Poor prognosis of young male patients with stage III colorectal cancer: A multicenter retrospective study
Abstract
Background and Objectives
The number of young patients with colorectal cancer (CRC) is increasing. However, sex-dependent differences in the prognosis of young CRC remain unknown.
Methods
We investigated patients aged <70 years with stage III CRC treated between January 2000 and December 2010 in 24 Japanese referral hospitals. Patients were divided into subgroups by age of 50 years (early-onset and late-onset groups) and sex, and clinical characteristics and survival outcomes were compared. Risk factors associated with poor survival outcomes were also analyzed.
Results
Among 4758 consecutive patients, 771 (16%) were <50 years. Regardless of sex, there were more patients with rectal cancer and treated with adjuvant chemotherapy in the early-onset group. Among males, tumors in the early-onset group were poorly differentiated (p < 0.001), and patients were diagnosed at an advanced N stage (p = 0.010). Among females, there were more patients with left-sided cancer in the early-onset group (p < 0.001). Relapse-free survival (RFS) and overall survival (OS) were worse in the early-onset group than in the late-onset group (5-year RFS rates: 58% and 63%, p = 0.024; 5-year OS rates: 76% and 81%, p = 0.041, respectively), while there were no age-dependent differences in the survival outcomes of female CRC patients. A multivariate analysis identified age <50 years as one of the independent risk factors associated with poor RFS in male stage III CRC patients (p = 0.032)
Conclusions
Young male patients with stage III CRC showed poorer survival outcomes than their older counterparts. Therefore, age- and sex-related differences in the incidence of CRC recurrence need to be considered.
1 INTRODUCTION
Colorectal cancer (CRC) is the fourth most commonly diagnosed and third most deadly cancer worldwide.1 The global trend indicates a steady increase in the incidence of CRC among young individuals over the last several decades.2, 3 Similarly, in line with this pattern, the incidence of CRC among young adults (<50 years) has been rising at a rate of 2% per year in Japan since 2000.4
Sporadic CRC in young adults is associated with specific clinicopathological characteristics, including poor histological differentiation and advanced tumors.5 Previous studies reported that CRC in the young had distinct molecular features from CRC in the elderly, resulting in different responses to various treatments.5 However, age-dependent differences in the prognosis of CRC after curative resection remain controversial. Previous studies suggested that young patients with CRC developed more aggressive disease and, thus, had a worse prognosis than older individuals.6, 7 In contrast, other studies indicated that the survival rate of young patients was similar to or better than that of older patients following adjustments for clinicopathological parameters.8, 9
Retrospective studies showed that sex affected differences in clinical outcomes between young and older CRC patients.10-13 An analysis of the SEER database suggested that the survival advantage of young CRC patients was more pronounced in women than in men.10 However, the effects of potential sex-age interactions on CRC survival remain unclear, particularly in advanced stages.
Approximately 30% of patients with stage III CRC develop recurrence despite receiving multimodal treatments, such as curative surgical resection and adjuvant chemotherapy.14 Age- and sex-dependent differences in clinical outcomes in patients with stage III CRC need to be clarified for appropriate follow-up. Therefore, the present study investigated the prognosis of stage III sporadic CRC patients according to age and sex and compared it with that of elderly patients.
2 MATERIALS AND METHODS
2.1 Patients and data extraction
We investigated consecutive patients collected by The Japanese Study Group for Follow-Up of Colorectal Cancer. All patients had pathologically diagnosed stage III colorectal cancer and underwent curative surgery at 24 Japanese referral hospitals between January 2000 and December 2010. Patients satisfying the following criteria were excluded from our retrospective study: histology other than adenocarcinoma, hereditary disease such as familial adenomatous polyposis, Lynch syndrome and Peutz–Jeghers syndrome, inflammatory bowel disease, insufficient clinical and pathological information, unknown follow-up information, multiple colorectal cancers, and multiple primary tumors. Moreover, since a pooled analysis of randomized control trials indicated that survival outcomes in stage III CRC patients ≥70 years were poorer than those in their younger counterparts,14 we also excluded patients ≥70 years.
We retrieved the following data: demographic data, the primary tumor location, histological type, the TNM pathological classification of tumors according to the American Joint Committee on Cancer (AJCC) manual 8th edition,15 and the presence of adjuvant therapy and preoperative chemoradiotherapy.
Patients were followed up every 3 months until death or the study cut-off date of January 2019, whichever came first. Patients were initially intended to be followed for a minimum of 5 years. After this period, additional follow-up was conducted based on either the patients' preference or at the discretion of the treating physicians.
The present study was approved by the Central Institutional Review Board (Tokyo Medical and Dental University [No. M2017–268]) and the Ethics Review Board of each institution, including the Ethics Committee of the University of Tokyo (No.3252-[15]).
2.2 Subgroup analysis
We divided male and female patients separately into subgroups according to an age of 50 years: the early-onset group (<50 years) and the late-onset group (≥50 years).5 To investigate differences in clinical outcomes according to tumor sidedness, patients were further divided according to their tumor sidedness. A right-sided tumor was defined as a tumor of the appendix, cecum, ascending colon, or transverse colon. A left-sided tumor was defined as a tumor of the descending colon, sigmoid colon, or rectum. Overall survival (OS) and relapse-free survival (RFS) in these subgroups were reviewed.
2.3 Statistical analysis
Statistical analyses were performed with JMP Pro 16.2.0 (SAS Institute). All variables were summarized as medians (range) or numbers (percentages). Quantitative variables were compared using the Mann–Whitney U-test. Qualitative variables were compared using the chi-square test. OS was defined as the time from the first operation to death. RFS was calculated from the time of curative surgery to any type of recurrence or death. The Kaplan–Meier method was used in the survival analysis, and differences in survival outcomes were assessed by the Log-rank test. Univariate and multivariate regression analyses were performed to identify predictive factors for survival outcomes. Variables with a p-value < 0.05 using a univariate analysis were further evaluated using a multivariate analysis, and the Cox proportional regression model was performed to assess prognostic factors. Results are reported as hazard ratios and 95% confidence intervals.
All reported p-values were two-sided, and results were considered to be significant if the p-value was less than 0.05.
3 RESULTS
3.1 Patient characteristics
A total of 4758 patients met the inclusion criteria. Among them, 771 patients (16%) were <50 years (Figure 1).
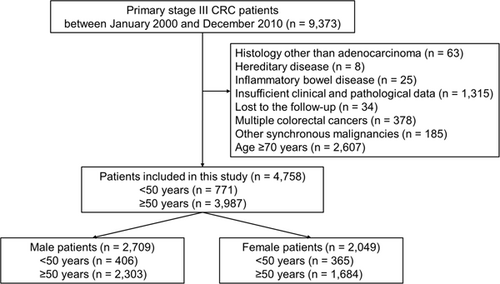
Table 1 summarizes the characteristics of patients divided according to sex and an age of 50 years. Regardless of sex, more rectal cancer patients were included in the early-onset group (p = 0.023 in males, and p < 0.001 in females, respectively), and patients in the early-onset group received adjuvant chemotherapy more frequently than those in the late-onset group (p < 0.001 in males, and p = 0.039 in females, respectively). Among male patients, tumors in the early-onset group were poorly differentiated (p < 0.001) and an advanced N stage (p = 0.010). In contrast, there were more patients with left-sided cancer in the early-onset group than in the late-onset group (p < 0.001) among females. The distribution of tumor pathological stages did not significantly differ between the two age groups in male or female patients (p = 0.091 in males and p = 0.089 in females, respectively).
| Variable | Male patients (n = 2709) | Female patients (n = 2049) | ||||
|---|---|---|---|---|---|---|
| Early-onset | Late-onset | p-Value | Early-onset | Late-onset | p-Value | |
| Demographic data | ||||||
| Age, years | 44 (20–49) | 62 (50–69) | N/E | 44 (23–49) | 61 (50–69) | N/E |
| Tumor location | 0.023 | <0.001 | ||||
| Appendix | 1 (0%) | 0 (0%) | 0 (0%) | 3 (0%) | ||
| Cecum | 21 (5%) | 110 (5%) | 13 (4%) | 109 (6%) | ||
| Ascending colon | 25 (6%) | 207 (9%) | 16 (4%) | 219 (13%) | ||
| Transverse colon | 29 (7%) | 121 (5%) | 24 (7%) | 111 (7%) | ||
| Descending colon | 15 (4%) | 90 (4%) | 22 (6%) | 58 (3%) | ||
| Sigmoid colon | 70 (17%) | 525 (23%) | 94 (26%) | 454 (27%) | ||
| Rectum | 245 (60%) | 1250 (54%) | 196 (54%) | 730 (43%) | ||
| Tumor sidedness | 0.89 | <0.001 | ||||
| Right-sided | 76 (19%) | 438 (19%) | 53 (15%) | 442 (26%) | ||
| Left-sided | 330 (81%) | 1865 (81%) | 312 (85%) | 1242 (74%) | ||
| Tumor histology | <0.001 | 0.33 | ||||
| Well- to moderately differentiated | 356 (88%) | 2155 (94%) | 342 (94%) | 1559 (93%) | ||
| Poorly differentiated | 22 (5%) | 89 (4%) | 14 (4%) | 57 (3%) | ||
| Mucinous/signet | 28 (7%) | 59 (3%) | 9 (2%) | 68 (4%) | ||
| Lymphatic invasion | 314 (77%) | 1776 (77%) | 0.92 | 285 (78%) | 1295 (77%) | 0.63 |
| Venous invasion | 304 (75%) | 1768 (77%) | 0.41 | 256 (70%) | 1211 (72%) | 0.52 |
| Pathological TNM classification | ||||||
| T stage | 0.44 | 0.18 | ||||
| T1 | 29 (7%) | 125 (5%) | 32 (9%) | 103 (6%) | ||
| T2 | 44 (11%) | 281 (12%) | 53 (15%) | 222 (13%) | ||
| T3 | 248 (61%) | 1381 (60%) | 202 (55%) | 947 (56%) | ||
| T4 | 85 (21%) | 516 (22%) | 78 (21%) | 412 (24%) | ||
| N stage | 0.010 | 0.11 | ||||
| N1 | 260 (64%) | 1623 (70%) | 243 (67%) | 1192 (71%) | ||
| N2 | 146 (36%) | 680 (30%) | 122 (33%) | 492 (29%) | ||
| Pathological stage | 0.091 | 0.089 | ||||
| IIIa | 40 (10%) | 268 (12%) | 46 (13%) | 199 (12%) | ||
| IIIb | 268 (66%) | 1583 (69%) | 225 (62%) | 1133 (67%) | ||
| IIIc | 98 (24%) | 452 (20%) | 94 (26%) | 352 (21%) | ||
| Adjuvant chemotherapy | 323 (80%) | 1646 (71%) | <0.001 | 296 (81%) | 1281 (76%) | 0.039 |
| Preoperative chemotherapy and/or radiotherapy | 11 (3%) | 51 (2%) | 0.54 | 4 (1%) | 20 (1%) | 1.0 |
- Note: Values are presented as the number of patients (%) or median (range).
3.2 Patient prognosis
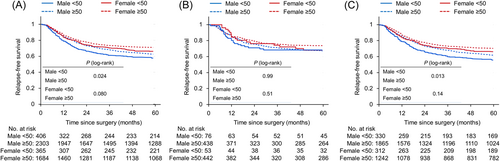
We compared the prognosis of CRC patients according to an age of 50 years, sex, and tumor sidedness. The mean follow-up time was 76.0 months in the early-onset group and 76.8 months in the late-onset group, respectively. Figure 2 shows RFS in stage III CRC patients. In male patients, RFS in patients with overall or left-sided CRC was worse in the early-onset group than in the late-onset group (5-year RFS rates: 58% vs. 63%, p = 0.024; 56% vs. 62%, p = 0.013, respectively), whereas the impact of age on RFS with right-sided colon cancer was limited (5-year RFS rates: 68% vs. 67%, p = 0.99). In female patients, RFS was slightly poorer in the early-onset group of overall CRC (5-year RFS rates: 67% vs. 71%, p = 0.080). However, when classified by tumor sidedness, RFS was similar in the early-onset and late-onset groups of female CRC patients (5-year RFS rates: 68% vs. 74% in right-sided cancer, p = 0.51; 66% vs. 70% in left-sided cancer, p = 0.14). In the subgroup analysis based on the risk of recurrence in male patients with stage IIIb CRC, RFS was slightly worse in the early-onset group than in the late-onset group (5-year RFS rates: 58% vs. 63%, p = 0.076; Supporting Information: Figure S1).
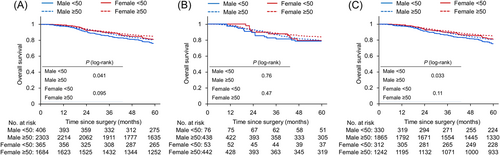
We then analyzed OS in stage III CRC patients (Figure 3). Similar to RFS, male patients with overall or left-sided CRC showed shorter OS in the early-onset group than in the late-onset group (5-year OS rates: 76% vs. 81%, p = 0.041; 76% vs. 81%, p = 0.033, respectively). In contrast, OS in female patients did not significantly differ between the two age groups, independent of tumor sidedness (5-year OS rates: 81% vs. 85% for overall, p = 0.095; 79% vs. 84% in right-sided cancer, p = 0.47; and 81% vs. 85% in left-sided cancer, p = 0.11). Among male patients with stage IIIb CRC, OS was also slightly poorer in the early-onset group than in the late-onset group (5-year OS rates: 77% vs. 82%, p = 0.070; Supporting Information: Figure S2).
In the subgroup analysis based on adjuvant chemotherapy (Supporting Information: Figure S3), male patients with stage III CRC who underwent adjuvant chemotherapy exhibited shorter RFS and OS in the early-onset group compared to those in the late-onset group (5-year RFS rates: 59% vs. 65%, p = 0.011; 5-year OS rates: 75% vs. 83%, p < 0.001, respectively). Conversely, there was no significant difference in the prognosis of male patients who did not receive adjuvant chemotherapy between the two age groups. Among females, age did not impact survival outcomes in patients, irrespective of whether they received adjuvant chemotherapy.
3.3 Risk factors for poor outcomes in male CRC patients
To analyze risk factors for poor RFS in male patients with stage III CRC, we performed a univariate analysis and found that significant predictors of RFS were age, tumor sidedness, tumor histology, lymphatic invasion, venous invasion, T stage, N stage, adjuvant chemotherapy, and preoperative chemotherapy and/or radiotherapy (Table 2). Multivariate Cox regression models revealed that age <50 years, left-sided tumor, histology of por/muc/sig, positive lymphatic invasion, positive venous invasion, ≥T3 stage, N2 stage, no experience of adjuvant chemotherapy, and treatment history of preoperative chemotherapy/radiotherapy were independent factors associated with worse RFS in male patients (Table 2).
| Variable | Univariate regression coefficient (95% CI) | p-Value | Multivariate regression coefficient (95% CI) | p-Value |
|---|---|---|---|---|
| Age (<50 vs. ≥50 years) | 1.21 (1.03–1.43) | 0.024 | 1.20 (1.02–1.42) | 0.031 |
| Tumor sidedness (left vs. right) | 1.21 (1.03–1.43) | 0.025 | 1.26 (1.06–1.49) | 0.008 |
| Tumor histology (por/muc/sig vs. tub1/tub2) | 1.60 (1.30–1.97) | <0.001 | 1.38 (1.11–1.70) | 0.003 |
| Lymphatic invasion (positive vs. negative) | 1.59 (1.35–1.88) | <0.001 | 1.35 (1.14–1.61) | <0.001 |
| Venous invasion (positive vs. negative) | 1.74 (1.47–2.06) | <0.001 | 1.43 (1.20–1.70) | <0.001 |
| T stage (≥T3 vs. ≤T2) | 3.01 (2.39–3.78) | <0.001 | 2.46 (1.95–3.11) | <0.001 |
| N stage (N2 vs. N1) | 2.18 (1.93–2.48) | <0.001 | 1.88 (1.66–2.14) | <0.001 |
| Adjuvant chemotherapy (none vs. received) | 1.22 (1.07–1.40) | 0.004 | 1.43 (1.24–1.64) | <0.001 |
| Preoperative chemotherapy and/or radiotherapy (none vs. received) | 0.58 (0.42–0.82) | 0.002 | 0.63 (0.45–0.89) | 0.009 |
- Abbreviations: CI, confidence interval; CRC, colorectal cancer.
4 DISCUSSION
Despite intensive studies on young-onset CRC, the prognosis of young patients with sporadic CRC remains unclear. A recent analysis of the IDEA database identified age <50 years as a negative prognostic factor in high-risk stage III CRC that was associated with a higher relapse rate even though young patients received higher intensities of adjuvant chemotherapy.16 These findings suggest that poor survival in young patients is attributable to a more aggressive tumor biology. Patients <50 years consistently had poorly differentiated CRC more frequently than those ≥50 years in our cohort. In comparisons with older patients, survival outcomes in younger patients differed according to sex in the present study; male patients in the early-onset group had shorter survival than those in the late-onset group, whereas survival outcomes in female patients did not significantly differ between the age groups. To the best of our knowledge, this is one of the largest cohort studies to investigate the effects of sex on survival in young stage III CRC patients and compare it with that in elderly patients.
The importance of sex as a CRC prognostic factor remains inconclusive. However, in comparisons with older or male patients, a survival benefit was reported for young female patients.10-13, 17, 18 A possible explanation for the survival benefit of young women is endogenous female sex hormones; previous studies reported that female sex hormones decreased the risk of CRC.19 Furthermore, postmenopausal hormonal replacement therapy was shown to reduce the risk of CRC and extend survival in CRC patients.20-24 Regarding the mechanisms underlying the antitumor activities of female sex hormones, recent studies showed that female hormones exerted stimulatory effects on immunological responses.25-27 These findings indicate that sex hormones in young female patients play a role in suppressing the recurrence of CRC and contribute to better survival outcomes, despite its unfavorable pathological characteristics.
Different survival outcomes among age or sex groups may also be attributed to the molecular profiles of CRC. Numerous studies reported biomolecular features in young sporadic CRC; young CRC patients were less likely to have KRAS or BRAF V600E mutations or the promoter methylation of CpG islands.28-31 In contrast, microsatellite stability (MSS), TP53 variants, or long interspersed nuclear element-1 (LINE-1) hypomethylation were more frequent in young patients.32, 33 Among these genetic or epigenetic variations, the BRAF V600E mutation, MSS, and LINE-1 hypomethylation were associated with poor survival outcomes.34-39 The incidence of these prognostic molecular features also differed according to the tumor location; the BRAF V600E mutation was more frequently observed in right-sided colon cancer,34 and MSS and LINE-1 hypomethylation in left-sided colon cancer.34-38 Based on these findings, the higher prevalence of MSS or LINE-1 hypomethylation may underlie the poorer survival of young patients with left-sided CRC.
Previous studies demonstrated that diet affects the prognosis of CRC through gut microbial changes.33, 40-44 Among dietary habits, a high intake of fat and red and processed meat was shown to promote dysbiosis and was identified as a risk factor for the recurrence as well as incidence of CRC.44-46 Animal studies recently showed that microbiome changes and host responses caused by a high-fat diet were also dependent on sex and age47, 48; myeloperoxidase, a marker of inflammation and oxidative stress, was concentrated in the colon mucosa due to a high-fat diet only in male rats. Moreover, a specific bacterial species that produces carcinogenic compounds, such as hydrogen sulfide, increased in response to a high-fat diet in young male rats but not in aged male rats.47 Furthermore, estrogen attenuated high-fat diet-induced dysbiosis in mice.48 These variations in the microbiome and bowel microenvironment may explain sex- and age-dependent differences in the survival outcomes of CRC. In Japan, the consumption of red or processed meat is more frequently observed in young males.49 Favorable dietary habits may markedly improve clinical outcomes in young patients after curative CRC resection.
There are several limitations that need to be addressed. We did not analyze the clinical information of patients, such as family history, comorbidities, performance status, or regimens of adjuvant chemotherapy, which may affect the survival outcomes of CRC. Moreover, molecular profiles were not extensively examined in detail.
5 CONCLUSIONS
In stage III CRC, young male patients showed poorer RFS and OS than older patients, whereas there was no age-dependent difference in RFS or OS in female patients. An age <50 years was identified as an independent risk factor for recurrence in male patients with stage III CRC. Young male patients need to be carefully followed up after curative resection for stage III CRC.
AUTHOR CONTRIBUTIONS
Kazuaki Okamoto, Kazuhito Sasaki, Hiroaki Nozawa, and Soichiro Ishihara developed the study design and concept, retrieved the data of patients, and carried out the analysis. Kazuaki Okamoto, Kazuhito Sasaki, Hiroaki Nozawa, Koji Murono, Shigenobu Emoto, Shinichi Yamauchi, Kenichi Sugihara, and Soichiro Ishihara participated in writing and revising the manuscript critically. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was based on data collected from the following referral institutions in the Japanese Study Group for Postoperative Follow-up of Colorectal Cancer: Sapporo Medical University (I. Takemasa), Hirosaki University (K. Hakamada), Niigata University (H. Kameyama), Niigata Cancer Center Hospital (Y. Takii), National Defense Medical College (K. Hase), Tochigi Cancer Center (K. Kotake), University of Tokyo (S. Ishihara), Tokyo Metropolitan Cancer and Infectious Disease Center Komagome Hospital (K. Takahashi), National Cancer Center Hospital (Y. Kanemitsu), Tokyo Woman's Medical University (M. Itabashi), National Center for Global Health and Medicine (H. Yano), Tokyo Medical and Dental University (K. Sugihara), Keio University (H. Hasegawa), Teikyo University (Y. Hashiguchi), Kyorin University (T. Masaki), Kitazato University (M. Watanabe), Fujita Health University (K. Maeda), Aichi Cancer Center (K. Komori), Kyoto University (Y. Sakai), Osaka Medical Center for Cancer and Cardiovascular Diseases (M. Ohue), Osaka Rosai Hospital (S. Noura), Hyogo College of Medicine (N. Tomita), and Kurume University (Y. Akagi).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Central Institutional Review Board (Tokyo Medical and Dental University [No. M2017–268]) and the Ethics Review Board of each institution, including the ethics committees of the University of Tokyo (No.3252-[15]).
Open Research
DATA AVAILABILITY STATEMENT
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
SYNOPSIS
We demonstrated that male patients aged <50 years with stage III colorectal cancer showed shorter relapse-free survival compared with those ≥50 and <70 years. Age <50 was an independent factor associated with worse prognosis in male patients.




