PCA Atrophy and Synkinesis as the Main Factors for Persistent Vocal Fold Immobility in RLN Paralysis
Abstract
Objectives/Hypothesis
An immobile vocal fold due to recurrent laryngeal nerve (RLN) injury usually shows no gross signs of atrophy and lies near the midline. In 1881, Felix Semon proposed that this phenomenon was due to a selective injury of nerve fibers supplying the posterior cricoarytenoid muscle (PCA) and supported this with postmortem proof of selective PCA atrophy. In recent decades, evidence has emerged that the RLN regenerates after injury but does not always result in useful motion of the vocal folds. It has been proposed that this is caused by laryngeal synkinesis. Laryngeal synkinesis describes a random distribution of regenerated nerve fibers to opposing vocal fold muscles. This study was conducted to clarify the relative contribution of these two potential pathomechanisms in our patient population.
Study Design
Retrospective case analysis.
Methods
Retrospective analysis of laryngeal EMG results from cases with RLN paralysis of at least 6 months duration seen at our neurolaryngology clinic.
Results
Out of 118 PCA EMGs, there was not a single normal or near-normal tracing, whilst 33.3% of TA EMGs indicated normal or near normal innervation. PCA EMGs showed signs of persistent high-grade partial denervation (41.5%) as a sign of atrophy, moderate or strong synkinesis (21.2%), or a combination of both (37.3%).
Conclusions
In chronic RLN paralysis the intrinsic laryngeal muscles are affected to different extents either by atrophy or synkinesis or a combination of both. The PCA is always affected. The lesser damage to TA innervation explains the commonly seen maintenance of vocal fold muscle bulk.
Level of Evidence
4 Laryngoscope, 131:E1244–E1248, 2021
INTRODUCTION
Many cases of persisting RLN or vocal fold “paralysis” (VFP) do not show relevant signs of vocal fold atrophy.1-3 Further, discrete closing movements from the common paramedian resting position are sometimes preserved or may recover with time.2, 4 A permanent intermediate or lateral position of the immobile vocal fold is far less common.2, 5 This led the pioneers in laryngoscopy to term the condition “abductor paralysis.” Felix Semon was among the first. In 1881 he presented a case series of 22 patients which included evidence of selective atrophy of the posterior cricoarytenoid muscle (PCA) from post mortem dissections in seven of 10 cases. He created a lesion model based on a proposed higher vulnerability of abductor nerve fibers of the recurrent laryngeal nerve (RLN) to become preferentially affected in partial RLN lesions.1 For almost a century this lesion model was referred to as Semon's law.6, 7
With the advent of electromyography (EMG) and its use on the larynx (LEMG), another puzzling finding appeared. In some cases of RLN lesions abnormal muscular activity was recorded during antagonistic maneuvers; namely, thyroarytenoid (TA) activity in the vocal folds during forced sniff and PCA activity during phonation.7, 8 This usually coincided with the onset of improvement in patient's voices.5
Noting similarities with synkinetic phenomena in facial nerve paresis, these findings were termed by Roger Crumley as laryngeal synkinesis.9 This accurately described the electrophysiological findings, but in most cases, no “kinesis” (greek: κίνησις = motion) occurred. The more medial or paramedian position of the vocal folds, as opposed to a more intermediate (historically “cadaveric”) position in flaccid paralysis, could be explained by the overall higher strength of the closing muscles. The Wagner Grossmann hypothesis, which related to Semon's findings by postulating a role of the cricothyroid muscle in the paramedian vocal fold position was disproven on these grounds and experimental studies in cats.10 This further discredited Semon's findings. To account for different voice outcomes and vocal fold positions, a laryngoscopic classification system discriminating favorable and unfavorable types of synkinesis was proposed and continues to be used to this day because of its value in therapeutic decision making.11
Synkinesis alone, however, does not adequately explain persisting vocal fold immobility or “paralysis.” First, from a theoretical perspective, it is very unlikely that the forces of antagonistic synkinetic muscles would balance one another so perfectly as to eliminate any abnormal or residual macroscopic movements. Second, in many cases of persistent immobility, there is no relevant synkinesis to be found in LEMG. TA synkinesis rates between 10% and 50% have been reported.7, 12-14 Third, in fresh RLN lesions the damage to TA innervation is sometimes very low.15, 16 Limited impairment of TA innervation at the time of the initial lesion can explain the lower rate of aberrant synkinetic reinnervation to the TA but it cannot explain persisting focal fold immobility.
Other findings implicating the PCA's important role in persistent vocal fold immobility include a lower propensity of the PCA to reinnervate17 and preferential damage to PCA innervation in fresh RLN lesions, especially if iatrogenic.15 With respect to the seemingly contradictory hypotheses of selective abductor paralysis versus synkinesis we wanted to analyze LEMG data from patients with persistent vocal fold immobility longer than 6 months to search for further evidence of the role and nature of PCA damage in chronic vocal fold paralysis.
MATERIALS AND METHODS
LEMG data were obtained from our neurolaryngology clinic and appropriate inpatients from 2008 to 2019. Only LEMG data from vocal fold paresis/paralysis of 6 months duration or longer with no or minimal residual vocal fold movement were included. Malignancy related cases were excluded. Ethic committee approval for data collection and analysis in the form of a registry was obtained by the Medical Association of Thuringia.
All LEMGs were carried out as transcutaneous needle EMGs, TA with a direct puncture via the cricothyroid membrane, PCA with the transcutaneous-transluminal-transcricoidal or “midline” approach first described by Mu18 using bipolar disposable concentric needles (Neuroline® 75–65/25, Ambu, Ballerup, Denmark or Teca elite®, Natus, Middleton, USA). Patients were awake and sitting, local anesthesia with 2% lidocain was used in most cases. One pediatric case was done in the waking phase after general anesthesia with transoral bipolar hooked wires (Inomed, Emmendingen, Germany) for TA and the abovementioned method for PCA. Recordings were done with a four-channel EMG machine (Neuropack M1, Nihon Kohden, Tokyo, Japan) in the same session usually sampling the TA first and the PCA second. In some cases with bilateral VFP LEMG was limited to bilateral PCA recordings. In 2013 we started using multichannel recordings which included channels for a respiratory belt and a thermistor (Natus, Middleton, USA) to register nasal breathing and a contact microphone (Atmos, Lenzkirch, Germany) to record vocal maneuvers, commands, and comments.
From available LEMG parameters only two were used: 1) maximal effort interference pattern (IFP) during agonistic maneuver as an indirect measure for preserved/restored muscle bulk and 2) synkinetic activation during antagonistic maneuver as a measure for pathologic/random reinnervation with the subsequent effect of an autoparalysis or synkinesis.
IFP densities during agonistic maneuver (loud phonation of [a:] or [i:] for TA and forced sniff for PCA) were graded semiquantitatively in accordance with the European Laryngological Society (ELS) consensus paper19 and for further calculations scaled ordinally as a digit between “4” and “0”, 4 - “normal/dense”, 3 - “mildly rarefied”, 2 - “strongly rarefied”, 1 - “single fiber patern/activitiy”, or 0 - “no voluntary activity”.
Synkinetic activity during antagonistic maneuver (forced sniff for TA and normal-effort, normal-pitch sustained vowel phonation for PCA) was graded as “absent”, “mild” (just noticeable up to a mean peak amplitude ratio under 0.65), “moderate” (mean peak amplitude ratio of antagonistic and agonistic maneuver between 0.65 and 1), and “strong” (antagonistic maneuver stronger than agonistic manoeuver and with a density equivalent to a “mildly decreased” interference pattern density or denser). Because the TA exhibits physiologic antagonistic activity, only “moderate” and “strong” antagonistic activity of the TA was considered as positive synkinesis, whereby the cut off of 0.65 was chosen for its negative prognostic value.20 In PCA there seems to be no regular antagonistic activity with normal-effort, normal-pitch sustained phonation. Therefore, any antagonistic activity of the PCA was regarded as a sign of pathologic reinnervation.14, 21 We used the same grading for PCA as for TA for better comparison. All data were collected retrospectively and processed with Microsoft Excel (Redmond, USA). Statistics was descriptive.
RESULTS
Out of 473 patients, 94 met inclusion criteria (60 females; 34 males; age: 4 to 83 years; median 55 years; duration of paralysis: 6 to 640 months, median 18 months). 49 had unilateral VFP; 45 had bilateral VFP. In total, 118 data sets of immobile vocal folds with complete IFP and synkinesis grading of PCA were obtained. Several patients with bilateral paralysis had only PCA recordings done (to avoid glottic swelling), so the number of complete TA recordings was lower (n = 81). Causes of VFP were iatrogenic (n = 66), congenital (n = 4), traumatic (n = 6), idiopathic (n = 15), and neurologic (n = 3).
Out of 118 PCA LEMGs, there were no normal or near-normal LEMG traces, whilst 33.3% of TA EMGs (27 of 81) showed normal or near-normal innervation (see Figs. 1a and 1b). PCA EMGs showed signs of persistent high-grade partial denervation with IFP “strongly decreased” or lower in 49 (41.5%), high-grade synkinesis with “moderate” or “strong” synkinetic activation in 25 (21.2%), or both in 44 (37.3%). The overall rate of high-grade synkinesis was 58.5% (69) and the overall rate of high-grade partial denervation was 78.8% (93). High-grade TA lesions (66.6%) were due to high-grade partial denervation in 27.2%, high-grade synkinesis in 24.7%, or a combination of both in 14.8%.
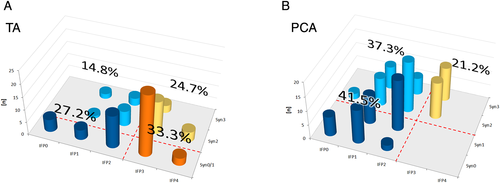
On average, IFP of the TA was higher than that of the PCA with only little lower values on the left side. Synkinesis rates tended to be higher on the right side and in the PCA. In cases of longer-duration paralysis, average IFP and synkinesis rates were higher, whereas the differences between right- and left-sided values became smaller. There were some differences between iatrogenic and noniatrogenic lesions, but the median duration of paralysis of both groups was different, which might have influenced results (17 months in noniatrogenic vs. 48 months in iatrogenic lesions) (see Table I).
| TA | PCA | |||
|---|---|---|---|---|
| Average IFP | Synkinesis | Average IFP | Synkinesis | |
| All, right side | 2.55 | 40.4% | 1.84 | 88.7% |
| (±0.97, n = 47) | (19 of 47) | (±0.97, n = 62) | (55 of 62) | |
| All, left side | 2.32 | 38.2% | 1.63 | 75% |
| (±0.96, n = 38) | (13 of 34) | (±0.97, n = 56) | (42 of 56) | |
| Iatrogenic, right side | 2.66 | 43.6% | 1.84 | 91.8% |
| (±0.78, n = 38) | (17 of 39) | (±0.94, n = 49) | (45 of 49) | |
| Iatrogenic, left side | 2.65 | 33.3% | 1.68 | 78.4% |
| (±0.63, n = 26) | (8 of 24) | (±0.85, n = 37) | (29 of 37) | |
| Noniatrogenic, right side | 2.11 | 33.3% | 1.85 | 76.9% |
| (±1.54, n = 9) | (3 of 9) | (±0.99, n = 13) | (10 of 13) | |
| Noniatrogenic, left side | 1.45 | 28.6% | 1.5 | 66.7% |
| (±1.13, n = 11) | (2 of 7) | (±0.79, n = 18) | (12 of 18) | |
| 6 to 12 months duration, right side | 2.41 | 40.9% | 1.46 | 75% |
| (±1.18, n = 22) | (9 of 22) | (±1.06, n = 24) | (18 of 24) | |
| 6 to 12 months duration, left side | 2.12 | 16.7% | 1.33 | 47.6% |
| (±1.27, n = 17) | (2 of 12) | (±0.8, n = 21) | (10 of 21) | |
| Longer than 12 months duration, right side | 2.68 | 42.3% | 2 | 97.4% |
| (±0.75, n = 25) | (11 of 26) | (±0.84, n = 38) | (37 of 38) | |
| Longer than 12 months duration, left side | 2.48 | 42.1% | 1.8 | 91.4% |
| (±0.6, n = 21) | (8 of 19) | (±0.8, n = 35) | (32 of 35) | |
- IFP's of PCA are lower than of TA, synkinesis rates of PCA are higher than of TA; average IFP gradings and synkinesis rates increase with duration of paralysis, especially synkinesis rates of the left side, indicating a delayed nerve regeneration of left sided RLN lesions. In old lesions almost all PCA show at least some minor signs of synkinetic reinnervation; differences between noniatrogenic and iatrogenic cases are influenced by different median durations of paralysis; IFP grading: 0 – no voluntary activity, 1 – single fiber activity, 2 – strongly decreased IFP, 3 – mildly decreased IFP, 4 – normal/dense IFP; ± standard deviation and/or number of cases in brackets below.
DISCUSSION
Out of 118 PCA LEMGs performed in cases of persistent immobile vocal folds, none appeared close to normal, all PCA showed either a poor reinnervation or a high grade synkinesis or combinations of both (Fig. 1b). This was in contrast to TA LEMGs, of which one third were normal or near-normal (Fig. 1a). Persistent high-grade TA denervation was less common (42%). The latter corresponds to the common clinical observation of preserved vocal fold muscle bulk in most cases we see in our clinics.
These results correlate with Semon's findings1 and with findings of computed tomography studies.22, 23 Semon relied on clinical examinations of his own and other contemporary laryngologists but also had postmortem proof of atrophied PCA and sustained TA muscle bulks for some of his cases. None of those cases in 1881 were iatrogenic; rather, they were related to tumors, infections, were idiopathic, or believed to be of central origin. Nerve regeneration processes might have been unknown to the author and electrophysiologic testing was not yet available, so preferential damage or higher vulnerability of abductor fibers of the RLN was assumed.
Higher vulnerability of abductor nerve fibers was not something we saw in acute noniatrogenic or tumor-related cases.15 But both findings could be explained by a lower regeneration propensity of PCA nerve fibers, which was proven in animal studies and could apply to humans as well.17, 24
In recent years laryngeal synkinesis was advocated as the main reason for a persistent vocal fold immobility or vocal fold “paralysis.”7-9, 11 As mentioned above there are some flaws to the concept of synkinesis as the sole reason for this condition. First, the unlikeliness of always perfectly balanced forces in the tug of war between adductor and abductor muscles and, second, there are many cases who do not even have a relevant amount of synkinesis in LEMG.7, 12-14 The number of cases in the early studies was low, and because medical conditions and medical practice have changed since 1881, there were never post mortem proofs. In the present case series, high-grade synkinesis was more often present in the PCA (58.5%) than in the TA (39.5%). In the remainder of cases, there was not much pathologic reinnervation.
In most cases, persistent denervation and pathologic/synkinetic reinnervation occurred together and enhanced each other to varying degrees. That makes it difficult to estimate an exact threshold for IFP and synkinesis that would correlate with a loss of function at rest (as clinical examinations were done at rest). Such a threshold would also depend on the function of the antagonistic muscles. However, despite the uncertainties of LEMG grading in clinical reports and limited muscle sampling during routine LEMG examinations, we did not find any near-normal PCA LEMGs in our population of patients with persisting vocal fold immobility. As mentioned above, this contrasted with the findings from TA LEMGs, which commonly showed less severe damage.
One weakness of this study was that we - like the majority of LEMG users - did not include EMG of the lateral cricoarytenoid muscle (LCA). If PCA and TA commonly show different degrees of damage, LCA is likely to be different too. LCA could - from the origin of its innervation - range somewhere between the TA and PCA. As the main adductor, the LCA could have a major impact especially on vocal fold position and the voice outcome in VFP. Because reliable LCA sampling in LEMG is difficult to achieve, mainly due to its close proximity to TA, there is very limited data on the LCA in patients with VFP.8 Despite these difficulties it might be worthwhile to pursue more detailed studies of LCA EMG in selected cases in future.
Another way to assess the LCA function could be a thorough clinical examination with close-up high-resolution endoscopy under local anesthesia as described by Richardson and Bastian25 or just recently Fleischer and Hess.26 However, correlation of clinical findings with LCA-EMG needed to be established and are likely to depend on the overall muscle balance too. Ultrasound measurements of LCA are well established in horses as an early indicator of equine laryngeal neuropathy,27 but we have not yet been able to successfully visualize the muscle in humans in a similarly useful way.
Considering the higher vulnerability of PCA nerve fibers compared to TA innervation1, 15 together with the likely lower propensity for regeneration of PCA nerve fibers compared to TA17 we postulate an ascending lesion model for RLN damages similar to Semon's postulate: Involvement of intrinsic laryngeal muscles in RLN paralysis varies depending on the severity of the nerve lesion. PCA which receives its nerve branches first is involved always, LCA with its intermediate branch may be damaged to variable degrees, and TA receiving the terminal and mechanically best protected RLN branches is least damaged. This is most obvious for mechanical (i.e. iatrogenic) RLN lesions but may partially hold true for noniatrogenic lesions too. The reasons for this worse prognosis for PCA innervation remain unclear today as in 1881.
CONCLUSION
In persistent focal fold paralysis, different laryngeal muscles are affected to different extents either by a persistent high-grade partial denervation or high-grade pathologic (synkinetic) reinnervation or both. Therefore, atrophy and/or synkinesis may be limited to few or even a single intrinsic laryngeal muscle.
PCA is always affected. TA only in two thirds of cases. The state of the LCA is not known from this study and should be further investigated. Depending on severity an ascending lesion model for RLN damages is postulated.
The term “abductor paralysis” in the sense of loss of normal function of the PCA seems technically correct. However, it would imply a general noninvolvement of the other intrinsic muscles which is not the case most of the time. We, therefore, leave it to the discussion of the scientific community whether this term should be revived as the standard term for immobile vocal folds or not.
Data of this case series support the findings of Semon and other early laryngologists as well as those who pioneered LEMG and the concept of laryngeal synkinesis such as Hirano, Crumley, Blitzer, Maronian and others.
ACKNOWLEDGMENTS
We thank our colleagues at our neurolaryngology clinic, Kathleen Klinge and Ahmed I. Nasr as well as our technician staff. MED-EL financially supported this research as part of the preclinical research performed by SRH Wald-Klinikum on the laryngeal pacing project. Open access funding enabled and organized by Projekt DEAL.




