Home Sleep Testing to Direct Upper Airway Stimulation Therapy Optimization for Sleep Apnea
Editor's Note: This Manuscript was accepted for publication on August 03, 2020.
a.s., p.m.b., and k.h. are study investigators and received honoraria and travel expenses for invited talks on behalf of Inspire Medical, Inc., outside the submitted work. This study contains data from the industry sponsor Inspire Medical, Inc.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives/Hypothesis
Selective upper airway stimulation (sUAS) is a well-established treatment option for obstructive sleep apnea (OSA). This study aimed to determine if there are benefits in performing a home sleep test (HST) to evaluate postoperative sUAS effectiveness after patient acclimatization compared to the generally used polysomnography (PSG) titration, as measured by long-term follow-up outcomes.
Study Design
Retrospective comparative cohort analysis.
Methods
We conducted an analysis of consecutive patients at our center who had completed a 6-month follow-up (month 6 [M6]) and recorded data from M6, month 12 (M12), and month 24 (M24). After device activation, we performed an HST with the patient's stimulation settings, and measured the apnea-hypopnea index (AHI), Epworth Sleepiness Scale (ESS), and device usage. These values were compared to patients who had undergone PSG-based device titration.
Results
Baseline values of the initial 131 patients show high ESS and moderate OSA. At the 2-month time point of the HST, nearly half of the patients (46.2%) reached an AHI ≤15/hr, and approximately a fifth (19.2%) reached <5/hr. The PSG and HST groups differed in median ESS at M24, but no other differences were observed for ESS at M6 and M12. Both groups showed similar AHI, oxygen desaturation, and usage hours per week.
Conclusions
Adjusting therapy by using the HST technique after device activation and acclimatization has clinical and economic advantages. These advantages are contingent on several conditions being met when deviating from the standard device protocol, including precise communication with the referring sleep medicine physicians, especially their role in helping with long-term follow-up.
Level of Evidence
4 Laryngoscope, 131:E1375–E1379, 2021
INTRODUCTION
Selective upper airway stimulation (sUAS) is a well-established treatment option for positive airway pressure (PAP)-intolerant patients suffering from obstructive sleep apnea (OSA). After a large phase III trial with 5-year follow-up,1, 2 there have been several prospective multicenter studies in Central Europe that utilized long-term follow-up periods, economic benefits analyses,3-5 and reports from large registry cohorts.6, 7
The patients were initially counseled and evaluated on the inclusion and exclusion criteria, particularly if they had undergone drug-induced sleep endoscopy (DISE) to rule out complete centric collapse.8 The implantable pulse generator was placed in the lateral subclavian groove, with the stimulation cuff electrodes applied around the protrussor branches of the hypoglossal nerve in the submental area.9 The respiratory-sensing lead was placed in the fourth, fifth, or sixth intercostal space. The device was activated approximately 1 month after implant, and the patient was trained to self-adjust the degree of stimulation at home for approximately 2 months, followed by an in-laboratory polysomnography (PSG) evaluation to confirm or adjust the stimulation settings. However, access to in-lab PSG testing is limited by the sleep laboratory's capacity and the willingness of patients to travel or lose working days. Furthermore, many patients have problems with falling asleep in the unfamiliar sleep-laboratory environment and prefer recording techniques that can be used at their own home with this second-line treatment modality.
Over the past 2 years, we utilized a 2-month (M2) home sleep test (HST) instead of a titration PSG as an initial evaluation of therapeutic progress with 6-month (M6), 12-month (M12), and yearly follow-ups (e.g., M24). Cases with insufficient apnea-hypopnea-index (AHI) reduction, inadequate clinical benefits, or low therapy usage were addressed with repeat office-based readjustments, PSG therapy titration, or repeated DISE with sUAS reprogramming. In some isolated cases, additional upper airway surgery was performed.10-13
This study attempted to show that our center's approach of using HSTs for the initial sUAS evaluation would be similar to results using PSG titration at the M6, M12, or M24 evaluations.
MATERIALS AND METHODS
The goal of our analysis was to compare the 2-month postoperative outcomes using two different methods: PSG for therapy titration or HST followed by additional visits if necessary. The first sUAS was implanted in November 2012 at our university's department of otorhinolaryngology, which was supported by a department-owned sleep laboratory that utilized the PSG and HST techniques. We initially used PSG for sUAS titration, but in May 2017, we began to use HST during the initial therapy accommodation phase, which was approximately 2 months after the implantation. This approach was supported by improved insurance procedure reimbursement in 2016, which led to increased patient numbers and growing referrals from the sleep-medicine networks, especially for patients who did not live in the area. However, the standard PSG technique was still used in patients with high proportions of baseline central sleep apnea, longer periods of apnea with frequent awakening, or other special situations.
One month after the device was activated, we began therapeutic adjustments to the device that were based on the patient's individual voltage thresholds and needs, such as the duration of the start delay. At this time point (M2) or even earlier, we asked the patients to undergo an HST from our center if they were from the local area, so they could remain at home for testing. HST results and implant usage hours, read out from the sUAS device, would be discussed in an office visit at a later time. For remote patients, they were contacted by mail or phone to recommend the next steps, which could be a normal M6 follow-up, recommending increased amplitude, secondary PSG titration, or DISE with sUAS reprogramming.
We attempted to follow up every patient at M6, M12, and annually thereafter. The annual follow-up included an HST using the stimulation settings that the patient is currently using, the Epworth Sleepiness Scale (ESS) questionnaire, and a telemetry usage readout that was based on the accumulated usage hours and the calculated usage per week since the last telemetry readings. The HST was scored using the following criteria: hypopneas defined as 30% reduction of airflow and 4% oxygen desaturation, the AHI, and the oxygen desaturation index. Some HSTs also included peripheral arterial tonometry-based measurements. If an external sleep practitioner had already performed an HST, we used their results instead of repeating the test. The external HST results are assumed to use the same criteria as those that were previously described.
Statistical Analysis
We used version 22.0 of the Statistical Package for the Social Sciences software (IBM, Armonk, NY) for all analyses. Descriptive statistics were calculated for the demographic variables. Continuous variables were compared with a Mann-Whitney U test. The results are reported as the median and interquartile range. P values ≤.05 were considered statistically relevant.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The local ethics committee (AZ 17-300A; Ethics Committee, University of Lübeck, Lübeck, Germany) approved this retrospective chart review. Informed consent was obtained from all individual participants included in the study.
RESULTS
Cohort Description
From November 2012 to November 2019, there were 141 consecutive sUAS implants. The main reasons for PAP noncompliance were mask leak, panic under the mask, and removing mask at night. There were 131 who had at least a 6-month follow-up. Of these implants (Fig. 1), only 122 of the M6 data points were available due to one patient who was lost to follow-up, five who missed their M6 time point, and one patient who declined to participate because of insurance coverage issues. There were two patients who had longer hospital stays not related to the implant procedure during the M6 visit but were available again for the M12 time point.

There were 117 potential datasets at M12, but only 107 patients participated due to one patient who was lost to follow-up, one who died from non-OSA, cardiovascular or car accident–related causes, and six patients who had not yet completed their M12 time-point. Of these six patients, four are scheduled for their M12 time point outside of the study endpoint. There were 85 potential datasets at M24, but only 69 of the patients could be evaluated due to one who had died from a heart attack, five who were lost during follow-up, and 10 who had not yet completed their M24 time-point.
The two cohorts—standard PSG versus alternative HST adjustment at the M2 time point—were compared, and there were no significant differences in age, body mass index, ESS, or sleep apnea severity (Table I). Both groups consisted predominantly of moderate OSA (PSG 66.7%, HST 61.5%), with a lower incidence of severe OSA (PSG 33.3%, HST 38.5%).
| PSG and HST | Entire Initial Assessment, N = 131 | Implantation, PSG (71), HST (53), n = 124 | Month 6 Assessment, PSG (71), HST (51), n = 122 | Month 12 Assessment, PSG (78), HST, (39), n = 107 | Month 24 Assessment, PSG (61), HST (8), n = 69 |
|---|---|---|---|---|---|
| Age, yr, median (quartiles) | 55.0 (48.0; 61.0) | 55.0 (48.0; 60.0) | ± | ± | |
| 56.0 (49.5; 64.0) | |||||
| Gender, M/F, no. | M/F 112/19 | M/F 65/7 | ± | ± | |
| M/F 43/12 | |||||
| BMI, kg/m2, median (quartiles) | 29.3 (26.5; 32.0) | 29.5 (26.8; 32.0) | 29.7 (26.7; 32.6) | 29.4 (26.5; 32.8) | 29.3 (25,0; 32.1) |
| 29.0 (26.0; 32.0) | 28.1 (25.9; 32.6) | 28.9 (26.0; 32.6) | 28.4 (24.1; 32.9) | ||
| ESS in points, median (quartiles) | 14.0 (10.0; 17.0) | 13.0 (10.0; 16.8) | 8.0 (5.0; 12.0) | 6.0 (3.0; 11.0) | 6.0 (3.0; 9.0) |
| 14.0 (10.3; 16.8) | 8.0 (0.0–19.0) | 7.0 (4.8; 12.0) | 11.5 (8.5; 17.3) | ||
| AHI, events/hr, median (quartiles) | 26.0 (22.0; 36.0) | 25.5 (22.0; 36.0) | 10.0 (5.0; 20.4) | 11.4 (6.0; 19.6) | 13.0 (6.0; 19.8) |
| 25.0 (21.4; 38.4) | 12.8 (7.0; 23.9) | 13.9 (7.7; 28.6) | 12.0 (3.0; 15.0) | ||
| ODI, events/hr, median (quartiles) | 14.9 (10.0; 23.0) | 16.0 (10.0; 26.0) | 5.8 (2.5; 15.0) | 9.0 (3.8; 20.5) | 8.0 (4.0; 20.9) |
| 14.4 (9.0; 21.5) | 7,0 (3.3; 13.3) | 8.9 (4.4; 20.2) | 3.0 (1.0; 21.0) | ||
| Usage, hr/wk, median (quartiles) | N/A | N/A | 47.0 (38.5;52.0) | 43.0 (34.0; 48.5) | 39.0 (32.0; 51.0) |
| 49.5 (39.0; 55.5) | 40.5 (26.5; 52.0) | 44.5 (25.8; 48.8) |
- Bold print for P value <.05 between PSG and HST groups.
- AHI = apnea-hypopnea index; BMI = body mass index; ESS = Epworth Sleepiness Scale; F = female; HST = home sleep test; M = male; N/A = not applicable; ODI = oxygen desaturation index; PSG = polysomnography.
HST Analysis
We began using HST, rather than PSG, for the initial testing at M2 in March 2017. Since then, 53 patients were activated and included in these analyses. In March 2017, there were no established changes to the therapy-adjustment protocol, and five patients received a PSG as standard device optimization protocol after several weeks of activation, including two who came from a new referral (a remote sleep center with good access to PSGs) and three who suffered from severe comorbidities (heart or renal failure or a high-dosage opiate prescription for chronic back pain), which led us to use PSG to make therapeutic adjustments. Four patients were excluded due to missing M6 data. One patient had no HST or PSG data due to a miscommunication during a longer hospital stay.
At the time of activation, the majority of the HST group of patients were adjusted to a bipolar electrode configuration (45 patients), with a subset tailored to a unipolar configuration to improve tongue protrusion appearance (two patients at off minus off, two patients at minus off minus, and no patients at minus minus minus). All patients were initially set to the standard stimulation rate and pulse width configuration (90 microseconds, 33 Hz) and the typical start delay settings (20 to 30 minutes), except for five patients with start delays set to 45 minutes and four with start delays set to 15 minutes or less. Functional voltage levels and amplitude enhancement are strongly dependent on electrode configuration settings.12 Therefore, the amplitude that was set during activation was different compared to HST at M2: at bipolar with 1.4 to 1.9 volts, at minus minus minus with a mean of 0.7 to 1.2 volts, at off minus off with 0.6 to 1.3 volts, and at minus off minus with 0.8 to 1.2 volts. At the M2 time point, the median AHI was 18.5/h, with a range of 0.5/hr to 67.7/hr. About half of the patients (46.2%) achieved an AHI ≤15/hr, and approximately a fifth (19.2%) reached less than 5/hr. The actions taken from the M2 results include the following: 13 patients with sufficient AHI and no need for other interventions were counseled for their regular M6 visit, 11 were motivated to increase amplitude, eight underwent office-based changes for electrode configurations, four were recommended to repeat HST, two were scheduled for PSG adjustments, one was counseled for general therapy considerations, and 11 were scheduled for DISE with activated stimulation to identify persistent obstruction sites and/or testing effects of the altered electrode configurations.
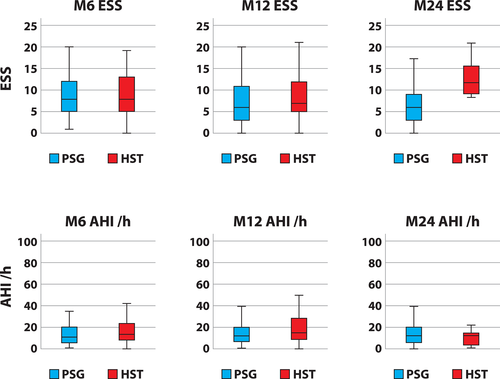
Comparisons between the study groups (Table I) showed no significant differences in age, weight, daytime sleepiness in ESS, or in objective OSA parameters. There was a higher proportion of women in the HST group. Excluding the M24 assessment for ESS (P < .001), there were no differences between the two groups during later follow-ups (Fig. 2, Table II).
| Month 6 Assessment | Month 12 Assessment | Month 24 Assessment | |
|---|---|---|---|
| AHI ≤15/hr | PSG: 66.2% | PSG: 54.3% | PSG: 63.3% |
| HST: 56.9% | HST: 52.8% | HST: 85.7% | |
| AHI ≤5/hr | PSG: 31.0% | PSG: 20.0% | PSG: 21.7% |
| HST: 17.6% | HST: 13.9% | HST: 42.9% |
- AHI = apnea-hypopnea index; HST = home sleep test; PSG = polysomnography.
DISCUSSION
This is the first study focusing on the use of PSG or HST for the treatment-adjustment phase of sUAS in OSA. We showed that there were no significant differences between PSG- or HST-controlled postimplantation therapeutic effects at 2 months and beyond, excluding a difference in ESS at the M24 assessment. The treatment effects are comparable in subjective and objective therapeutic outcomes and usage, and this pattern was observed in both the short-term and the long-term 1- and 2-year follow-ups.
There were several conditions that made the reported results possible:
1. The development of this therapeutic process at our center was streamlined for patient selection, implantation, and therapy adjustment. We began our HST-based therapy adjustments after the 65th implantation.
2. We believe that there are still cases with indications for an initial PSG-based adjustment, which should always be kept in mind. We also described cases with a higher initial proportion of central sleep apnea or unclear respiratory sensing during activation. Improper sensing during implantation should be identified and corrected during the surgery, because respiratory sensing can affect therapy outcomes.10
3. Good communication between the otorhinolaryngology center performing the implant, the local sleep lab, and the patient is essential. Every participant in this network should be committed to dialogue, particularly in cases of persistent severe OSA symptoms or tests that indicate the required next steps for individual adjustments. This includes encouragement for increased therapy usage or voltage, repeated HST or PSG, or DISE with stimulation13 to optimize electrode configurations or identify persistent obstruction sites, such as the soft palate.11 It is also our center's experience that recommending weight-loss programs is effective for overweight patients with persistent OSA problems.
4. New participating sleep centers should start with PSG-based standard device optimization protocol to gain experience on device titration and programming, even if the case selection included DISE and the implantation was performed by an experienced professional.
Nonetheless, the HST-based therapy adjustments in sUAS have several benefits. HST allows therapeutic control at the patient's home. This treatment should be highlighted as a second-line therapy, so many PAP-intolerant patients feel restricted by cables in PSG, which leads to disturbed recordings. There are also many sleep medicine providers in Germany that perform HST but not PSG, meaning that accessibility is simple, and it is easier to accommodate the patient's schedule, especially in a group that is primarily of working age (Table I). In this cohort, the majority of the sUAS candidates already experienced occupational problems with OSA-related symptoms at work or with the first-line PAP therapy. Due to PSG waiting lists in Germany, there is a need to prioritize patients with severe problems, and approximately half of the HST group in this study have a postoperative AHI below 15/hr. In other health insurance environments, using HST reduces the need for prior insurance authorization, decreasing the delay in patient care. Because some issues with sUAS titration could not be resolved in a single-night PSG, a broader range of therapeutic tools is beneficial that includes office-based interventions and DISE. These points taken together, patients appear more compliant, and the HST-based AHI results are more reliable, as they represent a full-night AHI rather than a titrated AHI portion of a sometimes short sleep period.
Our data include several limitations. First, this case series was performed on patients based on clinical intentions and needs. Therefore, the analysis was performed retrospectively and contained out-of-center data that did not undergo our center's quality checks, especially scoring from HSTs ordered from outside clinics. The PSG group outcomes have limitations, as there were new staff who are recently learning the titration technique and also includes German postmarket study cases,3 which were intensively addressed. There are also lost follow-up time points to be considered, but the rates of loss were lower than in comparable reports.4 There was an increase in daytime sleepiness in the ESS that needs to be discussed (Table I), although usage was comparable. The low case number of eight patients needs critical consideration. In this instance, the small group size should be taken into account compared with the large size of the entire group and, in comparison, with the largest registry reports that included 500 to 1,600 cases.6, 7 The response rate (Table II) was also lower in percentage, but the initial distribution showed higher rates of severe OSA cases in the HST group (38.5%) compared with the PSG group (33.3%). Finally, these data contain results from a period when UAS was not as widely adopted as a second-line treatment as today. Whether this explains the gender disparity in both cohorts is uncertain.
CONCLUSION
Therapy adjustments using the HST technique during the postimplant phase of sUAS had clinical advantages. HST is useful as a second-line titration control method, but several conditions are required to justify deviations from the PSG-based standard device optimization protocol.
ACKNOWLEDGMENTS
The authors are grateful to Quan Ni and Kent Lee, Inspire Medical, Inc., Minneapolis, Minnesota, U.S.A., for the initial idea to look closer at M2 results. Open access funding enabled and organized by Projekt DEAL.




