Upregulated METTL3 in nasopharyngeal carcinoma enhances the motility of cancer cells
Zhi-Feng Liu, Jing Yang, and Shu-Pei Wei contributed equally to this study.
Funding information: Guangdong Provincial Department of Science and Technology, Grant/Award Number: 2018A030313525; Hunan provincial Health and Family Planning Commission, Grant/Award Number: B20180186
Abstract
The roles of RNA m6A modification in carcinogenesis have attracted much interest recently. However, the dysregulation of RNA m6A regulators (writers, readers, and erasers) in nasopharyngeal carcinoma (NPC) has never been reported. In this study, we showed that METTL3, one of the writers, was upregulated in NPC. Functional studies revealed that METTL3 promoted the migration and invasion of NPC cells. However, METTL3 knockdown reversed this effect and inhibited the migration, invasion and metastasis of NPC cells. METTL3 activated the luciferase activity of TOPflash (a reporter for beta-catenin/TCF signaling), and downregulation of METTL3 inhibited the expression of beta-catenin/TCF target genes vimentin and N-cadherin, which are two regulators of epithelial-mesenchymal transition. Moreover, dominant negative beta-catenin blocked the migration and invasion of NPC cells. Further mechanistic studies showed that METTL3 silencing decreased the m6A methylation and total mRNA levels of Tankyrase, a negative regulator of axin. Moreover, Tankyrase overexpression abrogated the repressive effects of METTL3 silencing on the migration of NPC cells. Collectively, our study demonstrates the oncogenic roles of METTL3 in NPC, and suggests that METTL3 might be a therapeutic target for NPC.
1 INTRODUCTION
Nasopharyngeal carcinoma (NPC) is prevalent in southern China.1 Metastasis is one of the major causes of recurrence and death related to this malignancy.2 A better understanding of the mechanisms of NPC would benefit therapy.
Methylation at the N6 position of adenosine (m6A) is a highly prevalent and reversible modification within eukaryotic mRNAs that has been linked to many stages of RNA processing and fate.3 METTL3 and METTL14 are the writers for m6A modification, and FTO and ALKBH5 act as the erasers to remove the m6A modification. m6A modification is recognized by the readers (YTHDF1-3 and YTHDC1-2).4, 5 With regard to biological functions, m6A modification plays important roles in embryonic stem cells and mammalian spermatogenesis.6-9 Recently, dysregulation of m6A regulators has been observed in cancer,10 suggesting that targeting m6A modification might be a promising therapeutic approach.
METTL3, the major writer for m6A modification, is involved in various cancer types.11-15 The oncogenic activity of METTL3 is dependent on the cancer type. For example, METTL3 is a tumor suppressor in renal cell carcinoma,16 and an oncogene in breast cancer, pancreatic cancer and hepatocellular carcinoma.12, 14, 15 METTL3 has been reported to affect various aspects of malignant behavior of cancer cells, including chemo- and radioresistance, proliferation and apoptosis and migration.12, 15, 17 Moreover, METTL3 upregulates EGFR and YAP in lung cancer independent of its enzyme activity.18 However, the expression pattern and functions of METTL3 in NPC remain unknown.
In this study, we examined the expression pattern of METTL3 in NPC, and studied its roles in the progression of this malignancy.
2 MATERIALS AND METHODS
2.1 Cell culture and transfection
Cancer cell lines were obtained from Shanghai Cell Bank (The Chinese Academy of Science) and were cultured in DMEM (Invitrogen) with 10% FBS (Sigma). All cell lines were passaged at 37°C with 5% CO2. Cells were transfected with lipofectamine 2000 according to the instructions of manufacture.
2.2 NPC sample collection
NPC tumor samples from NPC patients were obtained from The First Affiliated Hospital of University of South China, Hengyang, China. All samples collected were from patients admitted to the hospital for NPC from 2012 to 2018. These patients included 21males and 16 females, and the age ranged from 47 to 73 years old. The tumor stages of the patients were: 6 stage I, 22 stage II, and 9 stage III. The patient did not receive any form of treatment (eg, chemotherapy and radiotherapy) before surgery. This study was approved by the ethical committee of The First Affiliated Hospital of University of South China (E2018-03). The organization collected permission from the hospital's biomedical ethics committee and informed consent from patients.
2.3 Quantitative real-time PCR
Total RNA was extracted from the tissues using TRIzol (Invitrogen, Rockville, MD) according to the manufacturer's instructions. First-strand cDNA was synthesized using a Random Primers kit (Promega, Madison, WI). The ribosomal protein S18 mRNA level acted as the internal control. The primer sequences for METTL3 were: Forward, 5′-cagcattgtc tccaaccttc-3′; Reverse, 5′-ggttacaagagtaggatgtg-3′.
2.4 Immunohistochemistry
Tissues were cut into 3 to 5 μm sections for immunohistochemistry (IHC) staining. Sections were deparaffinized in turpentine and rehydrated through a series of graded ethanol. Antigen retrieval was performed in a sodium citrate buffer (pH 6.0) in 98°C for 20 minutes. Endogenous peroxidase was inactivated by incubating the slides with 3% hydrogen peroxide. Nonspecific protein binding was blocked with normal goat serum (ZSGB-BIO, Beijing, China) for at least 1 hour. The sections were incubated with the primary antibody at 4°C overnight. Bound antibodies were detected by using the HRP coupled secondary antibody. The signals were visualized by DAB, and nuclei were counterstained using hematoxylin.
2.5 me-RIP assay
The methylated m6A RNA immunoprecipitation (me-RIP) was performed as described previously.19 The methylated LEF1RNA was evaluated by qRT-PCR.
2.6 Cell migration assay
Cell migration assay was performed using Boyden chamber. Cells (2 × 105) suspended in 0.05 mL medium containing 1% FBS were placed in the upper chamber, and the lower chamber was loaded with 0.152 mL medium containing 10% FBS acting as the chemoattractant. Eight hours later, cells migrated to the lower surface of filters was detected with traditional hematoxylin and eosin (H&E) staining. The experiments were repeated for three times. Five random visual fields were counted for each sample and the average was determined.
2.7 Cell invasion assay
An in vitro cell invasion assay was performed using Transwell chambers with polyethylene terephthalate membranes (24-well inserts, 8.0 μm; Corning). Medium (200 μL, 2 × 105 cells) was added to the upper chamber, which had been coated with Matrigel (BD Biosciences). Meanwhile, medium (500 μL) supplemented with fetal bovine serum was added to the bottom chamber to induce invasion. After a 36-hour incubation, the cells were stained with 0.1% crystal violet and counted. The experiments were repeated three times and the data are presented as the means ± SD.
2.8 Immunoblot
To measure the protein levels of the target molecules, the proteins from tissues and cell lines were extracted using RIPA lysis buffer. Proteins were separated by SDS-PAGE. The proteins were transferred to the PDVF membrane. After blocking with the 5% BSA solution for 1 hours at room temperature, the membrane was incubated with primary antibodies overnight. Then, the membranes were washed with TBST solution and incubated with the secondary antibody for 1 hour at the room temperature. The protein was visualized by ECL kit.
2.9 Immunoprecipitation assay
Cells were treated with MG132 (10 μM) for 10 hours and then harvested with RIPA buffer. After centrifugation, the supernatant was immunoprecipitated with antiaxin antibody (Abcam) overnight. The protein G beads were added to the supernatant and incubated for another 4 hours. The beads were washed with RIPA buffer and boiled with 1×loading buffer at 100°C for 5 minutes. Then, the supernatant was examined using western blot with antiubiquitin antibody.
2.10 Reporter assay
NPC cells were grown to a subconfluent density. 16 hours later, the reporter assays were performed using 0.1 μg of Topflash, 0.5 μg of expression vector, and 0.05 μg of TK Renilla luciferase (internal control for transfection efficiency). Forty-eight hours later, cells were treated with Wnt3a (100 ng/mL) protein for 8 hours. Then, cell lysates were prepared and the reporter activity was measured using the dual-luciferase reporter assay system (Promega).
2.11 Metastasis animal model
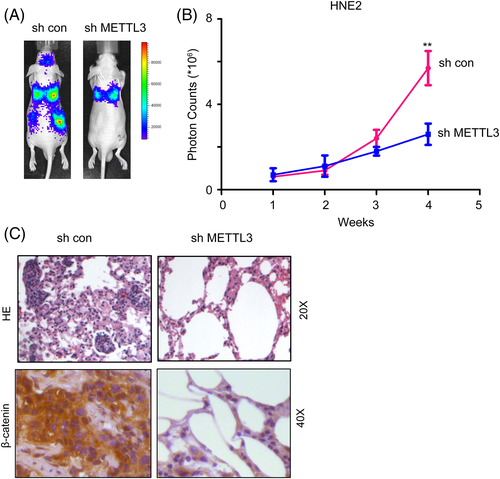
The metastasis of NPC cells was evaluated using the in vivo image systems in vivo. This study was approved by the ethical committee of The First Affiliated Hospital of University of South China. 1 × 105 HNE2 cells (with or without METTL3 knockdown) were labeled with luciferase gene and injected into the tail vein of the nude mice. Mice aged 5 weeks were selected, 4 in each group. The fluorescence intensity of HNE2 cells in the lung was traced for the metastatic foci for 4 weeks. After experiment, mice were sacrificed the lung tissues were fixed, embedded and assayed using HE staining.
2.12 Statistical analysis
The results are expressed as the mean ± SD. For analysis, we used Student t test and One-way ANOVA, and P < .05 was thought to be statistically significant.
3 RESULTS
3.1 The mRNA and protein levels of METTL3 were elevated in NPC
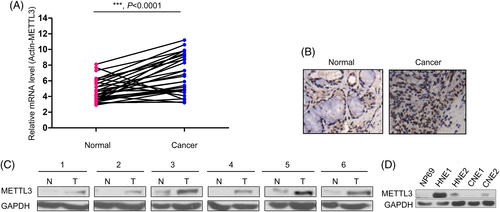
To better understand the mRNA and protein levels of METTL3 in NPC tissues, qPCR was performed to examine the mRNA levels of METTL3 in 37 NPC tissues and paired noncancerous tissues. Statistical analysis revealed that upregulation of METTL3 mRNA in NPC tissues compared to paired normal tissues (Figure 1A). In the following study, we examined the protein level of METTL3 in NPC tissues by IHC and western blot (Figure 1B,C). The results of IHC and western blot analysis suggested that the protein levels of METTL3 were elevated in NPC. In addition, we detected the protein levels of METTL3 in normal NP69 cells and NPC cell lines (HNE1, HNE2, CNE1 and CNE2). METTL3 protein level in NP 69 cells was lower than that in NPC cells (Figure 1D). Taken together, these data suggest that METTL3 is upregulated in NPC.
3.2 METTL3 promoted the migration and invasion of NPC cells
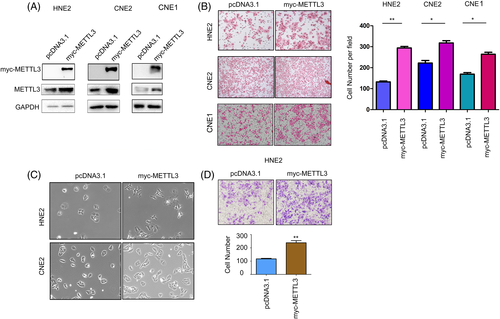
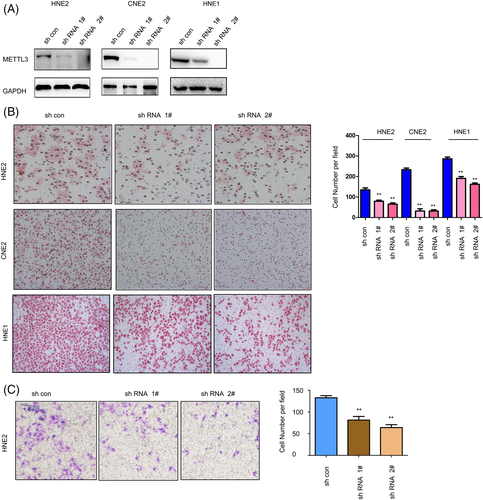
The effects of METTL3 on the malignant behaviors of NPC cells were first examined by gain-of-function assays. Myc-tagged METTL3 (myc-METTL3) was overexpressed in HNE2, CNE2 and CNE1 cells compared to normal controls, which was confirmed by western blotting (Figure 2A). Then, the effects of METTL3 on the mobility of NPC cells were examined by cell migration and invasion assays. Forced expression of METTL3 promoted the migration of HNE2, CNE2, and CNE1 cells (Figure 2B). In addition, overexpression of METTL3 changed the morphology of HNE2 and CNE2 cells to a fusiform shape (Figure 2C). Moreover, METTL3 promoted the invasion of HNE2 cells (Figure 2D). Next, we assessed the functions of endogenous METTL3 in NPC cells. METTL3 expression was knocked down by two shRNA sequences (Figure 3A). Knocking down the expression of METTL3 inhibited the migration and invasion of NPC cells (Figure 3B,C). In summary, these results suggest that METTL3 enhance the motility of NPC cells.
3.3 METTL3 activated beta-catenin/TCF signaling in NPC cells
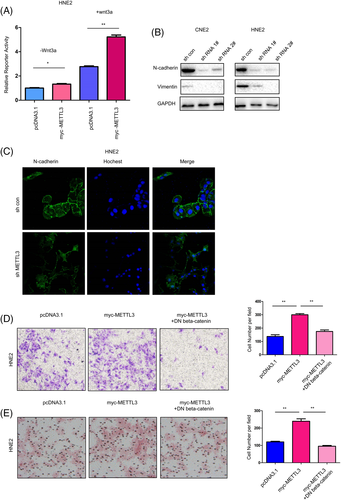
We next examined the reporter activities of various signaling pathways in HNE2 cells after transfection with METTL3. As shown in Figure 4A, METTL3 activated TOPflash (a reporter for beta-catenin/TCF signaling) at the basal level and upon treatment with Wnt3a (Figure 4A), and METTL3 knockdown in HNE2 and CNE2 cells decreased the expression of N-cadherin and vimentin, two epithelial-mesenchymal transition regulators (Figure 4B); these results were further confirmed by immunostaining (Figure 4C). Moreover, dominant negative beta-catenin (DN beta-catenin) significantly blocked the migration and invasion of HNE2 cells induced by METTL3 (Figure 4D,E). Collectively, these results suggest that METTL3 promotes the motility of NPC cells by activating beta-catenin/TCF signaling.
3.4 Downregulation of METTL3 impaired the metastasis of NPC cells
We further explored the roles of METTL3 in the metastasis of NPC cells in vivo. HNE2 cells were labeled with luciferase, METTL3 was knocked down, and cells were injected into the tail vein of nude mice. The fluorescence intensity of HNE2 cells in vivo was traced for 4 weeks using an in vivo imaging system. As shown in Figure 5A,B, knocking down METTL3 expression inhibited the distant seeding of NPC cells. Consistent with these observations, downregulation of METTL3 reduced metastatic foci formation as well as beta-catenin expression in the lungs of nude mice (Figure 5C).
3.5 METTL3 promoted the m6A modification of Tankyrase (the negative regulator for axin) in NPC cells
To elucidate the molecular mechanisms through which METTL3 activated beta-catenin/TCF signaling, we first examined m6A modification in NPC cells after METTL3 overexpression or knockdown. As shown in Figure 6A,B, forced expression of METTL3 in NPC cells increased the m6A modification level, and knockdown yielded the opposite result. It has been reported that several components of Wnt/beta-catenin, such as LEF and beta-catenin, are modified by m6A. We next examined whether METTL3 affected the protein levels of other components. The overexpression of METTL3 in NPC cells increased the protein level of Tankyrase and decreased the protein level of axin (Figure 6C). However, knockdown of METTL3 decreased the protein level of Tankyrase and increased the protein level of axin (Figure 6D). These observations suggested that METTL3 might activate Wnt/beta-catenin signaling by upregulating the expression of Tankyrase. Consistent with this hypothesis, overexpression of Tankyrase rescued the defects in cell migration caused by METTL3 knockdown (Figure 6E). Further me-RIP assays revealed that the m6A modification of Tankyrase was impaired upon METTL3 knockdown (Figure 6F). To further analyze, the effect of the decrease in m6A level on the stability of Tankyrase mRNA, we conducted RNA stability assays and found that knockdown of METTL3 shortened the half-life of the Tankyrase mRNA transcript. Thus, METTL3 knockdown-induced repression of Tankyrase expression is at least partly due to the decreased stability of the Tankyrase mRNA transcript upon METTL3-mediated m6A methylation of Tankyrase mRNA (Figure 6G).
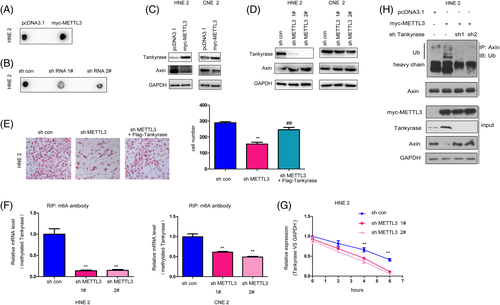
We next examined the roles of Tankyrase in METTL3-mediated regulation of beta-catenin/TCF signaling. As shown in Figure 6H, overexpression of METTL3 promoted the ubiquitination of axin, which was attenuated by the knockdown of Tankyrase, suggesting that METTL3 promoted the degradation of axin by upregulating Tankyrase.
4 DISCUSSION
Recently, dysregulation of m6A regulators (writers, readers, and erasers) has been reported in several cancer types, including colorectal cancer, prostate cancer, hepatocellular carcinoma, pancreatic cancer, and so on.12, 15, 20, 21 However, the expression pattern and the functions of m6A regulators in NPC have never been reported. In this study, we showed that METTL3 expression was elevated in NPC tissues. In a functional study, METTL3 promoted the migration, invasion and metastasis of NPC cells. The mechanistic study showed positive regulation of beta-catenin/TCF signaling by METTL3. These observations clearly demonstrated the oncogenic roles of METTL3 in NPC.
One of the interesting findings of this study is the regulation of beta-catenin/TCF signaling by METTL3. METTL3 was found to activate TOPflash, and knocking down the expression of METTL3 decreased the expression of vimentin and N-cadherin. It has been reported that the mRNAs of some components in the beta-catenin/TCF pathway have m6A modifications.22 Based on these studies, we identified Tankyrase as a substrate of METTL3 in the beta-catenin/TCF signaling pathway and helped to uncover the mechanisms.
Axin is a concentration-limiting factor in the beta-catenin degradation complex.23 Tankyrases are physiological regulators of axin protein homeostasis and Wnt signaling that act by promoting the ubiquitination of axin.24 Moreover, it was found in our study that METTL3-mediated ubiquitination of axin attenuated the knockdown of Tankyrase. These reports and our study provide a good explanation for the regulation of beta-catenin/TCF by METTL3.
In addition, this study demonstrated the upregulation of METTL3 in NPC. METTL3 has been reported to be upregulated in hepatocellular carcinoma (HCC), and its oncogenic functions in HCC are dependent on its enzyme activity.15 However, the oncogenic functions of METTL3 in lung cancer were independent of its methyltransferase activity and were mediated by its regulation of the protein levels of EGFR and YAP.18 These studies suggested that METTL3 might promote the progression of cancer through different mechanisms.
In this study, we evaluated the expression of METTL3 in NPC tissues, and examined its role in regulating NPC cell migration, invasion, metastasis, and beta-catenin/TCF signaling. Our data collectively have clearly demonstrated the oncogenic roles of METTL3 during NPC progression and suggest that METTL3 might be a promising target for future NPC therapeutics.
ACKNOWLEDGMENTS
We thank the colleagues from Department of Otorhinolaryngology of the First Affiliated Hospital of University of South China, for the NPC sample collection from patients.
CONFLICT OF INTEREST
All authors declare no conflict of interest.




