Cancer-Specific Survival of Trimodal Therapy Versus Radical Cystectomy in T2N0M0 Non-Urothelial Bladder Cancer
ABSTRACT
Introduction
We hypothesized that, within organ-confined (OC, T2N0M0) non-urothelial carcinoma of urinary bladder (non-UCUB) patients, trimodal therapy (TMT) use does not differ from radical cystectomy (RC) regarding cancer control outcomes.
Methods
Within the SEER database (2004–2021), rates of TMT versus RC use in OC non-UCUB patients were calculated. Nearest-neighbor 1:1 propensity score matching (PSM) for age, sex, race/ethnicity, and histological subtype was applied. Cumulative incidence plots depicted 5-year cancer-specific (CSM) and other-cause mortality (OCM) rates. Multivariable competing risks regression (CRR) models were fitted. Sensitivity analyses were performed within squamous cell (SCC), neuroendocrine (NEC), and adenocarcinoma (ADK) and other histological subtypes.
Results
Of 814 OC non-UCUB patients, 310 (38%) received TMT versus 504 (62%) RC. After PSM, 5-year CSM rate was 50% after TMT versus 29% after RC and TMT was associated with 2.1-fold higher CSM relative to RC (multivariable HR [mHR]: 2.1, p < 0.001). In sensitivity analyses within 229 (28%) SCC, TMT patients had higher 5-year CSM rates relative to their RC-counterparts (67% vs. 22%, mHR: 4.3, p < 0.001). Similarly, within 314 (39%) NEC, TMT patients had higher 5-year CSM rates relative to their RC-counterparts (mHR: 1.8, p < 0.001). Conversely, within 118 (28%) ADK and 153 (19%) other subtypes, CSM after TMT did not differ from CSM after RC (33% vs. 15%, mHR: 1.4 and 43% vs. 33%, mHR: 1.4; p = 0.4).
Conclusion
In OC non-UCUB patients TMT is associated with significantly higher CSM than RC. Most pronounced survival disadvantage was recorded within SCC and NEC patients.
1 Introduction
Non-urothelial carcinoma of the urinary bladder (non-UCUB) accounts for up to 20% of all bladder cancer subjects [1]. Although radical cystectomy (RC) represents standard of care in these population [2-6], previous studies reported the use of trimodal therapy (TMT) in non-UCUB patients, particularly in those harboring (OC, T2N0M0) stage [7-9]. For instance, De Angelis and colleagues reported that, among 5130 TMT-treated patients, 425 (8%) harbored non-UCUB [7]. This management represents an exception to the guidelines recommendations, where TMT should be reserved to OC UCUB [2-4]. Nevertheless, the rates of TMT versus RC in OC non-UCUB patients, along with their respective oncological outcomes, have not been systematically evaluated in comparison to RC [7-11]. To address this gap, we assessed cancer-specific mortality (CSM) in OC non-UCUB patients relative to RC-treated counterparts, using detailed matching and additional adjustments for other-cause mortality (OCM). We hypothesized that the use of TMT—defined as a combination of transurethral resection, chemotherapy, and radiotherapy—did not change over time and that CSM rates do not significantly differ between TMT and RC. To test these hypotheses, we analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database (2004–2021).
2 Materials and Methods
2.1 Study Population
Within the SEER database (2004–2021), we retrieved patients with histologically confirmed, nonmetastatic (M0), organ-confined (stage T2N0), non-urothelial carcinoma of the urinary bladder (International Classification of Disease for Oncology [ICD-O] site code C67.0–67.9). All patients underwent either RC and pelvic lymph node dissection or TMT. TMT was defined as a combination—without discrimination of the specific sequence—of transurethral resection of the bladder tumor (TURBT), chemotherapy (CT) and external beam radiotherapy (EBRT) [9, 12]. Patients with unknown age, vital status, TNM stage, and race/ethnicity were excluded, as well as autopsy-only or death-certificate-only cases.
2.2 Statistical Analyses
Utilization rates of TMT versus RC were calculated and temporal trends within the study period were assessed using the Estimated Annual Percentage Changes (EAPC). The primary endpoint for survival analyses consisted of CSM, defined as deaths attributable to non-UCUB, based on SEER mortality codes. To balance baseline characteristics between TMT and RC groups, Propensity score matching (PSM) with 1:1 case-to-control ratio according to the nearest neighbor was applied for age, sex, and histological subtypes. Five-year CSM rates according to treatment (TMT vs. RC) were depicted using cumulative incidence plots. Univariable and multivariable competing risks regression (CRR) models were fitted to evaluate the impact of treatment modality (TMT vs. RC) on CSM, after adjusting for OCM. Covariates consisted of age, sex, race/ethnicity, household income (≥ $70 000 vs. < $70 000) and histological subtypes. Additionally, sensitivity analyses were performed within the most frequent non-UCUB histological subtypes: squamous cell carcinoma (SCC), neuroendocrine carcinoma (NEC), adenocarcinoma (ADK), and other non-urothelial histological subtypes. Within each of the four sensitivity analyses, PSM was reapplied between TMT and RC patients and CRR models were refitted. All tests were two-sided, with a significance level p < 0.05. R software environment for statistical computing and graphics (R version 4.4.2, R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
3 Results
3.1 Descriptive Characteristics of the Study Population
Within 814 patients harboring OC non-UCUB, 310 (38%) underwent TMT and 504 (62%) RC. Relative to their RC counterparts, patients treated with TMT were older (75 vs. 68 years, p < 0.001) and less frequently male (63% vs. 71%, p = 0.014). Relative to RC-counterparts, TMT patients more frequently harbored NEC (50% vs. 32%, p < 0.001) histological subtype (Table 1). After 1:1 PSM, 289 (93%) OC non-UCUB patients treated with TMT were matched with 289 (57%) RC patients, and no residual differences for age, sex, and histological subtypes were recorded between TMT versus RC patients (Table 1 and Figure S1).
| Characteristics | Overall | Before PSM | After 1:1 PSM | |||||
|---|---|---|---|---|---|---|---|---|
| TMT (N = 310, 38%) | RC (N = 504, 62%) | pa | TMT (N = 289, 93%) | RC (N = 289, 57%) | pa | |||
| Age (years) | Median (IQR) | 70 (62, 78) | 75 (66, 80) | 68 (60, 75) | < 0.001 | 73 (64, 80) | 72 (66, 78) | 0.071 |
| Sex (male) | n (%) | 551 (68) | 194 (63) | 357 (71) | 0.014 | 182 (63) | 193 (67) | 0.2 |
| Histological subtypesb | n (%) | < 0.001 | 0.8 | |||||
| SCC | 229 (28) | 77 (25) | 152 (30) | 76 (26) | 86 (30) | |||
| NEC | 314 (39) | 154 (50) | 160 (32) | 134 (46) | 127 (44) | |||
| ADK | 118 (15) | 32 (10) | 86 (17) | 32 (11) | 30 (10) | |||
| Other | 153 (19) | 47 (15) | 106 (21) | 47 (16) | 46 (16) | |||
| Radiotherapy deliveredc | n (%) | NA | NA | 235 (47) | NA | NA | 154 (53) | NA |
| Chemotherapy deliveredc | n (%) | NA | NA | 16 (3.2) | NA | NA | 7 (2.4) | NA |
| Race/ethnicity | n (%) | 0.8 | 0.11 | |||||
| Caucasians | 679 (83) | 254 (82) | 425 (84) | 233 (81) | 253 (88) | |||
| Hispanics | 48 (5.9) | 20 (6.5) | 28 (5.6) | 20 (6.9) | 15 (5.2) | |||
| Asians/Pacific Islanders | 38 (4.7) | 15 (4.8) | 23 (4.6) | 15 (5.2) | 11 (3.8) | |||
| African Americans | 49 (6.0) | 21 (6.8) | 28 (5.6) | 21 (7.3) | 10 (3.5) | |||
| Median household income | n (%) | 0.8 | 0.8 | |||||
| ≥ $70k/year | 534 (66) | 205 (66) | 329 (65) | 192 (66) | 195 (68) | |||
| < $70k/year | 280 (34) | 105 (34) | 175 (35) | 97 (34) | 94 (33) | |||
- Note: The significant values are highlighted in bold.
- a Wilcoxon rank sum test; Pearson's χ2 test.
- b Abbreviations: SCC, squamous cell carcinoma; NEC, neuroendocrine carcinoma; ADK, adenocarcinoma.
- c Relative to RC-treated patients; all patients treated with TMT, by definition, received both modalities.
3.2 Rates of TMT vs. RC in OC Non-UCUB Patients Over Time
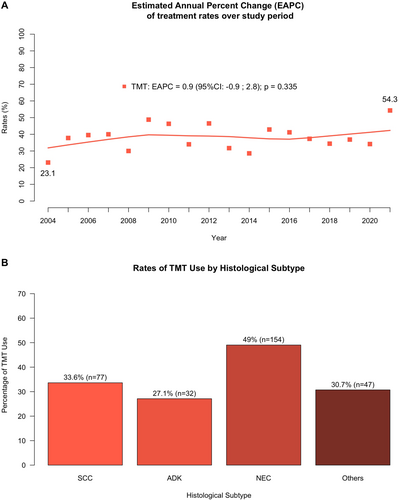
Rates of TMT relative to RC were plotted in OC non-UCUB patients between 2004 and 2021. Over the study period, use of TMT remained unchanged (EAPC: +0.9, 95% confidence interval [CI]: −0.9, +2.8; p = 0.3, Figure 1A).
3.3 Survival Analyses
3.3.1 TMT Versus RC CSM Rates in OC Non-UCUB Patients
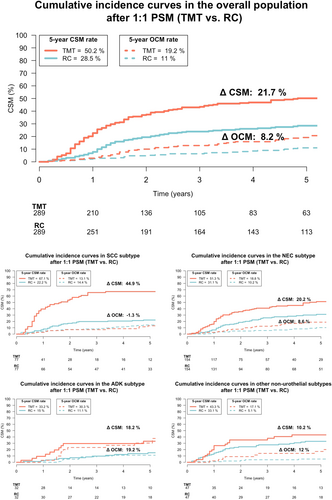
In cumulative incidence plots, 5-year CSM rates were 50% in TMT-treated patients versus 29% in RC-treated patients (Figure 2). In univariable CRR model addressing CSM, after accounting for OCM, TMT predicted 2-fold higher CSM relative to RC (hazard ratio [HR]: 2.0, 95% CI: 1.6–2.7, p < 0.001, Figure 3). In multivariable CRR models addressing CSM after adjusting for covariates, TMT independently predicted 2.1-fold CSM relative to RC (HR: 2.1, 95% CI: 1.6–2.8, p < 0.001, Figure 3).

3.4 Sensitivity Analyses Within Non-UCUB Histological Subtypes
3.4.1 TMT Versus RC CSM Rates in OC SCC of Urinary Bladder Patients
Within 229 SCC patients, 77 (34%) underwent TMT (Figure 1B). After 1:1 PSM, 77 (100%) OC SCC of urinary bladder patients treated with TMT were matched with 77 (51%) RC patients, and no residual differences for age and sex were recorded between TMT versus RC (Table S1). In cumulative incidence plots, 5-year CSM rates were 67% in TMT-treated patients versus 22% in RC-treated patients (Figure 2). In multivariable CRR models addressing CSM, after adjusting for covariates and OCM, TMT independently predicted 4.3-fold higher CSM relative to RC (HR: 4.3, 95% CI: 2.5–7.4, p < 0.001, Figure 3).
3.4.2 TMT Versus RC CSM Rates in OC NEC of Urinary Bladder Patients
Within 314 SCC patients, 154 (49%) underwent TMT (Figure 1B). After 1:1 PSM, 154 (100%) OC NEC carcinoma of urinary bladder patients treated with TMT were matched with 154 (96%) RC patients, and no residual differences for age and sex were recorded between TMT versus RC (Table S1). In cumulative incidence plots, 5-year CSM rates were 31% in TMT-treated patients versus 51% in RC-treated patients (Figure 2). In multivariable CRR models addressing CSM, after adjusting for covariates and OCM, TMT independently predicted 1.8-fold higher CSM relative to RC (HR: 1.8, 95% CI: 1.2–2.6, p = 0.003, Figure 3).
3.4.3 TMT Versus RC CSM Rates in OC ADK of Urinary Bladder Patients
Within 118 ADK patients, 32 (27%) underwent TMT (Figure 1B). After 1:1 PSM, 32 (100%) OC ADK of urinary bladder patients treated with TMT were matched with 32 (37%) RC patients, and no residual differences for age and sex were recorded between TMT versus RC (Table S1). In cumulative incidence plots, 5-year CSM rates were 33% in TMT-treated patients versus 15% in RC-treated patients (Figure 2). In the multivariable CRR model addressing CSM, after adjusting for covariates and OCM, TMT use was unrelated to CSM rates (HR: 1.4, 95% CI: 0.6–3.3, p = 0.5, Figure 3).
3.4.4 TMT Versus RC CSM Rates in NOC Other Non-UCUB Histological Subtypes Patients
Within 153 other non-UCUB patients, 47 (31%) underwent TMT (Figure 1B). Other non-UCUB histological subtypes consisted of 21 (14%) lymphoepithelial, 18 (12%) pseudosarcomatous, 16 (11%) large-cell, and 98 (64%) rare or non-otherwise specified non-UCUB carcinomas. After 1:1 PSM, 47 (100%) OC other non-UCUB patients treated with TMT were matched with 47 (44%) RC patients, and no residual differences for age and sex were recorded between TMT versus RC patients (Table S1). In cumulative incidence plots, 5-year CSM rates were 43% in TMT-treated patients versus 33% in RC-treated patients (Figure 2). In the multivariable CRR model addressing CSM, after adjusting for covariates and OCM, TMT was unrelated to CSM (HR: 1.4, 95% CI: 0.7–2.8, p = 0.4, Figure 3).
4 Discussion
TMT represents a guideline-recommended alternative to RC for carefully selected UCUB patients with solitary tumors, absence of hydronephrosis, and complete resection at TURBT [2-4]. Noteworthy, ~10% of TMT-treated patients harbor non-UCUB [7, 8, 10, 13, 14]. However, guideline recommendations are based almost exclusively on studies focusing on UCUB. Conversely, the impact of TMT on CSM rates compared to RC in OC non-UCUB remains unclear. To bridge this gap, we analyzed the rates of TMT versus RC use in OC non-UCUB patients within the SEER database (2004–2021). Furthermore, we evaluated CSM rates based on treatment approach and reported several key findings.
First, in 814 OC non-UCUB patients, the rate of TMT was 38% (n = 310) and did not significantly change over the study period (EAPC: 0.9, p = 0.3). These observations indicate that ~4 out of 10 OC non-UCUB patients are potentially treated with modalities that are not supported by established guidelines [2-4]. However, this percentage has to be considered with potential patient limitations, where some OC non-UCUB individuals may not be RC candidates or may not be willing to undergo RC. In consequence, in such instances, despite a lack of guideline recommendations, an alternative treatment modality to RC, such as TMT, is required. The current study represents the first analysis of TMT rates in OC non-UCUB patients within a framework of controlled comparison using advanced matching techniques, such as PSM, with RC patients. Previous comparisons performed within population-based data repository have not relied on a controlled methodology [7-10].
Second, we addressed cancer control outcomes associated with TMT relative to RC. Since very important patient characteristics differences may exist relative to RC patients, we relied on 1:1 PSM for age, sex, and histological subtypes to potentially eliminate those differences. In the current population, TMT patients were older (75 vs. 68 years, p < 0.001), less frequently male (63 vs. 71%, p = 0.014), and more frequently harbored NEC (50 vs. 32%, p < 0.001) relative to their RC counterparts. After 1:1 PSM, as well as additional multivariable adjustment for potential residual patient characteristics differences, an important increase in 5-year CSM rates was recorded for TMT relative to RC. Specifically, when 289 (93%) TMT-patients were compared with 289 (57%) RC patients, TMT was associated with 2.1-fold higher risk of CSM relative to RC (50% vs. 29%, multivariable HR (mHR): 2.1, p < 0.001). These findings should be considered at clinical decision-making prior to TMT use when such practice is chosen instead of RC in OC non-UCUB patients. Patients should be informed about a potentially highly appreciable survival disadvantage when TMT is chosen instead of RC especially in individuals who, based on age, performance status, and comorbidity profile, could undergo RC.
Third, four sensitivity analyses were performed within the most frequent non-UCUB histological subtypes, as well as within the subgroup of patients with other non-UCUB histological subtypes. Within each individual sensitivity analysis, PSM was applied relying on the same criteria as in the main analyses. The largest non-UCUB subtype consisted of NEC (n = 314, 39%), followed SCC (n = 229, 28%), ADK (n = 118, 15%), and finally a combination of other histological subtypes (n = 153, 19%; most frequent were lymphoepithelial [14%], pseudosarcomatous [12%], and large-cell [11%] carcinoma). The rates of TMT were highest in NEC (49%), followed by SCC (34%), ADK (27%), and other subtypes (31%). No data support more frequent use of TMT in NEC, SCC, ADK, or other histological subtypes [2-4]. In consequence, it is possible that patients and physician preferences resulted in those TMT rates [15, 16]. Equally different CSM rate comparisons between TMT and RC controls were recorded within each of the four examined histological subtypes groups. The most pronounced and highly significant difference between TMT and RC was recorded in SCC patients (45%) and was exhibited by a mHR of 4.3. This observation is expected, since SCC of the urinary bladder is known to be less sensitive to chemotherapy [5, 17-19]. The second most pronounced difference favoring better survival in RC was in NEC (20%) and was exhibited by a mHR of 1.8. Finally, a difference of 18% and 10% was recorded in ADK and other histological subtypes, respectively. However, based on small numbers of observations, these differences failed to reach statistical significance.
Taken together, the highest rates of TMT were recorded in NEC and SCC. In these two non-UCUB subtypes, TMT also exhibited the most pronounced disadvantage relative to RC. These observations deserve very strong consideration when TMT may represent an option, particularly in those two histological subtypes. However, in ADK, as well as other histological subtypes, CSM disadvantage of 18% and 10%, respectively, should be interpreted as an equally important contraindication, even though statistic significant differences were not reached due to small numbers of observations. However, the lack of variables that may have dictated the use of TMT in patients that were included in the current study precludes a truly objective evaluation of both TMT rates and TMT survival rates relative to RC controls.
Despite our findings provide valuable new insights, several limitations must be considered. Differences in stage and grade classification set TMT patients apart from those undergoing RC. Specifically, TMT patient data on T and N stage, as well as tumor grade, rely on clinical assessment [20, 21], whereas RC patients, who constitute the control group, benefit from pathological staging. However, such a discrepancy has been a limitation in all previous retrospective studies comparing TMT and RC [7-9, 12, 13]. The only exception is prospective randomized trials evaluating bladder preservation versus RC [22, 23]. However, no such trials have been conducted in OC non-UCUB patients, as current guidelines do not endorse TMT for this population. Second, key details regarding radiotherapy and chemotherapy, including type, dose, timing, and intent (curative vs. palliative), were not available. Furthermore, histologic classification was based on initial TURBT specimens without central pathology review, which may introduce diagnostic variability [24, 25]. As a result, the findings of this study are limited to population-based analyses. Similarly, information on the administration of immune checkpoint inhibitors is absent from the SEER database. Consequently, OC non-UCUB patients treated with TMT reflect real-world clinical practice without the ability to break down treatment components, such as chemotherapy and radiotherapy regimens, as would be possible in a prospective study [26]. Additionally, the lack of baseline comorbidity data in SEER prevented more detailed analyses incorporating patient health status. Despite these important limitations, which are shared with previous reports, comparisons between clinical and pathological staging are essential, as no alternative methodology allows meaningful evaluation. Therefore, all studies that attempt to compare TMT relative to RC are particularly valuable until data from prospective designs becomes available.
5 Conclusions
In 814 OC non-UCUB patients within the SEER database, a significant use of TMT relative to RC was recorded. Although TMT was on average associated with higher CSM compared to RC, subgroup analyses according to histological subtypes suggest that the most pronounced survival disadvantage was recorded in SCC and NEC subtypes. These findings emphasize the importance of accurate patient evaluation and thorough preoperative counseling about oncological outcomes when choosing TMT instead of RC in patients harboring OC non-UCUB.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in SEER at https://seer.cancer.gov.
References
Synopsis
Within the SEER (2004–2021), up to 3 out of 10 patients with T2N0M0 non-urothelial bladder cancer received trimodal therapy (TMT).TMT is associated with significantly higher 5-year cancer-specific mortality (CSM) than radical cystectomy (RC) in organ-confined non-urothelial carcinoma of the urinary bladder (50% vs. 29%).The survival disadvantage of TMT was most pronounced in squamous cell and neuroendocrine subtypes.




