Hydrodynamic Effects on Drug Dissolution and Deaggregation in the Small Intestine—A Study with Felodipine as a Model Drug
Abstract
The aim of this study was to understand and predict the influence of hydrodynamic effects in the small intestine on dissolution of primary and aggregated drug particles. Dissolution tests of suspensions with a low-solubility drug, felodipine, were performed in a Couette cell under hydrodynamic test conditions corresponding to the fed small intestine. Dissolution was also performed in the USP II apparatus at two paddle speeds of 25 and 200 rpm and at different surfactant concentrations below critical micelle concentration. The experimental dissolution rates were compared with theoretical calculations. The different levels of shear stress in the in vitro tests did not influence the dissolution of primary or aggregated particles and experimental dissolution rates corresponded very well to calculations. The dissolution rate for the aggregated drug particles increased after addition of surfactant because of deaggregation, but there were still no effect of hydrodynamics. In conclusion, hydrodynamics do not influence dissolution and deaggregation of micronized drug particles in the small intestine of this model drug. Surface tension has a strong effect on the deaggregation and subsequent dissolution. Addition of surfactants at in vivo relevant surface tension levels is thus critical for in vivo predictive in vitro dissolution testing. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2969–2976, 2015
INTRODUCTION
For orally administrated drugs in solid form, dissolution in the gastrointestinal fluids is a prerequisite for absorption. Bioavailability for oral administration of such drugs can thereby be deficient because of slow and incomplete dissolution from the dosage form.1 This has become even more important as the majority of small organic drugs in modern drug development are highly hydrophobic and poorly soluble in water.2 Dissolution is not only a consequence of the molecular properties such as hydrophobicity, but also strongly dependent on drug solid state form, excipients, and formulation manufacturing process. The understanding and prediction of the drug dissolution in the gastrointestinal (GI) tract are key aspects of pharmaceutical product development to design products with desired clinical performance and to ascertain consistent quality in commercial supply.
The drug dissolution rate is affected by composition of the GI fluids. The contents in the stomach and small intestine are well characterized, including factors such as pH, and bile salts. This has allowed development of various dissolution test fluids mimicking the content of GI fluids.3 The dissolution is also potentially influenced by the hydrodynamic conditions in the GI tract. Fluid movements is created by the gut wall motility aimed to grind, mix, and transport material in the GI tract.4 This creates convection and shear that may influence the rate of drug particle dissolution. Previously, it has been shown in in vitro experiments that the drug transport in the diffusion layer surrounding solid particles and consequently the dissolution rate indeed is affected by hydrodynamic conditions.5-8 However, although these studies provided mechanistic insight in drug particle dissolution, the translation of those hydrodynamic effects observed in vitro to the conditions in the GI tract has been missing. Another sparsely investigated factor involved in the dissolution process is deaggregation of clusters of drug particles that can be formed during handling and pharmaceutical processing of adhesive materials. Aggregates have a smaller surface area than primary particles and are therefore a critical factor for the determination of the dissolution rate. Aggregates that are suspended in a fluid disintegrate when the hydrodynamic forces exceed the cohesive bonds between the particles primarily determined by the Brownian motion, electrostatic, and van der Waals interactions.9
The focus of the current study was therefore to evaluate the influence of hydrodynamics on drug particle dissolution at test conditions representative of the fed small intestine including the aspect of deaggregation. The fed state was selected as this represents a continuous state of mixing in the GI tract and thereby could potentially provide pronounced influence on drug dissolution. The present study combined in vitro drug particle dissolution experiments of a low-solubility model drug, felodipine, performed under well-controlled hydrodynamic conditions and previously performed computer simulations of hydrodynamics in the GI tract,11, 12 allowing to link in vitro and in vivo intestinal hydrodynamics. The simulations of the hydrodynamic conditions experienced by drug particles in the upper small intestine was performed by a unique computer simulation model based on the Lattice–Boltzmann algorithm describing gut wall motility patterns determined by in vivo magnetic resonance imaging (MRI) images.4 The hydrodynamic conditions for the in vitro experiments were calculated allowing to define in vitro test conditions, by varying agitation, that mimicked the in vivo situation. Thereby, drug particle dissolution and deaggregation could for the first time be studied in vitro under in vivo relevant conditions. These in vitro experiments were performed in a Couette cell and thereafter repeated and further extended in the USP II paddle dissolution apparatus.
The aim of the present study was to investigate the effect of in vivo relevant hydrodynamics conditions on drug particle dissolution and deaggregation in order to allow definition of in vivo-predictive tools.
MATERIAL AND METHODS
Overall Study Design
The fluid shear rates at drug particles in the small intestine at fed-state motility were calculated by a previously developed computer model.11, 12 The simulations used motility patterns from in vivo MRI images obtained in rats under fed conditions. Physiological parameters such as dimensions and motility frequencies were transposed from the rat to the human situation. The simulations applied a modified Lattice–Boltzmann algorithm with a three-dimensional geometric model used to set boundaries conditions.
Dissolution experiments using felodipine as a model substance was performed on drug suspensions containing micronized primary particles or aggregated clusters of the same micronized particles. These experiments were performed under very well-controlled hydrodynamic conditions allowing to calculate and subsequently match the shear rate on the particles to magnitudes relevant for the in vivo situation, as determined by above-described computer model. To obtain controls and predictable hydrodynamics, dissolution testing was performed in a Couette cell, which is a cylindrical beaker with an inner rotating cylinder. The density of the dissolution medium was increased to make the drug particles neutrally buoyant. The different shear rates were obtained by varying the rotation rate of the inner cylinder.
As the Coutte cell is not a practically useful method for product dissolution testing, additional experiments with felodipine suspensions were performed in the standard USP II paddle apparatus. In order to bridge the in vivo relevant results obtained in the Coutte cell, testing in the same medium was performed in USP II at different paddle speeds. Furthermore, the influence of surfactants on the deaggregation of particle clusters was studied in the USP II apparatus.
All in vitro dissolution data were compared with theoretical dissolution calculations by Eq. 1 to support interpretation of dissolution results. Particle size distributions in the test suspensions were determined as well as drug solubility in the test media and zeta potential for suspensions mixed with dissolution test media further underpinning mechanistic interpretations of results.
Test Formulations
A low solubility, BCS class II drug, felodipine (AstraZeneca, Södertälje, Sweden) was used in the present study. Suspensions with micronized drug as primary and aggregated particles were manufactured as described below.
Manufacturing
For both the primary and the aggregated particle suspensions, the total drug concentration was 2 mM. The same batch of micronized drug was used to obtain both the aggregated and primary particle suspensions. To stabilize the suspensions, 0.2% and 2% (w/w) polyvinylpyrrolidone (PVP) (Kollidon 30™; BASF, Geismar, LA, USA) were added for the primary and aggregated particles suspensions, respectively. The PVP concentration of 2% (w/w) has no influence on the drug solubility.12 The aggregated suspensions were prepared using a previously developed method where drug particles are added to half the suspending fluid and than the other half is added followed by gentle mixing.13 The primary particle suspensions were obtained by deaggregating drug particles in an ultrasonic bath (S2; Covaris, Woburn, MA, USA).
Felodipine suspensions with additional CsCl (ICN Biomedicals Inc., Irvine, CA, USA) were also manufactured for dissolution tests where this salt was included in the dissolution medium. CsCl was used to density match the dissolution medium with the drug particles to avoid sedimentation of the particles during the dissolution experiments. Because of the lower solubility for felodipine in the density-matched media, the suspensions with CsCl had a lower drug concentration of 1 mM compared with the 2-mM used for the suspensions without this salt.
The homogeneity of drug content in suspension samples added to the dissolution experiments was verified by measuring total drug concentration in such samples in triplicates for each batch prior to dissolution testing. The variation in drug concentration was less than 10% allowing accurate dose administrations to the dissolution experiments.
Drug Particle Size Distribution Measurements
The particle size distribution for the suspensions used in the dissolution experiments was determined by laser diffraction using the Mastersizer 2000 (Malvern Instruments, Worcestershire, UK).
Zeta Potential Measurements
The zeta potential representing electrostatic repulsion is a key indicator of electrostatic attraction or repulsion between particles. For particles that are small enough, a low zeta potential will favor aggregation. Zeta potential was measured for felodipine by a Zetasizer (Malvern Instruments, Worcestershire, UK) for 25 μM felodipine suspensions in 0.05% N,N-dimethylacetamide (DMA) (Scharlau Chemie S.A, Sentmenat, Spain) as a reference and with addition of different concentrations the surfactants sodium dodecyl sulfate (SDS) (0.01–1 mM) or 2.6 mM sodium taurocholate (Sigma–Aldrich, Saint Louis, MO, USA). All samples were prepared by using the primary particle suspension.
Dissolution Experiments
in the Couette Cell
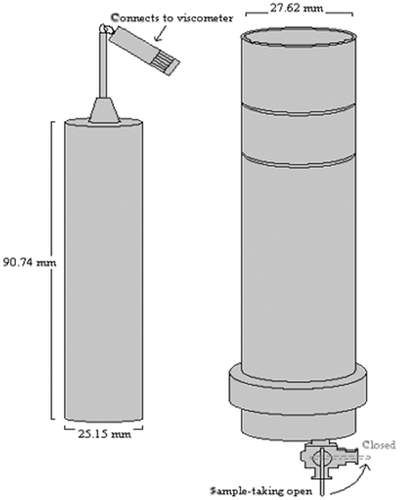
The Couette cell used was a modified UL-adapter connected to a digital Viscometer model LVDV-II+ (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA).14 The UL-adapter was modified by introducing a valve at the base for sampling (Fig. 1). The internal cylinder was connected to the viscometer that was used to rotate the internal cylinder at a constant speed. The total volume that could fit in the Couette cell was 12.3 mL.
The shear rate could be varied by altering the rotation velocity of the inner cylinder in the Couette cell between 5 and 100 rpm. A constant temperature of 37°C was obtained by a water bath.
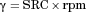 (1)
(1)To obtain similar shear rate as in the small intestine (determined by the computer model), the rotation of the inner cylinder in the Couette cell would be around 0.4–0.6 rpm. It was not practically possible to use such low rotations, and a lowest level of 5 rpm was selected. In order to determine influence of shear, another arbitrarily higher value of 100 rpm was selected.
The dissolution experiment was started by adding the 1-mM felodipine suspension to a water solution containing CsCl 38.6% (w/w) and 0.5% (v/v) DMA (Scharlau Chemie S.A.), such that final felodipine concentration was 0.5 μM in the cell. The low concentration of DMA was used to simplify filtration and obstruct adsorption to plastic, metal, and glass surfaces. This low amount of DMA did not affect the felodipine solubility. The addition of CsCl was used to density match the dissolution medium and felodipine particles, thereby avoiding sedimentation of the particles under the dissolution experiments.
To ensure that the diluted suspension would be homogeneous after mixing with the dissolution medium, the suspension droplet was put at the base of a glass cylinder with a pipette and the preheated medium was poured into the cylinder. A glass syringe was then used to blend the suspension by filling the syringe and inject it back into the cylinder again. Sampling was performed at different times after the start of the experiment (0, 5, 15, 25, 50, 80, and 100 min) with a glass syringe with a PTFE 0.2-μm filter. The first 6–6.5 mL was disposed and the rest of the volume in the cell was used for drug assay.
Dissolution in the USP II Paddle Apparatus
The dissolution tests were performed with a USP Dissolution Testing Apparatus II (Evolution 6100, Bathless Dissolution System; Distek, North Brunswick, NJ, USA). The volume of the dissolution media were 500 mL and the temperature was 37°C. The dissolution characteristics were examined at two extreme levels of paddle rotation, 25 and 200 rpm, to cover a maximal range of shear rate. A small volume of suspension (625 μL of felodipine) was added to the bottom of the beaker, followed of addition of the preheated dissolution media. Samples of 10 mL were withdrawn with a glass syringe via a cannula and filtered through a hydrophilic PTFE 0.2-μm filter. The cannula was put into a small hole in the lid, which assured that the sample was withdrawn from the same position each time. The first 7–8 mL of filtrate was disposed, subsequently approximately 1 mL of filtrate was put in a glass vial and used for drug assay.
- 0.5% DMA
- 0.5% DMA and 1 mM SDS
- 0.5% DMA and 0.1 mM SDS
- 0.5% DMA and 38.6% CsCl
One millimolar SDS gives a surface tension of approximately 36 mN/m,15 which is well in agreement with surface tension in the GI tract.16 The low concentration of DMA was added for practical experimental reasons as explained above. The addition of CsCl was used to density match the dissolution medium, and felodipine particles avoid sedimentation of the particles under the dissolution in the hydrodynamically well-controlled experiments in the Couette cell. The use of CsCl in some of the USP II experiments allowed to bridge USP II and Coutte cell dissolution results.
Drug Solubility in the Dissolution Media
To establish the solubility of felodipine at 37°C in the dissolution medium consisting of 0.5% DMA with and without 38.8% CsCl, solubility experiments were executed in duplicate. No solubility experiments were performed with SDS as the concentrations of SDS used was under CMC17 and thereby it is not affecting the solubility. The samples were put in a preheated water shaking table with the temperature, 37°C, and equilibrated for 3 days. The same sampling procedure was used as for the dissolution experiments.
Drug Assay
The drug samples obtained without CsCl were quantified with HPLC–UV (2695 Separations Module; a 2487 Dual λ Absorbance Detector; Waters, Milford, MA, USA), to determine the concentration of dissolved drug in the samples collected during the dissolution tests. The test conditions for the HPLC method are shown in Table 1.
| Mobile Phase | 55:45 AcCN/H2O (v/v) + 0.025% TFA |
|---|---|
| Column | Symmetry C18, 3.0 × 50 mm2 (3.5 μm) |
| Flow | 1 mL/min |
| Λ | 254 nm |
| Injected volume | 20 μL |
| Number of injections | 2 |
- ACN, acetonitrile (Fischer Scientific); TFA, trifluoroacetic acid (MERCK).
Quantification of felodipine in the presence of CsCl was not possible with the HPLC method because of precipitation of the salt in the mobile phase. Fluorescence measurements were therefore used for felodipine assay in the presence of CsCl with a luminescence spectrometer (LS55; Perkin Elmer Inst., Waltham, MA, USA) at an emission wavelength of 460 nm and with excitation at 370 nm. This setting optimized signal to background ratio.
A set of standard samples with a range of concentrations relevant for the current tests were prepared and underwent the same procedure as the samples in the dissolution and solubility tests to remove influence of any artifacts, such as adsorption or degradation, although such factors were minimized by careful method development including selection of suitable laboratory equipment and design of experiments.
The calibration curves used to quantify felodipine in the samples from the dissolution and solubility tests with HPLC–UV all had a relative standard deviation (RSD) value less than 15%. In addition, all standard calibration curves were linear with R2 values above 0.98.
Dissolution Calculations
It was essential to compare the results from the dissolution experiments with stringent theoretical calculations of dissolution to correctly interpret experimental dissolution results.
 is the particle radius, D is the monomer diffusion coefficient in the solvent, Vm is the molar volume, and Cs and Cb are molar concentrations for saturation solubility and bulk concentration, respectively.13 The mass transfer improvement because of stirring can, according to Nielsen18, be corrected by the terminal factor in Eq. 2 including the Péclet number (Pe). Pe depends as described in Eq. 3 on the gravitational acceleration, g (9.82 m/s2), the particle radius, R, the drug diffusion constant, D, the density differenceρ between the particles and the media with the viscosity η.
is the particle radius, D is the monomer diffusion coefficient in the solvent, Vm is the molar volume, and Cs and Cb are molar concentrations for saturation solubility and bulk concentration, respectively.13 The mass transfer improvement because of stirring can, according to Nielsen18, be corrected by the terminal factor in Eq. 2 including the Péclet number (Pe). Pe depends as described in Eq. 3 on the gravitational acceleration, g (9.82 m/s2), the particle radius, R, the drug diffusion constant, D, the density differenceρ between the particles and the media with the viscosity η.
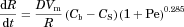 (2)
(2)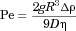 (3)
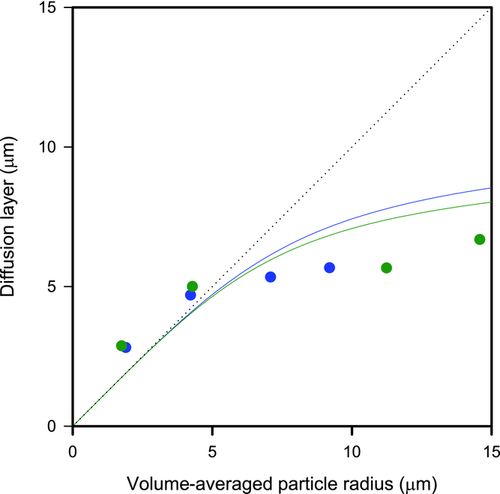
(3)A simulation study comparing dissolution calculations obtained according to Eq. 2 with an exact mathematical solution of Fick's first law showed that the equation we used in the current study is highly accurate.19 In addition to further confirm the validity of this equation, published dissolution data on oxazepam and digoxin6 was shown to correspond very well to calculations according to Eq. 2 (see Fig. 2).
The dissolution calculations based on Eq. 1 in the present study were performed by use of the software GI-SIM (AstraZeneca).20 The input variables for these calculations are given in Table 2. In addition, drug particle size distributions obtained from laser diffraction measurements were used in these calculations.
| Parameter | Without CsCl | With CsCl | Description |
|---|---|---|---|
| Ctot (M) | 2.5 × 10−6 | 0.5 × 10−6 | Total concentration of drug |
| S0 (M) | 3.99 × 10−6 | 0.89 × 10−6 | Solubility in dissolution medium |
| Vm (cm3/mol) | 260 | 260 | Drug molar volume |
| D (m2/s) | 6.7 × 10−3 | 6.7 × 10−3 | Drug diffusion coefficient |
| η (Pas) | 6.9 × 10−3 | 6.9 × 10−3 | Solvent viscosity |
| g (m/s2) | 9.82 | 9.82 | Gravitational acceleration |
| ρ (kg/m3) | 1000 | 1449 | Density of solvent |
| MW (g/mol) | 384.26 | 384.26 | Drug molecular weight |
RESULTS
Solubility
The mean (SD) (n = 2) solubility of felodipine in the dissolution media without and with 38.6% CsCl was 3.99 (0.49) and 0.89 (0.22) μM, respectively. The former corresponded very well with previously reported solubility data for felodipine, which provided aqueous crystalline drug solubility of about 4.0 μM estimated at 37°C,21 which confirmed that used amounts of DMA did not influence felodipine solubility. Because of the lower solubility in the presence of CsCl, the amount of felodipine added to the dissolution experiments with CsCl in the test medium was lower than corresponding tests without CsCl to maintain a similar level of sink condition.
Particle Size Distribution
The average ± SD (n = 3) volume weighted mean determined by laser diffraction for the aggregated felodipine particles in suspensions without CsCl was 13.0 ± 2.03 μm and with CsCl 10.4 ± 0.02 μm. The corresponding data for felodpine primary particle suspensions without CsCl was 3.79 ± 0.56 μm and with CsCl 5.11 ± 0.41 μm. Representative size distributions for felodipine primary and aggregate suspensions by use of the two different methods are shown in Figure 3.
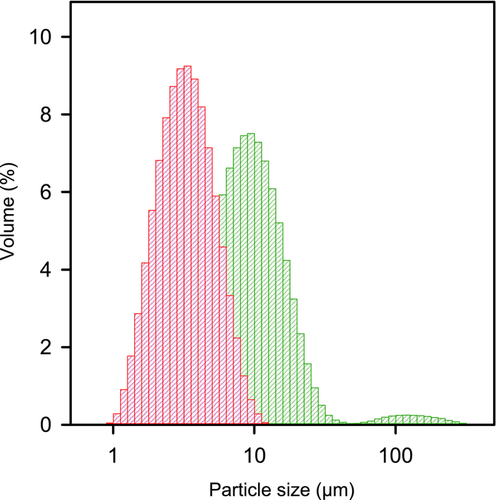
Thus, this confirmed that significant amount of drug clusters were present in the aggregated suspension as larger particle sizes were consistently measured for the aggregate suspensions and they were made of the same micronized drug material as the primary particle suspensions. The aggregates remained over time but smaller particles seemed to dissolve and add to the bigger particles leading to some particle growth. Therefore, suspensions was freshly prepared and characterized with respect to particle size distribution prior to the dissolution experiments.
Zeta Potential Measurements
Mean (SD) data for zeta potential for felodipine particles in different media are presented in Table 3. The values for felodipine without surfactant verifies that aggregation should be expected according to general interpretation of zeta potentials.22 It is clear that SDS has an impact on the zeta potential already at low concentration reducing tendency to aggregation and at high concentration the tendency to aggregation should be very low. Taurocholate at concentrations relevant for the GI tract showed an intermediate effect that would reduce propensity for aggregation but not completely prevent such effects.
| Zeta Potential (mV) | Medium |
|---|---|
| −40.97 | 0.05% DMA, 0.01 mM SDS |
| −38.83 | 0.05% DMA, 0.1 mM SDS |
| −44.27 | 0.05% DMA, 1 mM SDS |
| −34.80 | 0.05% DMA, 2.625 mM taurocholate |
| −22.9 | 0.05% DMA |
Dissolution
in the Couette Cell
The mean (n = 2) dissolution time profile for felodipine in density-matched media for primary and aggregated particle suspensions at low and high stirring rate together with theoretical prediction for the primary particle suspension are presented in Figure 4.
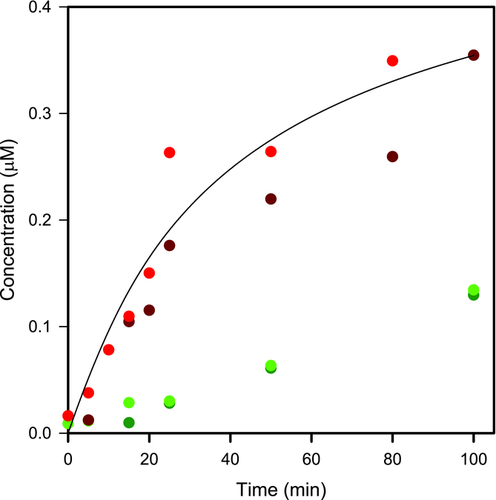
The dissolution from the primary suspensions was obtained in accordance with the theoretical predictions verifying the validity of these experiments. The dissolution of the aggregates was much slower compared with primary particles, and it was not affected by increasing the stirring rate indicating negligible deaggregation during the dissolution experiment. Interestingly, the stirring rate did not influence the dissolution rate of primary particles. Thus, the fluid flow seemed not to be a critical variable for predicting dissolution of well-suspended micronized felodipine particles.
USP II Paddle Dissolution
The dissolution experiments performed by using density-matched media in the USP II apparatus was overlapping with results obtained in the Couette cell both for primary and aggregated particle suspensions. This was not a surprising result given the insensitivity detected to different level of agitation noticed in the Couette cell experiments. This supported the use of USP II for continued testing in the present study to investigate in vivo relevance of different hydrodynamic factors.
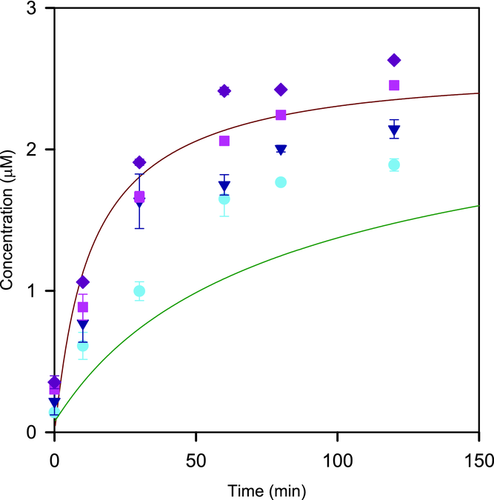
The mean dissolution profiles for felodipine from primary and aggregate particle suspensions in USP II paddle apparatus in water with 0.5% DMA at 25 and 200 rpm paddle stirring speed together with theoretical predictions are shown in Figure 5. The same pattern was obtained as in the previous experiments with the Couette cell, that is, substantially slower dissolution for the aggregates and no or very small influence of hydrodynamics. The excellent correspondence between predicted and experimental data obtained for both primary and aggregated suspensions confirmed that deaggregation did not contribute to the dissolution process. Thus, these results further confirmed that the hydrodynamics conditions did not have any significant impact on neither the dissolution of micronized primary felodipine particles or deaggregation of such particles.
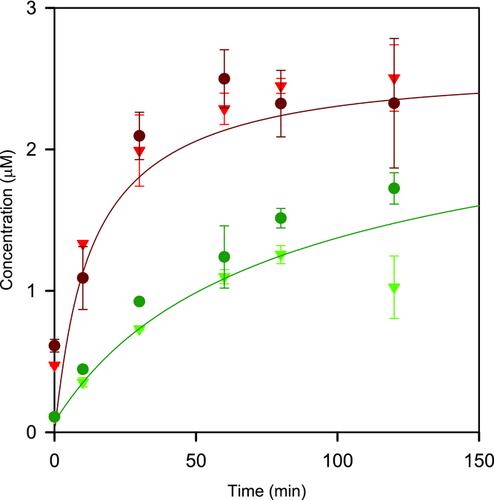
Mean dissolution profiles for felodipine aggregate particle suspensions in 0.5% DMA with different concentrations of SDS at 25 and 200 rpm paddle stirring speed together with theoretical predictions for primary and aggregate particles are shown in Figure 6. The aggregate particle dissolution increased significantly already at low SDS concentrations and at the highest level the dissolution corresponded to primary particle dissolution. The effect of agitation was minor also with the presence of surfactants.
DISCUSSION
This study applied a unique approach to investigate in vitro particle dissolution and deaggregation under hydrodynamic conditions that could be related to the in vivo situation. In the first stage, calculations of shear rate on particles in the fed small intestine were performed by use of an advanced computer model of small intestinal hydrodynamics. Thereafter, a novel experimental method for particle dissolution studies was designed with the aim to design a test with robust and predictable hydrodynamic conditions. This was obtained by use of a rotating cylinder in a slightly wider cylindrical beaker, a Couette cell normally used for viscosity measurements, using a dissolution medium with addition of a solute to increase density such that the drug particles became neutrally buoyant. With this design, it was possible to obtain a laminar flow and shear stress on the particles that could be very well controlled and easily calculated allowing to relate the in vitro situation to the corresponding values in the intestine. For practical reasons, it was not possible to run the in vitro experiments at such a low agitation as is the case in the intestine, but testing was performed at two different levels of about 10 and 200 times higher shear rates than is the case in the fed small intestine. The testing of the dissolution of micronized felodipine as suspensions of primary and aggregated particles under these settings showed that despite the more intense agitation than obtained in the intestine, it was not possible to break the aggregates. In addition, the dissolution of the primary particles was also unaffected by the agitation intensity and the dissolution profile corresponded very well to the theoretical prediction based on Fick's first law. Thus, the main conclusion from this study is that hydrodynamics in the fed small intestine is not a critical factor for predicting dissolution for micronized particles or deaggregation of drug aggregates. The shear stress experienced by drug particles in the intestine in the fasted state can be expected to be very different from the fed state. Unlike the motility associated with digestion in the fed state, the contractile activity is highly intermittent in the fed state and associated with migrating motor complex (MMC) patterns that repeat roughly every 1–2 h. During the third phase of the MMC, a strong contraction wave begins in the stomach and traverses the small bowels, likely creating a propagating wave of strong shear stress that might exceed the shear rates in the fed state. These events are intermittent and short lived, however, and their hydrodynamic influences on dissolution are unclear.
This study only included one model drug, felodipine. Zeta potential measurements showed that this drug has a high propensity for forming aggregates and the bonds between drug particles might have been particularly strong. However, similar but less extensive work was performed on another low-solubility drug, bicalutamide, with somewhat lower potential for aggregation as measured by the zeta potential. The results obtained for bicalutamide fully supported the results and conclusions shown in the present work (data on file at AstraZeneca).
The finding regarding lack of importance of hydrodynamics should not be extrapolated beyond the scope of this work. For example, GI hydrodynamics has shown to be critical for disintegration of larger objects such as tablets.23 In another work including detailed computer-based fluid modeling of the in vitro dissolution tests, a link between in vitro and intestinal hydrodynamics was established through a plasma concentration-based in vitro/in vivo correlation of different capsule formulations of a low-solubility drug including varying amounts of solubility enhancers.24 The effect of shear is also of greater importance for bigger particles than used in the current study,8 though for low-solubility drugs, use of such larger, unmilled drug particles is rarely normal practice.
In contrast to the negligible effect of different shear rates, the presence of surfactants significantly affected the dissociation and dissolution from aggregates. At 1% of SDS providing surface tension similar to levels obtained in the gastrointestine,15, 16 the dissolution of aggregates equaled the rate obtained for primary particles. The surfactant concentration was still below the critical micelle concentration so this increase in dissolution rate was not related to solubility enhancement but could be explained by deaggregation. The zeta potential of felodipine particles in biorelevant conditions of a model bile acid was just slightly lower than for 1% SDS, so the effect of surfactants on deaggregation must be considered in vivo relevant. Importantly, the use of dissolution media without surfactants can be expected to be highly overdiscriminating with respect to deaggregation and dissolution of drug aggregates. This is an undesirable situation probably leading to extensive additional costs in development and commercial manufacturing. To avoid this situation, low level of surfactants below the CMC is strongly recommended in all dissolution testing with hydrophobic drugs. For drugs that are dependent on solubilization in vivo, additional effects of surfactants above CMC, primarily by increasing solubility through solubilization and increasing transport in the diffusion layer,25 provides an additional, more well-established rationale, for including surfactants in the dissolution medium.
CONCLUSIONS
Hydrodynamic factors seem not to be critical for in vivo dissolution predictions of micronized drug particles and disintegration of drug aggregates. In contrast, use of surfactants to reduce surface tension to levels representative of the GI tract has a strong effect on the deaggregation and this approach is strongly recommended for low-solubility drugs to avoid artificial in vitro dissolution results.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Anders Borde, Johan Breitholtz, Sara Carlert, Pia Skantze, Urban Skantze at AstraZeneca R&D, and Mölndal for expert advice regarding the various experimental techniques.
This work was contributed to the EU IMI project OrBiTo (www.orbitoproject.eu) as sideground.




