The PK-Eye: A Novel In Vitro Ocular Flow Model for Use in Preclinical Drug Development
Abstract
A 2-compartment in vitro eye flow model has been developed to estimate ocular drug clearance by the anterior aqueous outflow pathway. The model is designed to accelerate the development of longer-acting ophthalmic therapeutics. Dye studies show aqueous flow is necessary for a molecule injected into the vitreous cavity to clear from the model. The clearance times of proteins can be estimated by collecting the aqueous outflow, which was first conducted with bevacizumab using phosphate-buffered saline in the vitreous cavity. A simulated vitreous solution was then used and ranibizumab (0.5 mg) displayed a clearance time of 8.1 ± 3.1 days, which is comparable to that observed in humans. The model can estimate drug release from implants or the dissolution of suspensions as a first step in their clearance mechanism, which will be the rate-limiting step for the overall resident time of a candidate dosage form in the vitreous. A suspension of triamcinolone acetonide (Kenalog®) (4.0 mg) displayed clearance times spanning 26–28 days. These results indicate that the model can be used to determine in vitro–in vivo correlations in preclinical studies to develop long-lasting therapeutics to treat blinding diseases at the back of the eye. © 2015 The Authors. Journal of Pharmaceutical Sciences published by Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3330–3342, 2015
INTRODUCTION
Prolonging therapeutic levels of a drug within the vitreous to treat blinding diseases is one of the most important goals in ophthalmic drug development. Intravitreal (IVT) injections of therapeutic proteins and the use of steroid implants in the vitreous are currently the best clinical methods to achieve prolonged exposure in the back of the eye. With increased life expectancy and an aging population, more people will require treatment for longer periods to manage blinding conditions that would otherwise rapidly progress.
Therapeutic biologics registered for ophthalmic use by IVT injection comprise a PEGylated-aptamer (Pegaptanib), antibody fragment (ranibizumab), and a Fc fusion (alfibercept).1 These medicines bind to VEGF and are administered by IVT injection every 1–2 months. Although IVT injections are a costly, invasive procedure that is associated with a low risk for vision threatening complications,2 many health systems around the world cannot cope with the increasing demands for IVT injection of the current anti-VEGF treatments,3, 4 even when administered every 1–2 months. The increased knowledge of the molecular mechanisms involved in blinding ocular diseases5, 6 will continue to drive the development of protein-based medicines,1 which tend to be potent and have a rapid onset of action. This will further drive the development7-11 of longer-lasting IVT injection dosage forms such as implants that require less frequent administration.
Mass exchange within the eye is dominated by the aqueous that is secreted into the vitreous from the ciliary body (2.0–2.5 μL/min).12-16 The vast majority of this aqueous passes the anterior hyaloid membrane and flows into the front of the eye (anterior chamber) to then leave the eye via trabecular and uveoscleral pathways.14-17 Drug elimination from the vitreous occurs by (1) the aqueous outflow into the anterior chamber and (2) permeation through the retina via retinal-choroid-sclera (RCS) pathways.14, 18 Therapeutic proteins clear predominantly through the anterior route because they are high-molecular-weight, charged molecules.7, 19-23
Proteins have longer half-lives (i.e., days to weeks)6, 7, 20, 24, 25 in the vitreous than RCS26 permeable molecules (e.g., lipophilic molecules ≤500 Da), which generally are a matter of hours.18, 27, 28 Reasons for this include (1) lower molecular weight permeable molecules diffuse more rapidly in the vitreous than proteins,18, 24, 27, 28 (2) the surface area of the retina is large compared to the anterior hyaloid membrane, and (3) permeable molecules can clear via several routes through the RCS (e.g., passive diffusion, active transport, binding to melanin, loss to conjuctival lymphatics and episcleral veins, and metabolism).18 In vitro cell permeation models have been developed to evaluate RCS permeability.29 It is thought that the physicochemical properties of a drug can often be used to predict RCS permeability.18, 29-31
Little has been reported to develop an in vitro model that accounts for the aqueous flow32 to estimate clearance times for molecules that exit the eye predominantly via the anterior route through the front of the eye. Preclinical pharmacokinetic research for new ophthalmic preparations is dominated by the use of animal models. There are a multitude of anatomical differences between animal models and human eyes (e.g., aqueous flow, vitreous volumes, diffusion pathways).28, 33-35 Young laboratory animals with an intact vitreous structure are often used, but much is still not understood about the vitreous36 between species, and animal models do not account for the variability and age-related differences of the human vitreous.24, 30, 37, 38 Correlating results from the literature can be complicated because different experimental techniques are often used to obtain PK data for each species.18 The formation of antidrug antibodies (ADAs) also results in accelerated clearance rates,39 making it impossible to accurately evaluate candidate therapeutic proteins.40-43 ADAs can also cause nonspecific inflammation to compromise efficacy. ADA formation is not predictive of the human immunological response and remains an intractable problem that delays development of humanized protein therapeutics.44
The use of in vitro models has been important to develop and optimize many dosage forms including pulmonary (deposition), percutaneous (diffusion), and oral (dissolution) preparations.45-48 These models are also used to establish in vitro–in vivo correlations and extrapolations (IVIVC and IVIVE),49-53 and for quality control.54, 55 For longer-acting ophthalmic preparations to be developed, preclinical candidates must display extended half-lives while under the influence of the aqueous flow within the eye. To address this need, we have developed an in vitro, two-compartment model scaled to human dimensions with aqueous flow designed to mimic the total aqueous mass transfer through the anterior route. The model (called the PK-Eye) is specifically designed to mimic the intraocular aqueous outflow to estimate (1) the clearance of therapeutic proteins, (2) the dissolution of drug suspensions, and (3) the release profiles of implants from the vitreous cavity. The design and flow properties of the in vitro model are described and then the model was used to estimate the intraocular residence times of ranibizumab, bevacizumab, and triamcinolone acetonide (TA) suspension, which were compared with published data from in vivo studies in animals and humans.
MATERIALS AND METHODS
Materials
Kenalog® (40 mg/mL) was purchased from Squibb. Bevacizumab (Avastin®, 25 mg/mL; Genentech, South San Francisco, California) and ranibizumab (Lucentis®, 10 mg/mL; Genentech, South San Francisco, California) were obtained from the pooled remaining contents of vials that had been used clinically. Sodium hyaluronate (solubility: 5.0 mg/mL; 1.8 MDa) was purchased from Aston Chemicals (Aylesbury, UK) and agar (solubility: 15.0 mg/mL) was obtained from Fluka Analytical (Gillingham, Dorset, UK). Dye (Coomassie Brilliant Blue, MW = 825.97 g/mol) was used to validate flow in the model. Visking dialysis membrane tubing (MWCO of 12–14 kDa) was purchased from Medicell International Ltd. (London, UK). Bovine serum albumin (BSA) was obtained from Sigma–Aldrich. A 16-channel Ismatec peristaltic pump (Michael Smith Engineers Ltd., Woking, Surrey, UK) was used to generate fluid flow into the PK-Eye. UV measurements were analyzed using Hitachi U-2800A spectrometer and a Wallac Victor2 1420 plate reader. HPLC was conducted using a Agilent 1200 series (Agilent, Wokingham, Berkshire, UK) equipped with Chemstation software (Agilent, Wokingham, Berkshire, UK) using a reverse-phase Synergi Polar-RP Phenomenex 4 μm, 15 cm column.
Methods
Design of the PK-Eye Flow Model
Efforts to develop the model were focused at the outset (1) to have the anterior and posterior cavities to be a similar size as in the human eye and (2) to use an aqueous inflow that mimics the aqueous outflow in humans. The model comprises two plastic pieces that are secured together with four screws (Fig. 1). The overall dimensions and volumes of the posterior (4.2 mL) and anterior (0.2 mL) cavities of the model are comparable to that of the human eye (Table 1). Several prototypes of the model were evaluated (Table 2) to best mimic the mass transfer caused by aqueous outflow. The prototyping efforts listed in Table 2 were focused to design a model that was both simple to fabricate and to use. It was found that a single inlet port (1.5 mm ID) with a flow of 2.0 μL/min15 was the most practical way to mimic aqueous flow in this model. The aqueous inlet port was placed in the vitreous cavity near the membrane barrier. An injection port was placed at the top of the vitreous cavity. A widely available dialysis membrane (MWCO 12–14 kDa) was fixed between the posterior and anterior cavities (Fig. 1). This membrane was used to provide a barrier to the anterior cavity for solubilized molecules residing in the posterior cavity. For outflow, a single outlet port in the anterior cavity extended upwards from the model to provide a small amount of backpressure. This was used to avoid the formation of air bubbles and to maintain the total liquid volume within the model.
| Average | PK-Eye | ||
|---|---|---|---|
| Dimensions | Anatomy | Human Eye56 | Model |
| Length (mm) | Cornea vertical length | 11.0 | 9.0 |
| Cornea horizontal length | 12.0 | 9.0 | |
| Volume (mL) | Posterior cavity | 3.9–5.0 | 4.2 |
| Anterior cavity | 0.2 | 0.2 |
| Ports | |||||
|---|---|---|---|---|---|
| Prototype | In | Out | Vitreous (mL) | Design Information | Observations During Validation |
| A | 1 | 1 | 5.6 | A spherical globe made of polyacrylate plastic | The volume was too large compared with the human eye |
| B | 2 | 1 | 4.2 | Volume was adjusted to match the average value reported in human eyes | Cavity lacked spherical shape |
| C | 2 | 4 | 4.2 | Incorporation of anterior (0.2 mL) and posterior cavities (4.2 mL) | Could not maintain a gel to water interface between posterior and anterior cavities |
| D | 2 | 10 | 4.2 | Fit cavity separating membrane and increase outlet ports | Unequal and insufficient flow in each port observed |
| E | 2 | 4 | 4.2 | Reduce the number of outlet ports | Input port diameters too large and too far away from the cavity separating membrane |
| F | 4 | 4 | 4.2 | Move input ports to replicate ciliary body location and flow | Inadequate and inconsistent flow from each outlet port |
| G | 4 | 1 | 4.2 | Incorporation of new outlet port in the anterior cavity | Too difficult to maintain an accurate flow rate using four inlet ports |
| H | 1 | 1 | 4.2 | One inlet to replicate ciliary body flow | Much more practical to use than with two inlet ports |
- The prototype that was used for these studies reported herein is H.
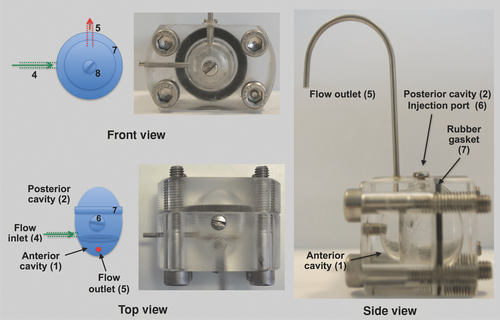
PK-Eye Dye Validation Studies
The inlet port was connected with tubing (1.5 mm ID) to a dispensing pump that provided aqueous (phosphate-buffered saline, PBS) inflow of 2.0 μL/min. Dye (Coomassie Brilliant Blue) was injected (100 μL, 1.2 mM) into the posterior cavity of the model in one experiment and then in the anterior cavity in another experiment to compare the effects of flow at ambient temperature. For the non-flow experiments, the model was full of liquid with its ports blocked with parafilm to replicate no flow. Images were obtained using a digital camera at various time points to visualize the dye clearance from the model.
Simulated Vitreous and Viscosity Measurements
Agar (0.4 g) and hyaluronic acid (HA) (0.5 g) were each separately mixed in 100 mL of stirred hot water.57 The agar solution was boiled to completely solubilize the agar. After boiling, the hot agar solution was mixed with HA and stirred to give a homogenous mixture to which a few drops of 0.02% sodium azide were added. The solution was left to cool for 24 h to ambient temperature (∼21°C) and formed into a gel-like consistency. Viscosity (n = 3) was measured using an AR 1000-N Rheolyst Rotational TA rheometer (TA Instruments, Elstree, UK) at 25°C. Approximately 2.0–3.0 mL of simulated vitreous was pipetted onto the rheometer plate while avoiding both over and under filling of the rheometer plate. The rheometer plate was washed with distilled water before each measurement and the temperature was kept constant (25°C.) The pseudo-linear viscoelastic region was determined using an oscillatory stress sweep over a broad torque range (1–10,000 uN.m). The pseudo-viscosity profile was obtained using a continuous flow test and an oscillatory time sweep was used to monitor whether the properties of the simulated vitreous changed over time.
Protein Clearance Studies
To coat the model with albumin solution, it was filled with 0.1% BSA solution and after securing the injection port, the model was incubated at 37°C for 12 h (n = 3 for each experiment). The model was then disassembled and washed with water and refilled with PBS (pH 7.4). To examine the effects of vitreous viscosity, the simulated vitreous was added to the posterior cavity by syringe and PBS was added to the anterior cavity through its injection port. Ranibizumab (0.5 mg) and bevacizumab (1.25 mg) were injected (50 μL) into the center of the vitreal cavity and sampling was generally accomplished by collection at the outflow port. Samples were stored at 4°C prior to analysis for protein content by the Bradford assay.
TA (Kenalog®) Clearance Studies
The posterior cavity of the model was filled with the appropriate media (PBS, pH 7.4 or simulated viscous vitreous). PBS (pH 7.4) was then added to the anterior chamber. The aqueous flow (2.0 μL/min) was started and the model was placed into a preheated oil bath at 37°C. Temperature was maintained using a probe connected to the hotplate heater. After approximately 2 h of heating the model, TA (Kenalog®, 4.0 mg in 100 μL) was injected into the posterior segment of the model. Sampling was accomplished daily by replacing the vial used to collect the outflow from the anterior cavity. Samples were stored at 4°C and then analyzed by HPLC (254 nm).
Quantification of Drug Release
The Bradford assay was used to determine protein concentration. Samples and standards (40 μL) were evaluated in triplicate using 96-well plates and mixed with Bradford Ultra reagent (110 μL). Plates were incubated for 10 min at room temperature and absorbance at 590 nm was measured using a plate reader. The calibration curve (R2 value of 0.99) was made using a solution of antibody (100 μg/mL) that was serially diluted (PBS, pH 7.4) to 10 μg/mL. Average measurements for blank (0 μg/mL of antibody) were subtracted from standards and unknown samples. The concentrations of unknown samples were estimated based on the standard curve. Samples from each time point was evaluated in triplicate (n = 3) and the mean and standard deviation were determined.
The concentration of TA (Kenalog®) was determined by HPLC with UV detection (254 nm) using methanol–water (70:30, v/v) as the mobile phase. A retention time of approximately 3.7 min was observed. The injection volume was 10 μL with a flow rate of 1.0 mL/min and a run time of 10 min. The calibration curve (R2 value of 0.97) was made starting with a TA suspension (1 mg/mL) that was serially diluted using PBS to 0.39 μg/mL.
Data Analysis
All results are presented as the mean and standard deviation, and data were plotted using OriginPro. Half-lives (t1/2) was calculated according to the best fitting model in OriginPro. First-order kinetic rate constants (k) were derived from the monoexponential curve and half-lives were calculated using the equation: 0.693/k. The rate constants (k) of zero-order release profiles were calculated as concentration–time and half-lives were calculated from initial concentration [A] using [A]/2k.
RESULTS AND DISCUSSION
Dye Experiments to Illustrate Flow Properties of the Model
Dye studies were conducted to illustrate the need for flow and compartmentalization of the model. With the aqueous inlet flow set at 2.0 μL/min, Coomassie blue dye was injected (100 μL, 1.2 mM) into the anterior cavity (Fig. 2). The amount of dye used was in large excess to what can be administered in an in vivo experiment by either injection or the use of eye drops. The dye cleared through the outlet port with only a trace amount being observed to migrate into the posterior cavity at about 24 h after injection (Fig. 2). In a second experiment, the dye was injected into the posterior cavity. With aqueous inflow (2.0 μL/min), the dye cleared from the posterior cavity into the anterior cavity and then through the outlet port of the model (Fig. 3, upper panel). When there was no flow (Fig. 3, lower panel), the dye remained concentrated only in the posterior cavity.

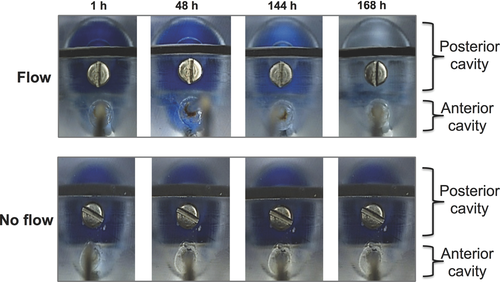
These dye experiments broadly mimic mass transfer in the eye that is caused by aqueous flow. When a drug is in the anterior chamber, very little of it will pass into the posterior cavity because of the aqueous outflow. And if a drug is in the posterior cavity, flow is necessary for the drug to clear anteriorly via aqueous outflow. It was conspicuous that the dye did not migrate between the two compartments unless flow was induced (Figs. 2 and 3), so the visking membrane did offer barrier properties to the dye. Coomassie blue has a relatively large molecular weight (∼825 g/mol) and is partially soluble in water because of its hydrophobic nature. The visking membrane is made from reconstituted cellulose. Water is thought to swell the membrane creating a hydrophilic barrier layer that can act to inhibit the diffusion rate of hydrophobic molecules through the membrane.58, 59
In addition to in vitro permeation models,26, 29 other in vitro models have been described for the preclinical development of IVT ophthalmic preparations.45, 60-63 For example, the dissolution of poorly soluble drugs and extended-release tablets62 were evaluated using a USP4 dissolution apparatus with a flow through of 1.5 mL/min in a chamber of 8–19 mL.64 Aspects of drug distribution in the vitreous have been evaluated using a model for saccades (i.e., eye movements), which can influence drug distribution in the vitreous.65 This single compartment, nonflow model was used to evaluate dye distribution in glycerol and polyacrylamide gels as vitreous substitutes.66 These in vitro models do not mimic the human eye in terms of its compartmentalization, scale, or mass transfer properties because of aqueous flow.
Computational modeling of the eye has also been used to evaluate drug distribution within the vitreous of animals and humans18, 67-74 after injection or release by implants,75, 76 and to develop vitreous diffusion IVIVCs.77, 78 The need to understand how a drug is distributed within the vitreous to access the retina has been a key driver for many of the studies focused on drug distribution within the posterior segment. However, knowledge of vitreous distribution can only be complete by first determining the residence time of a drug within the vitreous. Any preclinical candidate designed for prolonged action in the posterior cavity must be able to achieve extended PK properties in the presence of intraocular aqueous flow.
Preincubation of the Model with Albumin
There was a concern that a protein undergoing evaluation would adversely adsorb onto the internal plastic surfaces of the model. The model was preincubated with a 0.1% albumin solution, and then with the inflow set at 2.0 μL/min, bevacizumab was injected (1.25 mg in 50 μL) into the posterior cavity of the model.
This experiment was conducted using PBS in the model to allow rapid and complete distribution of bevacizumab throughout the posterior cavity. The use of PBS in the posterior cavity also acted to mimic the reduced viscosity of the vitreous after a vitrectomy. The viscoelastic properties of the vitreous are incompletely understood15, 34, 79 and there are many differences in the composition and microstructure of the vitreous.24, 30, 36-38 As a large molecule, the diffusion of a protein will be faster80 and will clear more rapidly from a vitrectomized eye.28, 81 It was also thought that faster and probably more complete antibody diffusion would occur in a low viscous solution that would make it easier to observe its propensity to adsorb onto the plastic surface.
The observed residence time of bevacizumab from the model after preincubation with albumin followed first-order kinetics and was 1.5 ± 0.3 days (Fig. 4a). When the model was not coated with albumin, the bevacizumab clearance half-life was 1.2 ± 0.1 days (Fig. 4b). Although this was not significantly different with the clearance half-life when the model was preincubated with albumin, there appeared a less discernable peak concentration, which suggests the bevacizumab reversibly adsorbed onto the plastic surface of the uncoated model. The presence of a peak concentration is characteristic for an intraocular injection73 and was more evident when bevacizumab clearance was measured in the albumin-coated model (Fig. 4a). If the model is fabricated from plastic and PBS is used in the posterior cavity, consideration should be made to preincubate with albumin or another blocking protein prior to evaluating a candidate therapeutic protein.
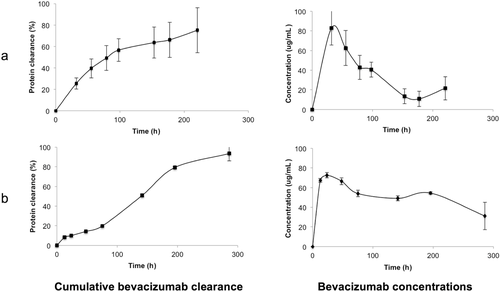
Bevacizumab is a monoclonal antibody that is widely used as an unlicensed, but more cost-effective anti-VEGF medicine3, 4 that is considered to be clinically equivalent to ranibizumab for the treatment of some indications.82, 83 It was encouraging that the half-life of 1.5 ± 0.3 days from the albumin-coated model (Fig. 4a) is comparable to the half-life of bevacizumab at the same dose in vitrectomized monkey eyes (1.5 ± 0.6 days; n = 6).84 There is a report that the half-life of bevacizumab at a 1.25-mg dose from a victrectomized human eye of a single patient was 0.7 days.85 In nonvitrectomized human eyes at this dose, the half-life of bevacizumab has mean values ranging from 4.9 to 10 days.81, 85-88 In contrast, the half-life of bevacizumab at this dose is 2.8 ± 0.6 days (n = 3)89 in normal monkey eyes that have an intact vitreous. Although less human data exists for the clearance of bevacizumab in vitrectomized eyes, clearance is expected to be faster than in nonvitrectomized eyes. There is also a significant variation in the half-lives for nonvictrectomized human patients, so the monkey and human data are also significantly different. On the basis of the more complete monkey data, the model could be used to estimate the vitrectomized clearance times by using PBS in the vitreous cavity. It is thought this would provide the fastest clearance time possible for a given preclinical formulation.
Clearance Profile of Ranibizumab Using Simulated Vitreous Fluid
Protein therapeutics will diffuse more slowly in vitreous fluid, which is generally more viscous than PBS. A simulated vitreous liquid was then used in the posterior cavity of the model. Reduced diffusion will prolong the time it takes for the protein to clear from the vitreous cavity. Simulated biological fluids are widely used to mimic aspects of an endogenous fluid during preclinical research.32, 90 Vitreous substitutes are also widely used, both clinically and for preclinical research.91, 92 We used a simulated vitreous fluid prepared from HA and agar56 that had a dynamic viscosity of approximately 0.6 Pa·s, which is comparable to human vitreous (∼0.5 Pa·s).93 Initial experiments established that none of the components of the simulated vitreous diffused into the anterior cavity of the model and that the simulated vitreous maintained its viscosity over time (∼2–3 weeks). No substrates were observed in the outflow unless ranibizumab was first injected.
Ranibizumab was then injected into the vitreous cavity of the model using the usual clinical dose (0.5 mg, 50 μL). The ranibizumab clearance half-life in the model followed first-order kinetics and was 8.1 ± 3.1 days (Fig. 5a), which is similar to the half-life in humans at this dose (∼7.294 and 9.095 days). Using simulated vitreous provides the means to estimate the time needed for a therapeutic protein to clear via the aqueous outflow pathway from a human eye. When PBS was used in the posterior cavity, the clearance half-life of ranibizumab was 1.5 ± 0.6 days (Fig. 5b). This was comparable to bevacizumab (Fig. 4a). When the model was pre-incubated with albumin and then rinsed, no protein was observed from the outflow. Protein was only observed when the sample, i.e. ranibizumab, was injected into the posterior cavity of the model.
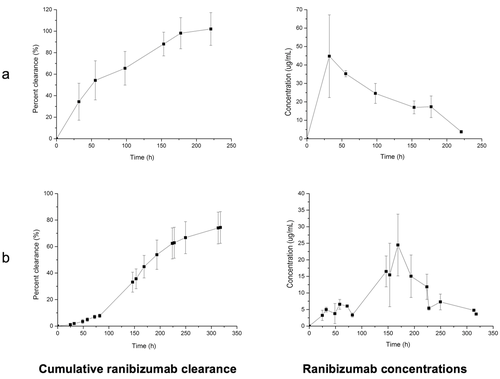
The visking membrane had a molecular weight cut off value of 12–14 kDa. This refers to the smallest number average molecular weight of a globular macromolecule that will be about 90% retained when dialyzed overnight,96 although there are no strict specification standards.55 It was anticipated that with experiments lasting several days to weeks, that passage through the membrane of small amounts of molecules with larger molecular weight would be possible. This is what was observed for both the ranibizumab and bevacizumab experiments (Figs. 4 and 5). It was also necessary for the membrane to ensure the simulated vitreous solution remained in the posterior cavity without allowing the gelled material to pass into the anterior cavity. The simulated vitreous should be made using very high-molecular-weight gelling components that form a stable viscous solution.
As a Fab, ranibizumab (∼48 kDa) is about 1/3 the molecular weight of bevacizumab (∼150 kDa), which is a full monoclonal antibody. In humans, these molecules display comparable mean half-lives with a similar therapeutic effect.21, 86, 94 The half-lives for ranbizumab span from about 7.2 to 9.0 days and for bevacizumab, the range is about 6.7–10.0 days.81, 85-88 As mentioned, these half-lives can be reduced because of medical conditions such as vitrectomy and retinal detachment.97 The usual clinical dose for ranibizumab (0.5 mg) is about 20% more than bevacizumab (1.25 mg) on a molar basis. Of the other biologics registered for ophthalmic use by IVT injection,1 there is no reported human half-life data for the Fc fragment fusion (alfibercept, ∼115 kDa),1, 81 but the PEGylated-aptamer (Pegaptanib, ∼50 kDa) displays a mean half-life of about 7–8 days for a IVT dose of 0.3 mg as determined by plasma measurements.98 Although the half-life of Pegaptanib is essentially the same as ranibizumab, its dose is about 60% of that is used for ranibizumab on a molar basis. Increasing the dose of Pegaptanib by 10 times of the US FDA-approved dose to 3.0 mg only increases the half-life to about 10 (±4) days.99, 100
These observations suggest that there is probably a “molecular weight threshold” after which most proteins and macromolecular drugs will display comparable half-lives in the posterior cavity due to clearance by the aqueous outflow pathway. New strategies are being developed to increase the vitreous resident times of macromolecular drugs.7, 10, 19, 34, 101-103 Some of these strategies may employ the use of implants (for example) where a release or dissolution step is required prior to clearance of the protein therapeutic. It is for these new strategies that we believe our model will find real utility in preclinical research. The strategy to increase the dose of a molecule as a means to increase its “therapeutic tail,” which is used with aflibercept, can also be evaluated in a realistic 2-compartment outflow model such as the PK-Eye. As more stable protein therapeutics are developed, much higher concentrated doses may be possible where exploitation of the therapeutic tail is difficult to evaluate in an animal model simply because of the generation of ADAs.
The need during preclinical research to obtain reliable pharmacokinetic information using animal models alone for protein therapeutics cannot be adequately met. The use of our model will avoid the problems associated with ADAs.39-43 The model also avoids the need to consider the anatomical differences28, 33-35 that exist between animal and human eyes. For example, globe sizes of the rabbit (New Zealand white) and monkey (cynomolgus) are similar, but the relative sizes of the anterior chamber and lens of the rabbit are much larger than the monkey.33 The rabbit vitreous is about 50% the size of the monkey vitreous (and about 30%–40% that of the human vitreous). But the aqueous flow of the rabbit (∼2.0 L/min104) and monkey (∼2.8 L/min105) are similar to that of the human. Although clearance of ranibizumab will be primarily driven by its diffusion through the vitreous,28 its clearance should be faster in the rabbit (less vitreous, larger anterior cavity, shorter diffusion path). However, the half-life of ranibizumab (0.5 mg) from the rabbit and monkey are about the same, being about 2.8–2.9 days22, 106, 107 and about 3 days,35 respectively. Even though the monkey eye is more similar to the human eye than is the rabbit eye,33 the half-life for IVT-injected ranibizumab (0.5 mg) from the monkey is much less than that observed in humans (∼7.294 and 9.095 days).
Although three of the four clinically used macromolecular therapeutics broadly display similar mean half-lives in humans, there is still range of clearance times that is indicative of how intraocular pharmacokinetics can vary in human eyes that are being treated. This variation may be because of how the IVT injection procedure is conducted,28, 108, 109 but more often there are complicating medical conditions86, 110, 111 in patients that require IVT injections that could facilitate greater RCS uptake of nonpermeable molecules. There are also variations in vitreous composition37 that can influence flow in the posterior chamber of human eyes68, 112 where aqueous outflow through the retina may increase.30 Variations in the vitreous occur because of (1) age (i.e., liquefaction),24, 28, 38 (2) whether a vitrectomy has been performed, and (3) due to the nature of the medical condition afflicting the eye. For example, small breaks in the inner-limiting membrane (ILM)113, 114 can cause retinal uptake of non-RCS permeable molecules such as therapeutic proteins.21, 87, 110, 111, 115 The intact ILM that is between the vitreous and retina can act as a barrier for macromolecules to the retina.7, 34 The pharmacokinetics of antibodies can further vary because of the presence of Fc receptors in the retinal tissue.115-117 Although some posterior aqueous outflow occurs through the retina, the relative amount may be higher in animal models than in humans17 and radiolabelled ranibizumab can be detected within the retina of the rabbit.118 However, quantifying the total dose that may clear through the retina119 or extrapolating data for retinal uptake from an animal model after IVT administration is not yet possible. The use of the model with PBS in the vitreous cavity can provide data for the fastest vitreous clearance time to be expected for a preclinical candidate. If considered along with the clearance times obtained with simulated vitreous, it will be possible to obtain minimum and maximum estimates for posterior cavity clearance times very early in preclinical research.
It is not possible with in vivo animal studies to mimic the wide range of conditions encountered in human eyes that may cause pharmacokinetic variation. For example, ranibizumab (and bevacizumab) clearance from vitrectomized rabbit eyes is about the same107 or at best slightly reduced compared with nonvitrectomized rabbit eyes.106 This may be because of the large rabbit lens making it difficult to remove a high enough proportion of the vitreous.107, 109 As longer-lasting preparations are developed, differences in human ocular pharmacokinetics will persist to obscure clinical outcomes. It is important in preclinical research to obtain robust PK data based on the aqueous outflow pathway because it appears to change less than RCS pathways for many of the intraocular diseases that require treatment by IVT injections. It is possible with our model to obtain good estimates of the upper limit for clearance early in preclinical studies. The many medical conditions that contribute to accelerate clearance times can then be accounted for to estimate the different clearance times that might occur clinically.120 Furthermore, as longer-lasting therapeutic protein preparations are developed, the use of our outflow model will allow for easy sampling to evaluate protein stability that will be important for preparations designed to last 2–3 months inside the vitreous cavity.
Clearance Profile of TA Suspension (Kenalog®)
The clinical use of steroid implants (e.g., Ozurdex® and Iluvien®) that release drug over several months into the vitreous are examples of IVT implants19 that may become increasingly more common in the clinic.19, 24, 34, 102, 103, 121-123 Many current implants utilize poorly soluble steroids124 so the nonsink conditions of the posterior cavity can be exploited to prolong the vitreous resident time. Steroids tend to be lipophilic and permeable, and so a significant amount of material solubilized in the vitreous would be expected to clear by both RCS and anterior outflow pathways. Once a permeable, low-molecular-weight molecule is solubilized in the vitreous, clearance would be expected to be relatively quick.18, 24, 27, 28 For example, the half-life of intravitreally injected dexamethasone phosphate, which is water soluble, is 3.48 h.27
Prior to the development of implants, the IVT injection of a suspension of a poorly soluble drug such as TA (Kenalog®)125, 126 was the best way to clinically prolong the presence of a drug in the vitreous cavity. TA solubility is in the range of approximately 21–30 μg/mL depending on solution media and temperature (25°C–37°C).127, 128 Kenalog® is the most commonly used TA preparation and has long been used for ophthalmic conditions125, 126, 129; however, there are other, preservative-free, TA formulations available that include Triesence® (Alcon) and Trivaris® (Allergan).129
Triamcinolone acetonide (Kenalog®, 4.0 mg in 100 μL) was injected into the posterior cavity of the model, which was heated to 37°C. The dissolution rate of the TA suspension is solubility controlled in the total liquid volume of the model and a near constant-release concentration (∼25–30 μg/mL) in both PBS and simulated vitreous was observed (Fig. 6). The clearance half-life of TA from the model was in the range of 26–28 days.
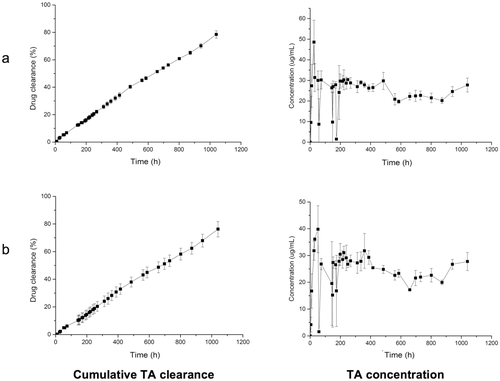
The mean half-life in humans for the IVT administration of TA at the same dose spans 15.4130–18.6 days.131 This clearance time is faster than what was observed in the model because of contributing clearance through RCS pathways. RCS permeable molecules once in solution will clear through both RCS and anterior outflow pathways. Although active transport by the RCS can be significant for some molecules,72 our model will provide an estimate of the upper time period for clearance. The model can be used to estimate drug release from implants or the dissolution of suspensions as a first step in their clearance. This will often be the rate-limiting step that defines the overall clearance time of a candidate preparation. Once the active is in solution, the model has the capacity to provide a good estimate of clearance by the anterior outflow route. As for other preparations designed for different modes of administration (e.g., oral, pulmonary), this information can be used with an in vitro26 or computational18, 29-31 permeability model to develop IVIVCs.49, 52, 53 There is intense effort to develop implants and other long-acting preparations from proteins, peptides and nucleotides,7, 10, 19, 34, 101, 103 and possibly other low-molecular-weight substances (e.g., the antiviral agent, Foscarnet18) that do not clear to a great extent by the RCS. In these cases, the preclinical use of our model would be expected to provide good estimates of useful clearance times.
CONCLUSIONS
The PK-Eye is a novel pharmacokinetic in vitro model designed for use during preclinical studies to accelerate the development of longer-lasting therapeutic dosage forms that are needed to treat chronic blinding conditions. Our low-cost, two compartment in vitro model is scaled to the human eye and the model mimics the mass transfer characteristics caused by anterior aqueous outflow. Therapeutic proteins are large hydrophilic molecules, so they are expected to clear primarily by aqueous outflow pathways. The formation of ADAs in animals in response to human therapeutic proteins and the anatomical differences in animal models make it difficult to correlate clearance profiles with that expected from human eyes. Animal studies also tend to be expensive with a large degree of variation because of the range of experimental protocols that are followed. Ethically, animal experimentation should be minimized when possible, especially when providing limited information.
Dye validation studies and clearance studies utilizing the model in different configurations (e.g., use of either PBS or simulated vitreous) indicate that relevant clearance profiles can be obtained using the model for both proteins and poorly soluble drugs injected as a suspension, or potentially formulated as an implant. Release of an active from an implant or its dissolution from a particulate or suspension will often be the rate-limiting step that defines the overall clearance time of a candidate preparation.
Obtaining PK data for molecules that clear predominantly by the aqueous outflow pathway is important because characteristics of aqueous outflow appear to change less than the permeability properties of the tissues at the back of eye. As longer-lasting preparations are developed, differences in human ocular pharmacokinetics will persist to obscure clinical outcomes. It is possible with our model to obtain good estimates of the maximum and minimum vitreous clearance times early in preclinical studies. This will allow faster optimization cycles during early preclinical studies while avoiding the needless use of animal models until absolutely necessary. Our model can be used with other in vitro or computational permeability models to develop IVIVCs that include RCS clearance pathways. This is analogous to strategies that are used to develop new dosage forms for oral and pulmonary administration. Our in vitro model can easily be used to evaluate protein function and stability. This is difficult to accomplish using animal models and is especially important for dosage forms that have longer clearance times that could extend over a 2–3-month period. Our studies show the PK-Eye model has many of the features needed to become a practical in vitro model with the capacity to contribute to research efforts focused on the development of new ophthalmic medicines.
ACKNOWLEDGMENTS
We are grateful for funding from the National Institute of Health Research (NIHR) Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, Moorfields Special Trustees, the Helen Hamlyn Trust (in memory of Paul Hamlyn), Medical Research Council, Fight for Sight and Freemasons Grand Charity. S.A. gratefully acknowledges funding from the UCL Overseas Research Student Fund. S.B. is also grateful for funding from the UK Engineering & Physical Sciences Research Council (EPSRC) for the EPSRC Centre for Innovative Manufacturing in Emergent Macromolecular Therapies. Financial support from the consortium of industrial and governmental users for the EPSRC Centre is also acknowledged. We thank Mr John Frost for all his advice and help to fabricate prototypes of the PK-Eye.




