging was classified according to the eighth edition of the AJCC/UICC TNM classification. The study was approved by the Ethics Committee of Kanagawa Cancer Center. Informed consent was obtained from all individual participants included in the study. The procedures complied with the Declaration of Helsinki.
Immunohistochemical Staining
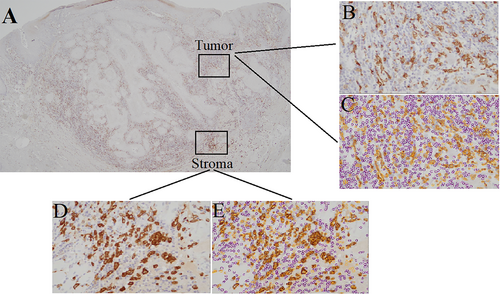
Resected tissues 4-μm thickness were fixed with 10% buffered formalin and embedded in paraffin. The paraffin-embedded sections were incubated with antibodies for pan-cytokeratin (clone AE1/AE3/PCK26, Ventana Medical Systems, Tucson, AZ; ready to use), CD4 (clone SP35, Ventana Medical Systems; ready to use), CD8 (clone SP57, Ventana Medical Systems; ready to use), forkhead box P3+ (FoxP3, clone 236A/E7, Nichirei, Tokyo, Japan; 1:200), CD163 (clone 10D6, Nichirei; 1:100), and CD45RO (clone UCHL1, Nichirei; ready to use) (Fig. 1).

Immunohistochemistry analysis of pan-cytokeratin, CD4, and CD8 was performed using the BenchMark ULTRA instrument (Ventana Medical Systems). Immunohistochemistry analysis of FoxP3+, CD163, and CD45RO was performed as follows. The tissue sections were deparaffinized in xylene, rehydrated with an alcohol gradient, and rinsed with a phosphate buffer solution (washing buffer; pH 9.0; Nichirei). The sections were quenched with peroxidase-blocking solution (Dako, Carpinteria, CA; ready to use) for 5 min, washed with the washing buffer, and incubated for 30 min with primary antibodies against FoxP3, CD163, and CD45RO. After rinsing with washing buffer, the sections were incubated for 30 min with the secondary antibody clone UCHL1 (Nichirei; ready to use) and washed again. The sections were immersed in a solution of 3,3′-diaminobenzidine (Dako) for visualization and counterstained with hematoxylin.
Evaluation of Staining
Histopathological review was performed by one of the authors and an experienced pathologist blinded to the patients' conditions (y.h. and t.y., respectively). In addition, the labels of the pathology specimens were de-identified. TB was assessed using pan-cytokeratin-immunostained sections. The number of immune cells was counted with a computerized image analysis system, Patholoscope ver.1.2.1 (Mitani Corporation, Fukui, Japan). Immune cells were identified by their specific markers, including CD4, CD8, FoxP3, CD163, and CD45RO. The software can recognize and count positive and negative cells separately in immunostaining image formats using a technical cell extraction algorithm. The count of immune cells was normalized with a semiautosystem of Patholoscope. For each marker, a maximum of one view (0.843 × 0.634 mm per one format) was examined at 20×. Two areas of parenchyma and stroma in the tumor (Tumor) and peripheral stroma at outside of the tumor invasion margin (Stroma) in which the highest number of each TIL subtype was found were selected to evaluate the staining (Fig. 2).
Statistical Analysis
All statistical analyses were performed using SPSS version 23.0 (IBM Corp, Armonk, NY). Regional control (RC) was defined as the period between the primary surgery and regional recurrences. Disease-free survival (DFS) was defined as the period between the primary surgery and first recurrence, last examination, or death due to any cause. The prognostic factors for lymph node metastases and DFS were analyzed by the Kaplan–Meier method and univariate analysis with log-rank tests using clinical characteristics (sex, pathological T stage, differentiation, budding score, and DOI) and each subtype of immune cells in the Tumor and Stroma. The median number of each immune cell subtype was used as the cutoff value to divide the samples into low- and high-infiltrating groups. A Cox proportional hazards model was used to test the risk factors of clinical characteristics and poorest prognostic subtype of immune cells detected by univariate analysis. Correlations for each factor were analyzed using Spearman's rank correlation coefficient. P-values <.05 were considered significant.
The subtypes of immune cells that showed significance in the multivariate analysis and budding score were evaluated. Combined evaluation was performed by categorizing the subtypes into three groups; group 1: low-density subtype and low-grade budding score, group 2: high-density subtype or intermediate/high-grade budding score, and group 3: high-density subtype and intermediate/high-grade budding score. The associations between each group and the DOI were evaluated using the Mann–Whitney U test.
RESULTS
Forty-three (69%) of the 62 patients were men and 19 were women, with a median age of 61 years (range, 31–87 years). The median follow-up duration was 68 months (range, 8–201 months). Thirty-five (56%) and 27 (44%) patients were classified as having stage I and II diseases, respectively (Table I). Eleven patients had tumors with DOI >5 mm. Neck recurrence was observed in 15 patients within 2 years after the primary surgery. Excluding one patient treated with definitive chemoradiotherapy, selective ND was performed in 14 patients (93%). Pathological N1, 2, and 3b recurrence was observed in 4 (29%), 4 (29%), and 6 (43%) patients, respectively. Three, eight, four, two, and one recurrence were localized from level I to V of the ipsilateral side, respectively. Finally, 10 patients were salvaged.
| RC | DFS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Number of Cases (%) | Median Survival (Mo) | 5-Yr RC (%) | HR | 95% CI | P-Value | Number of Cases (%) | Median Survival (Mo) | 5-Yr DFS (%) | HR | 95% CI | P-Value |
| Gender | |||||||||||||
| Male | 43 | 10 (23) | 30 | 75 | 1.16 | 0.40–3.39 | .788 | 17 (40) | 24 | 62 | 1.11 | 0.46–2.68 | .817 |
| Female | 19 | 5 (26) | 25 | 73 | 7 (37) | 25 | 72 | ||||||
| Pathological T stage | |||||||||||||
| T1 | 35 | 6 (17) | 66 | 82 | 2.11 | 0.75–5.94 | .144 | 11 (31) | 44 | 73 | 1.81 | 0.81–4.07 | .138 |
| T2 | 27 | 9 (33) | 22 | 64 | 13 (48) | 21 | 54 | ||||||
| Differentiation | |||||||||||||
| Well | 45 | 9 (20) | 44 | 79 | 2.14 | 0.76–6.03 | .136 | 16 (36) | 29 | 66 | 1.56 | 0.67–3.67 | .294 |
| Moderate | 16 | 6 (38) | 21 | 59 | 8 (50) | 21 | 59 | ||||||
| Poor | 1 | 0 (0) | 13 | N/A | 0 (0) | 13 | N/A | ||||||
| Budding score | |||||||||||||
| Low grade | 35 | 1 (3) | 66 | 97 | 25.09 | 3.29–191.46 | <.01 | 9 (26) | 65 | 85 | 3.03 | 1.32–6.95 | .005 |
| Intermediate/high grade | 27 | 14 (52) | 13 | 45 | 15 (56) | 12 | 37 | ||||||
| Depth of invasion | |||||||||||||
| ≤5 mm | 51 | 6 (12) | 62 | 82 | 3.48 | 1.24–9.82 | .011 | 16 (31) | 56 | 75 | 3.53 | 1.43–8.69 | .003 |
| >5 mm | 11 | 9 (82) | 20 | 38 | 8 (73) | 20 | 16 | ||||||
- CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; N/A = not applicable; RC = regional control.
Eight (13%) and two (3%) patients succumbed to the original disease and other causes, respectively. The 5-year RC and DFS rates were 74% and 65%, respectively. Based on univariate analysis, prognostic factors for both RC and DFS were identified as intermediate- or high-grade budding score (P < .01 for both) and DOI >5 mm (P < .05 and P < .01, respectively) (Table I).
The median numbers of Tumor and Stroma CD4+ T cells were 532 (4–3,727 cells) and 838 (128–3,059 cells), respectively; Tumor and Stroma CD8+ T cells were 370 (4–2,175 cells) and 638 (73–2,437 cells), respectively; Tumor and Stroma FoxP3+ T cells were 153 (5–548 cells) and 251 (9–1,139 cells), respectively; Tumor and Stroma CD163+ macrophages were 131 (0–602 cells) and 229 (10–660 cells), respectively; and Tumor and Stroma CD45RO+ T cells were 677 (9–1,978 cells) and 1,104 (145–2,828 cells), respectively.
Univariate analysis identified high densities of Tumor CD4+ (P < .05), CD8+ (P < .01), FoxP3+ (P < .01), and CD45RO+ T cells (P < .05) and CD163+ macrophages (P < .001) as risk factors for RC. Additionally, high densities of Tumor FoxP3+ T cells (P < .05) and CD163+ macrophages (P < .001) were identified as risk factors for DFS (Table II). Multivariate analysis identified a high density of Tumor CD163+ macrophages (hazard ratio [HR], 5.06; 95% confidence interval [CI], 1.12–22.88; P < .05) and an intermediate or a high grade of budding score (HR, 17.91; 95% CI, 2.28–140.73; P < .01) as independent risk factors for RC. Furthermore, a high density of Tumor CD163+ macrophages (HR, 3.34; 95% CI, 1.27–8.75; P < .05) was identified as an independent risk factor for DFS in multivariate analysis (Table IIIA,B). Between both of DOI and budding grade or Tumor CD163+ macrophages, and between budding grade and Tumor CD163+ macrophages, intermediate correlations (rs = 0.581, 0.595, and 0.403, respectively) were observed.
| RC | DFS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtype of TILs | Number of Immune Cells | Number of Cases (%) | Median Survival (Mo) | 5-Yr RC (%) | HR | 95% CI | P-Value | Number of Cases (%) | Median Survival (Mo) | 5-Yr DFS (%) | HR | 95% CI | P-Value |
| CD4+ T cells | |||||||||||||
| Tumor | Low | 4 (13) | 67 | 86 | 3.41 | 1.08–10.74 | .025 | 9 (29) | 65 | 79 | 2.11 | 0.92–4.85 | .067 |
| High | 11 (35) | 20 | 62 | 15 (48) | 15 | 49 | |||||||
| Stroma | Low | 7 (23) | 66 | 77 | 1.3 | 0.47–3.61 | .611 | 12 (39) | 65 | 73 | 1.47 | 0.64–3.63 | .355 |
| High | 8 (26) | 20 | 74 | 12 (39) | 15 | 55 | |||||||
| CD8+ T cells | |||||||||||||
| Tumor | Low | 4 (13) | 65 | 86 | 3.22 | 1.02–10.13 | .033 | 9 (29) | 62 | 78 | 2.16 | 0.94–4.96 | .058 |
| High | 11 (36) | 21 | 64 | 15 (48) | 20 | 51 | |||||||
| Stroma | Low | 10 (32) | 22 | 65 | 2.19 | 0.75–6.41 | .14 | 16 (52) | 20 | 51 | 1.98 | 0.84–4.67 | .106 |
| High | 5 (16) | 56 | 84 | 8 (26) | 44 | 79 | |||||||
| FoxP3+ T cells | |||||||||||||
| Tumor | Low | 3 (10) | 65 | 90 | 4.66 | 1.31–16.52 | .008 | 8 (26) | 62 | 78 | 2.63 | 1.12–6.20 | .02 |
| High | 12 (39) | 22 | 59 | 16 (52) | 21 | 51 | |||||||
| Stroma | Low | 7 (23) | 22 | 75 | 1.08 | 0.39–2.98 | .88 | 14 (45) | 20 | 60 | 1.44 | 0.64–3.26 | .37 |
| High | 8 (26) | 62 | 74 | 10 (32) | 62 | 69 | |||||||
| CD163+ macrophages | |||||||||||||
| Tumor | Low | 2 (7) | 66 | 93 | 8.28 | 1.86–36.84 | <.001 | 6 (19) | 64 | 81 | 4.37 | 1.73–11.05 | <.001 |
| High | 13 (42) | 17 | 57 | 18 (58) | 12 | 49 | |||||||
| Stroma | Low | 6 (19) | 65 | 80 | 1.69 | 0.60–4.74 | .314 | 10 (32) | 64 | 73 | 1.79 | 0.78–4.07 | .15 |
| High | 9 (29) | 21 | 69 | 14 (45) | 20 | 53 | |||||||
| CD45RO+ T cells | |||||||||||||
| Tumor | Low | 4 (13) | 66 | 87 | 3 | 0.95–9.43 | .047 | 10 (32) | 64 | 76 | 2.11 | 0.91–4.89 | .071 |
| High | 11 (36) | 21 | 61 | 14 (45) | 20 | 52 | |||||||
| Stroma | Low | 10 (32) | 24 | 65 | 2.18 | 0.74–6.37 | .143 | 14 (45) | 21 | 54 | 1.6 | 0.71–3.61 | .247 |
| High | 5 (16) | 58 | 84 | 10 (32) | 29 | 76 | |||||||
- CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; RC = regional control; TILs = tumor-infiltrating lymphocytes.
| A: Regional Control | |||
|---|---|---|---|
| Characteristics | Hazard Ratio | 95% CI | P-Value |
| Tumor CD163+ macrophages | 5.06 | 1.12–22.88 | .035 |
| Budding score | 17.9 | 2.28–140.73 | .006 |
| Depth of invasion | 1.16 | 0.41–3.33 | .782 |
| B: Disease-Free Survival | |||
|---|---|---|---|
| Characteristics | Hazard Ratio | 95% CI | P-Value |
| Tumor CD163+ macrophages | 3.34 | 1.27–8.75 | .014 |
| Budding score | 1.95 | 0.79–4.84 | .15 |
| Depth of invasion | 1.76 | 0.65–4.73 | .263 |
- CI = confidence interval.
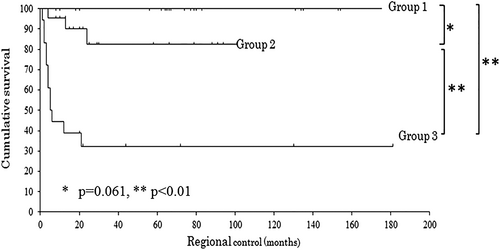
Combined evaluation of Tumor CD163+ macrophages and budding score, which were both prognostic factors for RC by multivariate analysis, was performed. Next, 22 (35%), 22 (35%), and 18 (29%) patients were classified into group 1 (low density of Tumor CD163+ macrophages and low grade of budding score), group 2 (either a high density of Tumor CD163+ macrophages or an intermediate/high grade of budding score), and group 3 (high density of Tumor CD 163+ macrophages and intermediate/high grade of budding score), respectively. Zero, four, and seven patients with a DOI >5 mm were included in each group. The 5-year RCs for patients in groups 1, 2, and 3, were 100%, 83%, and 32%, respectively. There was no significant difference between groups 1 and 2 (P = .061), as observed by the log-rank test; however, there were significant differences between groups 1 or 2 and 3 (P < .01 for both) (Fig. 3). For DOI, the Mann–Whitney U test showed an association between both groups 1 and 2 and groups 1 and 3 (P < .01 for both) but not between groups 2 and 3 (P = .066).
DISCUSSION
CD4+ T cells, CD8+ T cells, Tregs, tumor-associated macrophages (TAMs), and CD45RO+ memory T cells have been assessed as prognostic markers of malignant tumors in previous studies.19-24Particularly, CD8+ T cells, Tregs, and TAMs have been reported by many studies as key markers of the progression of a variety of cancers.25-29 Tregs are a small subset of CD4+ T cells and suppress the activation and proliferation of immune cells.28 FoxP3 is a transcription factor and specific marker of Tregs.28 TAMs are macrophages present in the tumor microenvironment. Upon stimulation, TAMs are polarized into one of two main types: M1 macrophages, which have proinflammatory and antitumor roles, and M2 macrophages, which have immunosuppressive and tumor-promoting roles.19 CD163 is a specific marker of M2 macrophages.29 CD45RO+ memory T cells are maintained for years in the body after encountering antigens; thus, CD45RO+ cells can respond to antigen re-exposure faster than after the first exposure.30
In the current study, high infiltration of Tumor CD163+ macrophages was the poorest prognostic marker for RC and DFS among all subtypes of TILs. Systematic reviews of macrophages and the prognosis of OSCC reported that an increased number of TAMs or CD163+ macrophages is a poor prognostic factor.19, 29 M2 macrophages may be novel prognostic markers of RC in the early stages, and consistently through advanced stages.
Most studies reported that a high density of CD8+ T cells in OSCC and head and neck SCC (HNSCC) predicts a favorable prognosis.20-22, 31-33 However, univariate analysis revealed infiltration of CD8+ T cells as a risk factor for RC in the current study. The immune activity of CD8+ T cells may be promoted depending on tumor progression in the early stages; the present study simply reflected the promoted activity of CD8+ T cells based on the tumor progression for lymph node metastasis, and the activity of CD8+ T cells had a limited influence on prognosis in the early stages. Watanabe et al also reported that CD8+ T cells are significant only in the advanced as opposed to early stages in 87 patients with OSCC.34
Some studies reported that a high density of CD4+ T cells is associated with tumor progression; thus, it is a poor prognostic marker.21, 35 However, increasing infiltration of FoxP3+ T cells tended to associate with poor tumor progression, which was consistent with the result of previous studies evaluating patients in the early and advanced stages of OSCC or HNSCC.24, 35 Although this topic has been widely examined, previous studies on the role of Tregs in prognosis revealed varying results, and the prognostic influence of Tregs remains unclear.21, 23, 33, 36 This could be probably because of the changing relationship between the number of infiltrating Tregs and prognosis with tumor progression, as O'Higgins et al36 and Hanakawa et al24 described in their systematic reviews.
There are a few studies on CD45RO+ T cells in OSCC. One study showed that a high density of CD45RO+ T cells is significantly associated with a favorable prognosis.37 A meta-analysis of solid tumors, such as colorectal, esophageal, and gastric tumors, reported that tumor-infiltrating CD45RO+ T cells led to favorable clinical outcomes.38 Almost all studies included in the meta-analysis evaluated all stages of solid tumors. Turksma et al analyzed blood samples of patients with HNSCC. Although the percentage of effector memory T cells does not differ significantly across the four tumor stages, it is higher in patients with stage I and II tumors than in healthy donors, and subsequently declines in patients with advanced stages, displaying the highest prominence in stage II.39 Univariate analysis revealed a high infiltration of CD45RO+ T cells as a poor prognostic factor of RC. This may be consistent with the mechanism in which memory T cell differentiation tends to accelerate with tumor progression in early stages.39, 40
In early oral tongue SCC, it is assumed that when a tumor appears to be more aggressive, a protective immune response is derived, and TILs infiltration increases. These are activated owing to the elevated invasiveness of tumors; thus, the differentiation to memory T cells is accelerated. Finally, the immunosuppressive activity and high infiltration of Tregs are important in the prognosis of oral tongue SCC.36, 40 The current study showed the status of each TIL subtype in the coexistence of both elimination and escape before and after microscopic metastases in early stages of oral tongue SCC. Although the prognostic significance of each subtype of TILs, such as CD4+ T cells, CD8+ T cells, Tregs, and memory T cells, may differ between the early and advanced stages, only M2 macrophages emerged as a consistent prognostic marker for all stages of the disease.
TB was a poor prognostic factor for both RC and DFS in univariate analysis and associated with poor RC in multivariate analysis, along with TILs. DOI was not a prognostic factor for RC or DFS in multivariate analysis. Moreover, the combination of TB and CD163 macrophages was suggested as a significant predictor of occult lymph node metastasis. Wang et al reported that the combination of invasion patterns and memory T cells is a prognostic marker for poor OS in early OSCC.17 Yu et al also reported that the iBD score, which evaluates inflammatory response and the BD model, is a risk factor for lymph node metastases and oral tongue SCC recurrence.18 Although TB and CD163+ macrophages were poor prognostic factors according to multivariate analysis, there was no significant difference between groups 1 and 2. This was partly due to the patients with occult lymph metastases almost belonging to group 3. This result also suggested that the acquisition of epithelial–mesenchymal transition and activation of subtypes of TILs are associated with tumor progression and metastases. Therefore, elucidating the mechanism of epithelial–mesenchymal transition and immunoediting for tumor progression may demonstrate the usefulness of TB as not only a prognostic factor but also a determinant for the treatment strategy.
Nevertheless, a few limitations exist to this investigation. First, this study was not cohort-based, and involved relatively few patients, as only patients with early oral tongue SCC from one institution were enrolled. Given that the number of deaths was low, poor prognostic markers for survival could not be sufficiently evaluated. Second, the methods for evaluating subtypes of TILs are controversial. Shimizu et al evaluated the density of CD8+ T cells from five different anatomic locations and showed the prognostic difference across the locations.20 In this study, two areas, in which a larger number of each TIL subtype was found, were selected; the number of immune cells was counted using a computerized image analysis system. Thus, there may have been selection bias. Therefore, our results must be validated in a prospective cohort study of a large number of patients and reassessed by comparison with the advanced stages of oral tongue SCC.
CONCLUSION
Each TIL subtype may have different mechanisms and predictive significances for early and advanced stages of oral tongue SCC. The current study showed that CD163+ macrophage were poor prognostic markers of both RC and DFS, and the combination of TB and CD163+ macrophages was a novel prognostic marker for regional recurrences in early oral tongue SCC. Identification of TIL subtypes in daily clinical practice may lead to more successful and individualized therapeutic approaches for early oral tongue SCC.
ACKNOWLEDGMENTS
We gratefully acknowledge the role of Sachie Osanai for her technical assistance with immunohistochemistry.
AUTHOR CONTRIBUTIONS
Conception and design of the study: y.h., a.k., t.y., n.o. Acquisition of data: y.h., a.k., t.y., m.f., t.m., n.k. Analysis and/or interpretation of data: y.h., a.k., t.y., n.o. Drafting the manuscript and revisiting the manuscript critically for important intellectual content: y.h., a.k., t.y., m.f., t.m., n.k., n.o. Approval of the version of the manuscript to be published: y.h., a.k., t.y., m.f., t.m., n.k., n.o. Agreed to be accountable for all aspects of the work: y.h., a.k., t.y., m.f., t.m., n.k., n.o.