Implementation of Hepatic Artery Infusion Pump Therapy: Real-World Single-Center Experience
Robert Connor Chick and Samantha M. Ruff contributed equally to this study.
ABSTRACT
Background and Objectives
Hepatic artery infusion pump (HAIP) therapy is an available option at highly specialized centers to treat unresectable liver tumors (e.g., colorectal liver metastases [CRLM]). This study describes the safety and outcomes of HAIP program implementation at an academic-based cancer center.
Methods
Patients who underwent HAIP placement (2021–2023) were included. Categorical and continuous variables were compared using Chi-square and Kruska–Wallis tests, respectively. Survival and variables associated with survival were calculated using the Kaplan–Meier method and Cox proportional hazards model, respectively.
Results
Of the 26 HAIP procedures for unresectable CRLM, four were done as adjuvant therapy. Median duration of HAIP therapy was 9.2 months and four patients subsequently underwent hepatectomy. Complication rate was 37.5%, with biliary complication rate of 23.1%. Median overall survival (OS) from date of diagnosis was 55.2 months. Concurrent primary tumor resection was associated with inferior OS (p = 0.030). Multivariable regression did not identify independent predictors of OS. Progression-free survival from time of HAIP placement was 7.8 months.
Conclusions
HAIP placement was technically successful in most patients with an acceptable complication rate. Survival outcomes were comparable with those described in the literature for HAIP therapy in combination with systemic therapy. The significant difference in outcomes for those with concurrent colectomy warrants further investigation.
1 Introduction
Colorectal cancer is one of the most common noncutaneous cancers in the United States, with prognosis heavily dependent on stage. Patients with early-stage colon cancer have a 5-year survival rate of > 90%, whereas those with metastatic disease have a 5-year overall survival (OS) of only 13% [1]. The liver remains one of the most common sites of distant metastasis for colorectal cancer. When technically feasible, resection of isolated liver metastases can be performed with curative intent [2]. However, only 15%–20% of patients with colorectal liver metastasis (CRLM) present with resectable disease [3]. This may be due to patient status, inadequate future liver remnant or anatomical constraints with biliary drainage, venous outflow, or arterial/venous inflow [4].
Earlier studies demonstrated a preferential perfusion of CRLM through the hepatic arterial system. This is contrary to liver parenchyma derivation of oxygen and nutrients from the portal venous system [5]. Hepatic artery infusion pump (HAIP) therapy takes advantage of liver specific dual inflow vascular anatomy in addition to this anatomic relationship to directly deliver high doses of chemotherapy to target lesions [6]. Moreover, regional delivery of cytotoxic therapy minimizes the systemic side effects. Currently, Floxuridine (FUDR), a drug derivative of the ubiquitously used antimetabolite 5-fluorouracil (5-FU), is predominantly utilized as a standard agent for HAIP therapy. Specific pharmacokinetics of FUDR with a short half-life and high first-pass metabolism in the liver makes it an ideal therapeutic agent for HAIP treatment [6].
HAIP chemotherapy is increasingly being utilized for the treatment of CRLM [7, 8]. Currently, HAIP therapy is limited to select high-volume centers due to complexity of the operation and, importantly, due to intensive postoperative management. However, the adoption is increasing across the United States and worldwide due to its potential efficacy [9]. Limited level 1 data exist to support its efficacy in the era of modern chemotherapy, but in phase II trials it shows favorable results regarding OS and conversion to resection [10]. The most frequent indication is in the setting of unresectable and of high-risk yet resectable CRLM, but additional indications include intrahepatic cholangiocarcinoma [11, 12]. Moreover, the role of HAIP therapy in the treatment sequence for unresectable CRLM is currently under investigation [13, 14]. Current recommendations are to utilize HAIP in the second-line setting in combination with systemic chemotherapy, or as adjuvant therapy after resection or ablation for CRLM [3]. HAIP can also be considered as a first or second line therapy for unresectable ICC [15].
Herein we describe the HAIP program experience of implementing a HAIP program at initial 2 years at a free-standing cancer hospital embedded within a major university.
2 Materials and Methods
2.1 Data Collection
All patients who underwent HAIP placement at The James Cancer Hospital at The Ohio State University, Columbus, OH, USA between January 2021 and October 2023 were included (IRB #2023C0177). The database was created by retrospective chart review by two authors (Julia Monasterio, Taylor Neilson) and verified by two separate authors (RCC, SMR). Demographic, clinical, pathologic, and treatment variables were collected and maintained in a deidentified database. Response in the liver was determined using RECIST criteria based on the radiology report in the patient's medical record [16].
2.2 Description of Technique
HAIP placement was performed by expert surgeons who were previously trained in the technique at a high-volume center (AT, ACK). The first 26 procedures were performed open and two were performed robotically [17]. The technique is similar to that described elsewhere [11]. The procedure begins with mobilization of the liver, cholecystectomy, common hepatic artery lymphadenectomy and identification of the gastroduodenal artery (GDA). The GDA is dissected out for a length of approximately 1–2 cm. Next, the pump pocket is created and the catheter is passed through a tunnel from the pump to the right upper quadrant of the abdomen. The pump is secured to the fascia. The GDA is test clamped and Doppler is used to confirm flow in the proper hepatic artery. The distal GDA is suture ligated, flushed through a transverse arteriotomy, and the catheter is placed with the tip ending at the bifurcation of the GDA from the common hepatic artery. Fluorescein or indocyanine green is injected into the catheter to confirm perfusion. The port is injected with heparinized saline. The pump is typically placed before primary tumor resection (PTR), but following any planned liver resection or ablation.
2.3 Statistical Analysis
Categorical and continuous variables were compared using Chi-square and Kruskal–Wallis tests, respectively. Survival estimates were calculated using the Kaplan-Meier method and comparisons were made with the log-rank test at α = 0.05. OS was determined from both date of diagnosis and from HAIP placement. Progression-free survival (PFS) was defined as documentation of progressive disease or death from any cause and was calculated from date of HAIP placement. Hazard ratios for survival were calculated with univariate and multivariable Cox regression models. Variables with a statistically significant hazard ratio for survival (p < 0.10) were used to construct a multivariate Cox regression model. Statistical analyses were conducted in SPSS (IBM).
3 Results
3.1 Demographics—Entire Population
Thirty-two patients underwent attempted HAIP placement. Median age was 51.2 years, most had an ECOG score of 0 or 1 (96.9%), were male (71.9%), and had no major comorbidities (90.6%, Table 1). Synchronous CRLM was the most common indication for HAIP (78.1%), followed by metachronous CRLM (15.6%) and unresectable cholangiocarcinoma (6.3%). The median Clinical Risk Score was 4; 16 (61.5%) had node-positive primary tumors, 23 (88.5%) had less than 12 months disease-free interval, all had multifocal disease, nine (34.6%) had carcinoembryonic antigen (CEA) over 200, and 12 (46.2%) had at least one tumor over 5 cm in diameter (Table 2) [18].
| Demographic | Category | n (%) |
|---|---|---|
| Age (years) | Median ± SE | 51.2 ± 1.94 |
| ECOG | 0 | 21 (65.6) |
| 1 | 10 (31.3) | |
| 2 | 1 (3.1) | |
| Race | Black | 2 (6.2) |
| Other | 1 (3.1) | |
| White | 29 (90.6) | |
| Gender | Female | 9 (28.1) |
| Male | 23 (71.8) | |
| BMI (kg/m2) | Median ± SE | 28.8 ± 1.13 |
| Charlson comorbidity index | 0 | 29 (90.6) |
| 1 | 0 (0.0) | |
| 2 | 2 (6.3) | |
| 3 | 0 (0.0) | |
| 4 | 1 (3.1) | |
| Inherited syndrome | No | 31 (96.9) |
| Yes | 1 (3.1) |
- Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; SE, standard error.
| Variable | Category | n (%) |
|---|---|---|
| CEA | Median ± SE | 202.6 ± 319.5 |
| Primary tumor location | Biliary Tract | 3 (9.4) |
| Left Colon | 18 (56.3) | |
| Right Colon | 5 (15.6) | |
| Rectum | 6 (15.6) | |
| Somatic mutation status | APC | 1 (3.1) |
| BRAF | 1 (3.1) | |
| KRAS | 8 (25.0) | |
| Multiple | 4 (12.5) | |
| None | 14 (43.8) | |
| Unknown | 3 (9.4) | |
| Mismatch repair deficiency | Yes | 3 (9.4) |
| No | 27 (84.4) | |
| Timing | Synchronous | 25 (78.1) |
| 6–24 months | 1 (3.1) | |
| > 24 months | 4 (12.5) | |
| N/A | 2 (6.3) | |
| Multiagent chemo before HAIP | Yes | 28 (87.5) |
| No | 4 (12.5) |
- Abbreviations: APC, adenomatous polyposis coli; BRAF, v-Raf murine sarcoma viral oncogene homolog B; CEA, carcinoembryonic antigen; HAIP, hepatic artery infusion pump; KRAS, Kirsten rat sarcoma virus; SE, standard error.
Ultimately, HAIP was performed in 28 patients (87.5%). Four patients failed to successfully undergo HAIP placement. One was aborted because the patient had a diminutive GDA and thus no target for catheter insertion, two were aborted due to discovery of peritoneal metastases, and one was aborted due to evidence of portal hypertension in the operating room in a patient with long-standing unresectable intrahepatic cholangiocarcinoma.
3.2 Technical Success and Complications
All patients who underwent HAIP placement were able to receive FUDR. Most often, complete perfusion was achieved before leaving the operating room (n = 21, 75.0%). Of those who had incomplete perfusion, four required postoperative embolization of the nonperfused hepatic artery branch (right or left) and subsequently had complete perfusion of the diseased hemi-liver.
The overall complication rate was 37.5%. Complication rate at 30 days was 0% and at 90 days was 3.6%. In patients who had HAIP placed for CRLM, the most common complication was biliary stricture (23.1%), followed by extrahepatic perfusion (3.8%) and superficial surgical site infection (3.8%). None of the patients who experienced biliary complications had concomitant perfusion complications. Additionally, none were on systemic bevacizumab during HAIP therapy. All patients with biliary complications received more than 6 months of HAIP therapy. No clinicopathologic features demonstrated a statistically significant association with risk of biliary complications. One pump had to be removed due to dislodgement during a subsequent operation, although the patient was no longer receiving FUDR at the time due to disease progression (Table 2).
3.3 Survival
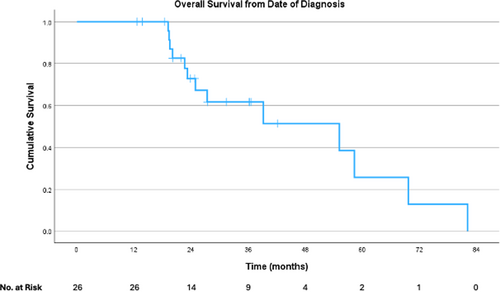
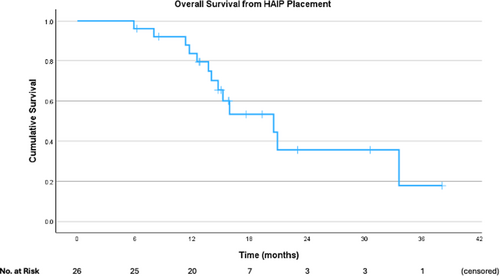
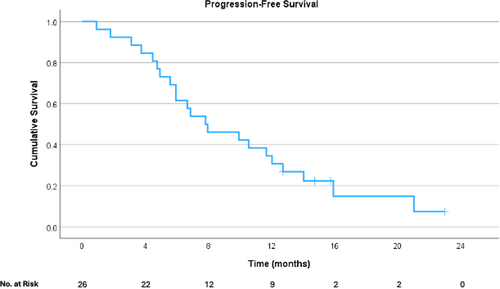
Survival analyses were performed only for those who had HAIP placed for CRLM. Median OS was 55.2 months (95% CI 20.3–90.0 months) from date of diagnosis (Figure 1). Median OS was 20.5 months (95% CI 12.2–28.8 months) from pump placement (Figure 2), and PFS from pump placement was 7.8 months (95% CI 3.8–11.9 months, Figure 3). Median duration of HAIP therapy was 9.2 months (SD + /− 8.1 months).
Factors examined on univariate Cox regression and their associated hazard ratios are summarized in Table 3. Only age > 70 (HR 5.6, 95% CI 1.13–28.2) and liver-specific disease progression (HR 2.56, 95% CI 0.13–1.18) were associated with worse OS. On multivariable Cox regression, neither age nor progression in the liver were significantly associated with OS.
| Variable | Category | n (%) | HR | p-value |
|---|---|---|---|---|
| Age | < 70 | 24 (92.3) | ref | — |
| > 70 | 2 (7.7) | 5.650 | 0.035* | |
| Gender | Male | 18 (69.2) | ref | — |
| Female | 8 (30.8) | 1.300 | 0.649 | |
| BMI | < 30 | 16 (61.5) | ref | — |
| > 30 | 10 (38.5) | 0.525 | 0.286 | |
| Side | Right | 5 (19.2) | ref | — |
| Left | 16 (61.5) | 1.450 | 0.642 | |
| Rectum | 5 (19.2) | 1.200 | 0.857 | |
| Charlson comorbidity index | 0 | 24 (92.3) | ref | — |
| 1 | 0 (0.0) | n/a | — | |
| 2 | 2 (7.7) | 2.230 | 0.453 | |
| CEA | < 200 | 9 (34.6) | ref | — |
| > 200 | 9 (34.6) | 1.765 | 0.406 | |
| Unavailable | 8 (30.8) | 1.221 | 0.780 | |
| Duration HAIP therapy | > 6mo | 22 84.6) | ref | — |
| < 6mo | 4 (15.4) | 2.217 | 0.325 | |
| Timing | Synchronous | 22 (84.6) | ref | — |
| Metachronous | 4 (15.4) | 1.324 | 0.675 | |
| Concurrent | Yes | 18 (69.2) | ref | — |
| No | 8 (30.8) | 1.003 | 0.996 | |
| Adjuvant | Yes | 4 (15.4) | ref | — |
| No | 22 (84.6) | 3.217 | 0.267 | |
| PD on HAIP | Yes | 21 (80.8) | ref | — |
| No | 5 (19.2) | 0.033 | 0.260 | |
| PD in liver | Yes | 10 (38.5) | ref | — |
| No | 16 (61.5) | 0.391 | 0.095* |
- Abbreviations: BMI, body mass index; CEA, carcinoembryonic antigen; PD, progressive disease.
- * denotes statistical significance at p < 0.10.
3.4 Liver-Specific Response
At 12 months after pump placement, there was one complete response, one without evidence of disease after concurrent ablation, and two partial responses. Of those who progressed, six patients had stable hepatic disease with extrahepatic progression, and 17 had progressive liver disease. Four patients were converted to resectable disease. Among those who progressed in the liver, median time to progression was 6.8 months.
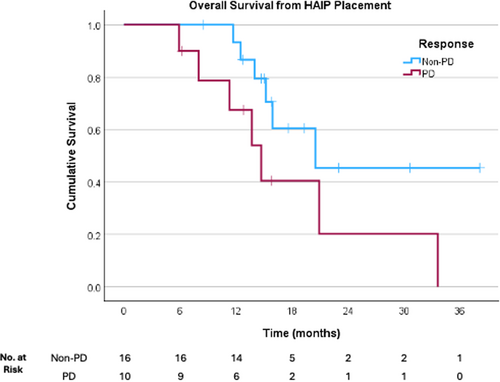
Adjuvant HAIP was planned in four patients. All four of these patients underwent concurrent ablation with or without parenchyma-sparing hepatectomy. There were no significant complications within this cohort. At last contact, two of these patients remained without evidence of disease. Patients who had progression in the liver had clinically inferior, but no statistically significant difference in OS (14.7 months vs. 20.5 months, p = 0.085, Figure 4).
3.5 Systemic Chemotherapy
All patients received systemic chemotherapy before or concurrent with HAIP. Of those with CRLM who had HAIP, 23 (88.5%) had previously been treated with systemic chemotherapy. The most common regimens were 5-FU with oxaliplatin and/or irinotecan, in combination with bevacizumab (50%). Four patients (15.3%) were treated with an epidermal growth factor receptor (EGFR) inhibitor and 5-FU backbone. The majority of patients (57.7%) were treated with HAIP and concurrent systemic therapy; the most common regimens for concurrent systemic chemotherapy were FOLFIRI (40%) and FOLFOX (20%). Only one patient was chemotherapy naïve before HAIP. There was no statistically significant difference in 24-month OS between those who received HAIP with or without concurrent systemic chemotherapy (75.0% vs. 67.3%, p = 0.924).
3.6 Concurrent PTR
Most of the 28 pump placements were performed for CRLM (92.9%). Of these, 18 (69.2%) underwent concurrent PTR. One patient still has their primary tumor intact, four had metachronous CRLM with previously resected primary tumor, and two with synchronous CRLM underwent emergent PTR. OS from date of diagnosis was significantly worse for those who had concurrent PTR (27.4 months) compared with those who did not (69.7 months, p = 0.030). When those with metachronous CRLM were excluded, there was no statistically significant difference in OS (27.4 months vs. 58.3 months, p = 0.263). There was no statistically significant difference in PFS between these cohorts (6.9 months vs. 9.9 months, p = 0.460). There was no difference in OS from date of HAIP placement (20.5 months vs. 20.9 months, p = 0.996).
4 Discussion
This single-center series represents a real-world example of implementation of a HAIP program in a university-based standalone cancer hospital by expert surgeons who were previously trained in the technique. HAIP programs should be managed by a multidisciplinary team within a select expert center. Coordination is required between the surgeon, operating room staff, medical oncology, pharmacy, nursing and outpatient staff, nuclear medicine, and interventional radiology. Additionally, the surgical trainees and advanced practice providers must be familiar with potential short- and long-term complications of HAIP when these patients present in the emergency department. Various other administrative, financial, or institutional barriers may be encountered by centers attempting to start a HAIP program [19].
In prior years, HAIP therapy was offered at our institution. In a series published by Chakedis et al., 60 patients were treated between 2012 and 2016, with a higher proportion treated in the adjuvant setting (43%) [20]. Overall complication rate was similar at 32%, but there was a higher rate of overall pump complications (23%) and lower rate of biliary complications (2%) compared with our current series. Time to liver progression was similar at 9.2 months. Chakedis et al. also examined the influence of sarcopenia and showed it was associated with worse OS (HR 2.4, 95% CI 1.1–5.0) [20]. This surgeon-led program ended in 2016 and there were no HAIPs placed at our institution between 2017 and 2019. Therefore, the series reported herein is distinct from and independent of this older series, and represents implementation of a new HAIP program.
These data demonstrate that HAIP was technically successful in most patients with an acceptable complication rate. The rate of biliary sclerosis among those with CRLM was 23.1%, whereas prior series describe an incidence of approximately 5% for adjuvant HAIP and even lower for unresectable CRLM [21]. However, this higher rate is similar to other contemporary single-institution series [5]. Concurrent bevacizumab can increase the risk of biliary toxicity, but none of the patients in this series were on bevacizumab while receiving HAIP therapy [22]. It is interesting that 83.3% of those with biliary toxicity received bevacizumab before HAIP therapy compared with 66.7% of those without biliary toxicity, though this difference was not statistically significant. There were very few pump pocket or catheter-related complications [11]. Duration of therapy was similar compared to prior series [5, 20, 23]. The rate of complications did not impact oncologic outcomes.
Survival outcomes were comparable with those described in the literature for HAIP therapy in combination with systemic chemotherapy. In a recent systematic review and meta-analysis by Sugumar et al., landmark survival estimates for HAIP with systemic chemotherapy were as follows: 12-month OS 80%, 24-month OS 54%, 36-month OS 35% [24]. Landmark survival estimates were generally similar in our cohort, with 12-month OS of 87.4%, 24-month OS of 71.6%, and 36-month OS of 17.8%. The sharp decrease from 71.6% 24-month OS to 17.8% 36-month OS may be due to relatively short follow-up to date. Only one patient in our series was chemotherapy naïve before HAIP therapy. This represents a potential area for improvement in the HAIP program, as some data suggests a significant benefit of up-front HAIP with systemic chemotherapy in unresectable CRLM [23]. However, the adoption of this treatment is currently being explored through ECOG EA2222 trial [25].
One unique finding in these data is the significant difference in OS from date of diagnosis when comparing those who had concurrent PTR with those who did not. This finding should be interpreted with caution, as the inclusion of patients with metachronous CRLM introduces a source of immortal time bias [26] Nevertheless, the distinct difference in OS between those who had PTR and those who did not is intriguing and raises the issue of the utility of PTR. PTR in the setting of metastatic colorectal cancer remains controversial. Randomized trials demonstrate no survival benefit of PTR in metastatic colorectal cancer [27, 28] However, PTR is still recommended by National Comprehensive Cancer Network (NCCN) guidelines before consideration of liver-directed therapy for CRLM [29] Furthermore, concurrent PTR with HAIP placement is widely performed and considered safe [30]. In light of the level 1 data showing no survival benefit for PTR, a liver-focused approach could be recommended for patients with unresectable CRLM with an asymptomatic primary tumor.
To date, there is very limited level 1 data to guide optimal use of HAIP within the broader treatment paradigm for patients with CRLM. The only level 1 data for HAIP therapy is the CALGB 9481 trial which randomized patients to systemic 5-FU or hepatic arterial infusion of FUDR [31]. Since then, observational studies such as this have described real-world outcomes of HAIP after or in conjunction with modern chemotherapy, which typically involves multiagent 5-FU based chemotherapy with or without other agents such as bevacizumab, EGFR inhibitors, or immune checkpoint inhibitors. The highly anticipated EA2222 PUMP trial (NCT05863195) is currently enrolling and will evaluate the benefit of HAIP following 3–6 months of standard multiagent 5-FU-based systemic chemotherapy. Adjuvant HAIP is being studied in the Netherlands for low-risk colorectal liver metastasis [13].
Limitations of this study include the single center, small number of patients, lack of control or comparison group, and heterogeneity of treatment regimens. Therefore, this analysis is limited to a descriptive nature and broad conclusions cannot be drawn from these results. However, the real-world data presented herein are similar to those of surgeon-directed HAIP programs, and may be generalizable to other centers that are beginning a HAIP program [5, 20]. Another limitation is that this database only captured patients who underwent an operation with a plan for HAIP placement. Therefore, we do not know how many patients at our institution were evaluated and determined not to be candidates for HAIP, eligible but not referred for HAIP therapy, did not elect to undergo HAIP placement, or were treated with other local therapies aside from resection. These therapies can include stereotactic body radiotherapy, transarterial radioembolization, and transarterial chemoembolization [32].
5 Conclusions
HAIP therapy can be safely implemented at institutions with limited prior experience. Survival outcomes were similar to those reported in larger series. The relationship between PTR and liver-directed therapy in colorectal liver metastasis warrants further investigation.
Disclosure
The authors have nothing to report.
Ethics Statement
This study was approved by the Institutional Review Board at The Ohio State University under protocol #2023C0177. Informed consent was waived due to the retrospective nature of the study. The study was conducted in accordance with the ethical standards of the IRB at The Ohio State University and with the Helsinki Declaration of 1975, as revised in 1983.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Synopsis
Hepatic artery infusion pump (HAIP) therapy may be used to treat unresectable colorectal liver metastases but requires specialized centers with multidisciplinary coordination. Implementation of a HAIP program at a single center was done safely and with acceptable oncologic outcomes.




