Controlled Release, Intestinal Transport, and Oral Bioavailablity of Paclitaxel Can be Considerably Increased Using Suitably Tailored Pegylated Poly(Anhydride) Nanoparticles
Abstract
The aim of the work was to evaluate in vitro and in vivo the effect of the addition of poly(ethylene glycol) (PEG) to paclitaxel (PTX)–cyclodextrin poly(anhydride) nanoparticles. For this, PTX in poly(anhydride) nanoparticles complexed with cyclodextrins (either 2-hydroxypropyl-β-cyclodextrin or β-cyclodextrin) and combined with PEG 2000 were prepared by the solvent displacement method. Intestinal permeability in vitro and in vivo pharmacokinetic studies in C57BL/6J mice were performed. Nanoparticle formulations containing PTX increased its apparent permeability through rat intestine in vitro in the Ussing chambers, enhancing its permeability 10–15 times compared with commercial Taxol®. In addition, in pharmacokinetic studies, drug plasma levels were observed for at least 24 h leading to a relative oral bioavailability between 60% and 80% for PTX complexed with cyclodextrin and loaded in pegylated poly(anhydride) nanoparticles after oral gavage. In all, PTX–cyclodextrin complexes encapsulated in pegylated nanoparticles managed to promote the intestinal uptake of the drug displaying sustained plasma levels after oral administration to laboratory animals with a more prolonged plasma profile compared with the formulation with no PEG at all. Therefore, pegylated poly(anhydride) nanoparticles represent a promising carrier for the oral delivery of PTX. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2877–2886, 2015
Abbreviations used
-
- i.v.
-
- intravenous(ly)
-
- PTX
-
- paclitaxel
-
- Pgp
-
- P-glycoprotein
-
- PEG
-
- poly(ethylene glycol)
-
- PMV/PA
-
- poly(methyl vinyl ether-co-maleic anhydride) or poly(anhydride)
-
- DCX
-
- docetaxel
-
- CD
-
- β-cyclodextrin
-
- HPCD
-
- 2-hydroxylpropyl-β-cyclodextrin
-
- EDTA
-
- ethylenediaminetetraacetic acid disodium salt
-
- PEG2000
-
- poly(ethylene glycol) 2000
-
- PBS
-
- phosphate-buffered saline
-
- PTX–HPCD
-
- PTX-2-hydroxylpropyl-β-cyclodextrin inclusion complexes
-
- PTX–CD
-
- PTX-β-cyclodextrin inclusion complexes
-
- PTX–HPCD–NP
-
- PTX–2-hydroxylpropyl-β-cyclodextrin inclusion complexes encapsulated in poly(anhydride) nanoparticles
-
- PTX–CD–NP
-
- PTX–2-hydroxylpropyl-β-cyclodextrin inclusion complexes encapsulated in poly(anhydride) nanoparticles
-
- PTX–CD–NP–PEG
-
- PTX–β-cyclodextrin inclusion complexes encapsulated in pegylated poly(anhydride) nanoparticles
-
- PTX–NP–PEG
-
- PTX encapsulated in poly(anhydride) nanoparticles
-
- PCS
-
- photon correlation spectroscopy
-
- SEM
-
- scanning electron microscopy
-
- cryo-EM
-
- cryo-electron microscopy
-
- SGF
-
- simulated gastric fluid
-
- SIF
-
- simulated intestinal fluid
-
- Papp
-
- apparent permeability coefficient
-
- R
-
- absorption ratio
-
- PD
-
- transmucosal potential difference
-
- Isc
-
- short-circuit current
-
- Cmax
-
- maximal plasma concentration
-
- Tmax
-
- time in which Cmax is reached
-
- AUC
-
- area under the plasma curve
-
- MRT
-
- mean residence time
-
- Cl
-
- clearance
-
- V
-
- volume of distribution
-
- t1/2
-
- plasma half-life
-
- Fr
-
- relative oral bioavailability
INTRODUCTION
Oral chemotherapy is an attractive approach for cancer treatment because of its convenience, safety, and ease of administration, especially as chronic regimens.1, 2 In several studies, patients stated that the oral route is the preferred route of administration claiming that orally administered medication interfered less with their daily life and they felt more freedom, which could be translated into an increase in their quality of life. In practice, the efficacy of the treatment is directly linked to the patient's attitude and behavior.3, 4
Many anticancer agents are administered by means of the intravenous(ly) (i.v.) route as they present a low oral bioavailability. Only 10% of the cancer treatments are provided as oral formulations. It is estimated that this percentage should increase to 25% in the close future.5 As for many other drugs, the poor oral availability of some major anticancer agents is mainly because of certain physicochemical properties of the molecules (solubility, lipophilicity, pKa), physiological factors (intestinal transit time, gastrointestinal pH, stability, absorption mechanisms, and local metabolism), and other aspects associated with the dosage forms. Paclitaxel (PTX) is a typical example of such a drug suffering from these drawbacks.
Paclitaxel, a natural diterpenoid obtained from the bark of the Pacific yew tree (Taxus brevifolia sp.), is one of the most used anticancer agents in clinics nowadays. It has proven to be useful against several types of cancers such as breast, refractory ovarian, and non-small cell lung cancers.6 It works by promoting the stabilization of microtubules, inhibiting cell proliferation, and finally inducing apoptosis.7 In spite of its potential, PTX presents two main limitations to its use. The former, which appears to have been solved in the last years, is the availability and cost of the drug. In fact, to obtain 1 g of PTX or its precursors, such as baccatin III and 10-deacetylbaccatin III, a large amount of bark from the yew was needed.8, 9 That is why the semi-synthesis of analogues has been the main solution.10 Secondly, because PTX presents a very low aqueous solubility (0.3 μg/mL), the commercially available formulations, Taxol® or the generic PTX injection formulations, include a mixture of ethanol and Cremophor EL® (1:1). Cremophor EL® (polyethoxylated castor oil) appears to be responsible for the severe hypersensitivity reactions that a significant number of patients present when treated with Taxol® or its generics. To minimize this effect, patients have to be pretreated with corticosteroids and antihistamines.11
Moreover, apart from its lipophilicity and low water solubility, the oral administration of PTX is also hampered because of the fact that it is substrate of the intestinal efflux pump P-glycoprotein (Pgp) and it is metabolized by the cytochrome P450 (isoform CYP3A4), both highly expressed in the gut.12
In order to overcome these drawbacks, numerous strategies have been reported and are under investigation attempting a safe and reproducible oral administration of this widely used anticancer agent.13, 14 These include the synthesis of taxane analogues (i.e., ortotaxel, larotaxel dihydrate),7, 15 the co-administration with Pgp and CYP3A4 selective inhibitors, that is, cyclosporin A or its analogues,16, 17 conjugation with low-molecular-weight chitosan,18 the formulation of self-emulsifying drug delivery systems,19 nanoemulsions,20 micelles,21, 22 lipid nanoparticles, and biodegradable polymeric nanoparticles.12, 23-25
Among nanoparticles, an interesting strategy recently proposed was the combination of poly(anhydride) nanoparticles and the complexes formed by cyclodextrins and PTX.23, 26, 27 These nanoparticles were able to establish bioadhesive interactions with the mucosa increasing the residence time of the drug-containing nanocarriers in the gut in close contact with the surface of the epithelium.28 In addition, cyclodextrins have been described as inhibitors of Pgp, which prevents the absorption of PTX at the intestinal surface.29, 30 Additionally, more recently, pegylated nanoparticles have been described as interesting carriers for PTX.31 Thanks to the multifunctional characteristics of these pegylated nanoparticles, including mucus diffusivity, bioadhesive properties, and the inhibitor effect of poly(ethylene glycol) (PEG) on Pgp,32 significant PTX plasma levels were observed for 72 h, achieving a relative oral bioavailability of around 70% in rat.31
In this context, therefore, the aim of this work was, in a first step, to investigate the effect of PEG on the loading of PTX-cyclodextrin complexes in poly(anhydride) nanoparticles and in a second step, to compare the different poly(anhydride) nanoparticles containing PTX in terms of intestinal permeability in vitro and in vivo pharmacokinetic profiles in C57BL/6J mice.
MATERIALS AND METHODS
Materials
Poly(methyl vinyl ether-co-maleic anhydride) or poly(anhydride) (PMV/MA) [Gantrez® AN 119; molecular weight 200 kDa] was purchased from ISP/Ashland Inc. (Barcelona, Spain). PTX (USP 26, grade > 99.5%) and docetaxel (DCX) (99.0%) were supplied by 21CECpharm (London, UK). Taxol® was provided by Bristol-Myers-Squibb (Princeton, NJ, USA). Verapamil, glutamine, glycine, polysorbate 80, β-cyclodextrin (CD), and 2-hydroxylpropyl-β-cyclodextrin (HPCD) were obtained from Sigma–Aldrich (St. Louis, MO, USA) and ethylenediaminetetraacetic acid disodium salt (EDTA) and PEG 2000 (PEG2000) were provided by Fluka (Buchs, Switzerland). Phosphate-buffered saline (PBS) was purchased from Medicago AB (Uppsala, Sweden). Acetone, ethanol, and acetonitrile were obtained from Merck (Darmstadt, Germany). Deionized water (18.2 MΩ resistivity) was prepared by a water purification system (Wasserlab, Pamplona, Spain). All reagents and chemicals used were of analytical grade.
Preparation of PTX–Cyclodextrin Complexes
The preparation of inclusion complexes of PTX and cyclodextrins (HPCD or CD) was performed, by an evaporation method, in the molar ratio 1:1 as described previously.23, 27 Ten milligrams PTX was dissolved in 2 mL ethanol and then added to 8 mL water containing the cyclodextrin. After magnetic agitation of the mixture for 72 h, ethanol was evaporated under reduced pressure (Büchi R-144, Postfach, Switzerland) and the resulting suspensions filtered through a 0.45 μm membrane filter. Finally, the obtained filtrate was evaporated under reduced pressure at a temperature of 50°C to obtain a solid dry residue.
Preparation of Nanoparticles
Paclitaxel–cyclodextrin inclusion complexes (PTX–HPCD or PTX–CD) were encapsulated in poly(anhydride) nanoparticles by a solvent displacement method as described previously.27 Pegylated nanoparticles with PEG2000 were prepared following the method published by Zabaleta et al.31 with minor modifications.
Preparation of Poly(Anhydride) Nanoparticles Loaded with PTX–Cyclodextrin Complexes
In brief, the inclusion complex of PTX with either HPCD or CD was dispersed in a solution of PMV/MA polymer in acetone. The mixture was magnetically stirred for 30 min at room temperature. Then, nanoparticles were formed by the addition of an ethanol/water mixture (1:1, v/v). The resulting suspensions were purified by centrifugation 27,000g, 20 min. The supernatants were removed and the pellets resuspended in water. Finally, the formulations were frozen and freeze-dried (Genesis 12EL; Virtis, Warminster, PA, USA) using sucrose (5%, w/w) as cryoprotector.
The developed formulations were named as follows: PTX–HPCD–NP (nanoparticles containing PTX–HPCD inclusion complex) and PTX–CD–NP (nanoparticles containing PTX–CD inclusion complex).
Preparation of Pegylated Nanoparticles Loaded with PTX–CD (PTX–CD–NP–PEG)
In this case, PTX (as inclusion complex with CD) was dispersed in a solution of acetone containing the poly(anhydride) polymer and 12.5 mg PEG2000. The mixture was magnetically stirred for 30 min at room temperature. Afterward, the nanoparticles were formed by the addition of a mixture of ethanol and water (1:1 by vol.). The resulting suspension was purified by centrifugation and finally, freeze-dried using sucrose (5%) as cryoprotector.
Preparation of Pegylated Nanoparticles Loaded with PTX (PTX–NP–PEG)
Briefly, PTX was incubated with Gantrez® AN and PEG2000 under magnetic stirring for 30 min in acetone. Then, nanoparticles were formed by the addition of a mixture of ethanol and water (1:1 by volume). The organic solvents were eliminated by evaporation under reduced pressure and the nanoparticle suspensions purified in Vivaspin tubes (300,000 molecular weight cutoff; Sartorius Group, Goettingen, Germany) at 3000g for 20 min. The purification step was repeated twice. Finally, the pellets were resuspended in water and the formulations were frozen and freeze-dried using sucrose (5%) as cryoprotector.
Characterization of the Different Nanoparticles
Physicochemical Characterization
The mean hydrodynamic diameter of the nanoparticles and the zeta potential were determined by photon correlation spectroscopy (PCS) and electrophoretic laser Doppler anemometry, respectively, using a Zetamaster analyzer system (Malvern Instruments Ltd., Worcestershire, UK). The diameter of the nanoparticles was determined after dispersion in ultrapure water (1:10) and measured at 25°C by dynamic light scattering angle of 90°C. The zeta potential was determined as follows: 200 μL of the samples was diluted in 2 mL of KCl solution (0.1 mM). The morphology of the nanoparticles was assessed by scanning electron microscopy (SEM) and cryo-electron microscopy (cryo-EM) techniques. The yield of the production of nanoparticles was calculated by gravimetry as published previously.28
PTX Content in Nanoparticles
The amount of PTX loaded into nanoparticles was quantified by HPLC–UV.23 Briefly, the equipment was an Agilent model 1100 series LC and a diode-array detector set at 228 nm. The chromatographic system was equipped with a reversed-phase 150 × 3 mm2 C18 Phenomenex Gemini column (particle size 5 μm). The mobile phase, eluted at 0.5 mL/min, was a mixture of phosphate buffer (0.01 M, pH 2) and acetonitrile (50:50, v/v). The column was placed at 30°C and the injection volume was 100 μL. DCX was used as internal standard. Calibration curves were designed over the range of 80–7000 ng/mL (r2 > 0.999). The limit of quantification was calculated to be 80 ng/mL.
For analysis, nanoparticles were solubilized with acetonitrile (1:5, v/v). Samples were transferred into auto-sampler vials, capped and placed in the HPLC auto-sampler. Each sample was assayed in triplicate and results were expressed as the amount of PTX (μg) per mg nanoparticles.
In Vitro Release Study
Release experiments were conducted under sink conditions at 37°C using simulated gastric fluid (SGF; pH 1.2; pepsin 0.32%, w/v) and simulated intestinal fluid (SIF; pH 6.8; pancreatin 1%, w/v) containing 1% of polysorbate 80 (Tween 80) as solubilizing agent for PTX. The studies were performed under agitation in a Vortemp 56TM Shaking Incubator (Labnet International Inc., Edison, NJ, USA) after the dispersion of the nanoparticles in the appropriate medium.
For each time point, 18 μg of PTX formulated in nanoparticles was resuspended in 1 mL of the corresponding simulated fluid. The different formulations were kept in the SGF for 2 h and then, for 20 h in SIF. At different time points, sample tubes were collected and centrifuged at 27,000g for 20 min. The amount of PTX released from the formulations was quantified by HPLC from the supernatants (calibration curves of free PTX in supernatants obtained from SGF and SIF, r2 > 0.999).
In Vitro Studies in Ussing Chambers
Studies were carried out after approval by the responsible Ethical Committee of the University of Paris-Sud (agreement number A092–019–01) in strict accordance with the European legislation on animal experiments.
Permeation Assays
Ussing Chambers (Warner Instruments, Harvard Apparatus, Hamden, CT, USA) were used to determine the permeability of fresh rat intestinal tissue to PTX formulated in nanoparticles or commercial Taxol®. The methodology and terminology used was previously described.33 Jejunum portions from fresh small intestine of sacrificed male Wistar rats (200–250 g; Charles River, Chatillon-sur-Chalaronne, France) were excised, rinsed with chilled NaCl 0.9%, and cut into segments of 2–3 cm length, discarding sections containing Peyer's patches. The selected portions were cut along the mesenteric border and mounted in Ussing chambers (apparent intestinal surface of 1 cm2) while bathed with PBS at pH 7.4 containing glutamine 0.2 M.
Each compartment (donor and receptor) was filled with 4 mL PBS, maintained at 37°C and continuously oxygenated with O2/CO2 (95/5%). In the receptor compartment, 0.1% (w/v) HPCD was also included as solubilizing agent for PTX. A 30-min equilibration time was allowed to achieve steady-state electrophysiological conditions. After equilibration, 2 mg PTX loaded in the different nanoparticle formulations or as Taxol® were diluted in 300 μL PBS and added to the donor compartment. Four tissue portions of four different animals were assessed for each formulation and experiments were repeated on different days.
Aliquots of 200 μL were withdrawn from the receptor chambers every 30 min up to 2 h and the volume was immediately replaced by fresh medium pre-equilibrated at the experimental temperature (37°C). In addition, at the beginning and end of the experiments, samples (200 μL) were also obtained from the donor compartments, in order to monitor any changes in this compartment during the experiment and to safeguard mass balance. All samples were immediately frozen and stored at −20°C until analysis. PTX amount was quantified by HPLC–UV as described above.
The cumulative amounts of drug permeated in the mucosal-to-serosal (M–S; absorptive transport, or apical to basolateral) and serosal-to-mucosal (S–M, excretory transport or basolateral to apical) directions were calculated. In addition, the influence of the presence of verapamil (selective Pgp inhibitor) was evaluated by adding 0.2 mM of the inhibitor to the PBS solution in the donor chamber.
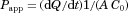 (1)
(1)In order to standardize calculations, the Papp values were estimated between 30 and 90 min after the addition of the formulation in study.
 (2)
(2)Measurement of Electrical Parameters
During all experiments, electrical parameters were recorded to determine tissue viability. Transmucosal potential difference (PD) was continuously recorded between two KCl saturated agar bridges connected to an MDVC-2C voltage clamp (Titis Bussines Corporation, Paris, France) via calomel electrodes filled with saturated KCl solution. Potential difference was short-circuited through the experiment by a short-circuit current (Isc) via agar bridges placed in each half-cell and adapted to platinum electrodes connected to an automatic MDVC-2C voltage clamp.
After 30-min equilibration, only tissues presenting PD > 2 × 10−3 V were maintained for study. As a further test of viability of the tissues, bumetamide DMSO stock solution (0.01 M) was added in the serosal compartment at the end of the experiment. Bumetamide is a specific inhibitor of the [Na+K+2Cl−] co-transporter and it decreases the secretion by cells and therefore, Isc also decreases.34 If no such decrease was observed in Isc, damages in tissues could be suspected and results from these samples were discarded.
In Vivo Pharmacokinetic Studies in C57BL/6J Mice
Pharmacokinetic Studies
Pharmacokinetic studies were performed in C57BL/6J mice (20–22 g) obtained from Harlan (Barcelona, Spain). Studies were approved by the Ethical Committee for Animal Experimentation of the University of Navarra (protocol number 147–11) in accordance with the European legislation on animal experiments.
Before the experiment, animals were adaptively fed for 1 week with free access to food and drinking water (22 ± 2°C; 12-h light and 12-h dark cycles; 50%–60% relative humidity). Previous to the oral administration of the formulations, animals were fasted overnight to avoid interference with the absorption, allowing free access to water.
For the pharmacokinetic study, mice were randomly divided into six groups. The experimental groups were: (a) PTX–HPCD–NP, (b) PTX–CD–NP, (c) PTX–NP–PEG, and (d) PTX–CD–NP–PEG. As controls, a group received the commercial formulation, Taxol®, i.v. and another group was treated with Taxol® orally. Each animal received the equivalent amount of PTX to a dose of 10 mg/kg body weight either orally with a blunt needle via the esophagus into the stomach or i.v. via tail vein as a slow infusion.
At established times after administration (0 min, 10 min, 30 min, 1 h, 1.5 h, 3 h, 6 h, 8 h, 24 h, 48 h, and 72 h); blood was obtained from three animals in each group. EDTA was used as anticoagulant agent. Blood volume was recovered intraperitoneally with an equal volume of normal saline solution pre-heated at body temperature. Samples were immediately placed on ice and centrifuged at 2500g for 10 min. Plasma was separated into clean tubes and kept frozen at −20°C until HPLC analysis.
Determination of PTX Plasma Concentration by HPLC–UV
The amount of PTX was determined in plasma by HPLC–UV.23 Calibration curves were used for the conversion of the PTX/DCX chromatographic area to the concentration. Calibrator and quality control samples were prepared by adding appropriate volumes of standard PTX in ethanol solution to drug free plasma. Calibration curves were designed over the range 80–3200 ng/mL (r2 > 0.999). An aliquot (100 μL) of plasma was mixed with 25 μL of internal standard solution (DCX, 4 μg/mL in ethanol, previously evaporated). After vortex mixing, liquid–liquid extraction was accomplished by adding 4 mL of tert-buthylmethylether following vortex gentle agitation (1 min). The mixture was centrifuged for 10 min at 4500g, and then, the organic layer was transferred to a clean tube and evaporated until dry (Savant, Barcelona, Spain). Finally, the residue was dissolved in 125 μL of reconstitution solution (acetonitrile–phosphate buffer 0.01 M pH 2; 50:50, v/v) and transferred to auto-sampler vials, capped and placed in the HPLC auto-sampler. A hundred microlitre-aliquot of each sample was injected onto the HPLC column. The limit of quantification was calculated to be 80 ng/mL. Accuracy values during the same day (intraday assay) at low, medium and high concentrations of PTX were always within the acceptable limits (less than 5%).
Pharmacokinetic Data Analysis
The pharmacokinetic analysis of plasma concentration plotted against time data, obtained after administration of the different PTX formulations, was performed according to a non-compartmental model with the WinNonlin 5.2 software (Pharsight Corporation, Princeton, NJ, USA). The following parameters were estimated: maximal plasma concentration (Cmax), time in which Cmax is reached (Tmax), area under the concentration–time curve from time 0 to ∞ (AUC), mean residence time (MRT), clearance (Cl), volume of distribution (V), and half-life in the terminal phase (t1/2).
 (3)
(3)Statistical Analysis
Data are expressed as the mean ± SD of at least three experiments. The non-parametric Kruskall–Wallis followed by Mann–Whitney U-test was used to investigate statistical differences. In all cases, p < 0.05 was considered to be statistically significant. All data processing was performed using GraphPad Prism 4.0 statistical software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Preparation and Characterization of Poly(Anhydride) Nanoparticles
Table 1 summarizes the main physicochemical properties of the different poly(anhydride) nanoparticles containing PTX. When nanoparticles were loaded with the PTX–cyclodextrin complexes, the mean size of the resulting carriers was significantly higher than when PTX was formulated with PEG2000 (p < 0.05). Herein, pegylation of nanoparticles significantly decreased the mean size of the resulting nanoparticles. Thus, PTX–CD–NP–PEG displayed a mean diameter about 25% lower than PTX–CD–NP (Table 1). Concerning the zeta potential of nanoparticles, all formulations were formed by nanoparticles with negative surface charge. However, again, when nanoparticles were pegylated with PEG2000, the zeta potential appeared to be slightly more negative, around −55 mV. For the formulation with just the PTX–CD complexes, the surface charge was −48 mV. On the other hand, the yield of the production of nanoparticles was calculated to be between 60% and 75% for the PEG and cyclodextrin containing formulations, respectively.
| Formulations | Size (nm) | Zeta Potential (mV) | PDi | PTX Loading (μg PTX/mg NP) | Yield (%) |
|---|---|---|---|---|---|
| PTX–HPCD–NP | 298 ± 9 | −47 ± 1 | 0.215 | 151 ± 5 | 65 ± 5 |
| PTX–CD–NP | 265 ± 9 | −48 ± 4 | 0.210 | 46 ± 5*** | 76 ± 8 |
| PTX–CD–NP–PEG | 215 ± 4 | −56 ± 2 | 0.181 | 65 ± 6* | 62 ± 5 |
| PTX–NP–PEG | 190 ± 5 | −55 ± 2 | 0.154 | 110 ± 4 | 60 ± 4 |
- Data expressed as mean ± SD (n = 4).
- PTX–HPCD–NP, paclitaxel complexed with 2-hydroxylpropyl-β-cyclodextrin and loaded in poly(anhydride) nanoparticles; PTX–CD–NP: paclitaxel complexed with β-cyclodextrin and loaded in poly(anhydride)nanoparticles; PTX–NP–PEG: paclitaxel loaded in poly(anhydride) nanoparticles combined with PEG2000; PTX–CD–NP–PEG, paclitaxel complexed with β-cyclodextrin and loaded in poly(anhydride)nanoparticles combined with PEG2000.
- *p < 0.05: PTX–CD–NP–PEG versus PTX–HPCD–NP.
- ***p < 0.001: PTX–CD–NP versus PTX–HPCD–NP.
Regarding the drug loading, the amount of PTX encapsulated in the nanoparticles varied according to the excipient used. On one hand, looking at the amount of drug encapsulated, when PTX was complexed with HPCD, the drug loading (150 μg/mg nanoparticles) was approximately three times higher than when PTX complexed with CD was loaded in nanoparticles (46 μg/mg nanoparticles). On the other hand, when PEG was incorporated to the formulation, the amount of PTX encapsulated was around 110 μg/mg NP. In contrast, when PTX was complexed with CD and loaded in pegylated nanoparticles, there was around a 40% increase in the drug loading, from 46 μg/mg nanoparticles to 65 μg/mg nanoparticles, compared with PTX–CD–NP.
Figure 1 shows the microphotographs obtained by SEM and cryo-EM of the nanoparticles. Figure 1a corresponds to SEM image of the PTX–HPCD–NP, Figure 1b corresponds to cryo-EM image of PTX–CD–NP and Figures 1c and 1d belong to SEM images of PTX–CD–NP–PEG and of PTX–NP–PEG, respectively. Nanoparticles displayed spherical shapes and, in all cases, their apparent sizes were similar to the obtained values by PCS. It is noteworthy that formulations containing cyclodextrins (Figs. 1a–1c) presented more irregular and rough surfaces, whereas the nanoparticles formulated with PEG (Figs. 1c and 1d) showed more diffuse surfaces in the photographs.
In Vitro Release Study
Paclitaxel release kinetics from nanoparticles was evaluated in two different media: SGF and SIF fluids with polysorbate 80 as solubilizing agent for PTX. Figure 2 represents the release profiles of PTX from the different assayed formulations as cumulative percentage of drug released as a function of time.
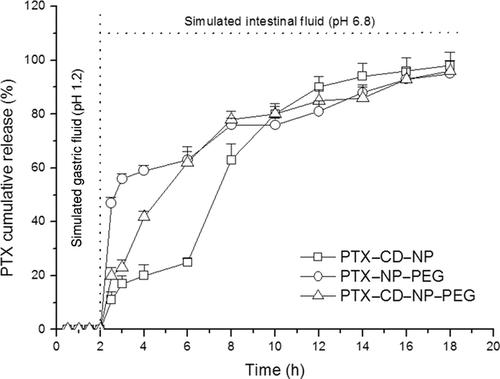
For all the evaluated formulations, there was no release of the drug when nanoparticles were dispersed in SGF. In contrast, when nanoparticles were dispersed in the SIF, the release behavior of PTX from the poly(anhydride) nanoparticles exhibited a biphasic pattern. There was an initial rapid drug discharge for PTX–NP–PEG (60% released) followed by a more sustained release up to 18 h. For the formulations containing cyclodextrins (PTX–CD–NP and PTX–CD–NP–PEG), there was a first slow release of the drug maintained for 6 h. After 6 h, there was a higher release rate prolonged for the next 10–12 h.
Focusing on the time to achieve 50% release of PTX from the nanoparticles, differences were observed. For pegylated nanoparticles (PTX–NP–PEG), 30 min of incubation in SIF appeared to be enough to promote the release of 50% of the loaded drug. However, when the nanoparticles contained CD, this time lapse appeared to be longer. Thus, for the PTX–cyclodextrin complexes loaded in the nanoparticles, the time to achieve the 50% release was around 5 h of incubation in the intestinal simulated fluid. For the combined strategy incorporating CD and PEG in the nanoparticles (PTX–CD–NP–PEG), the release rate appeared to increase because just 2 h of incubation in the SIF was necessary to release 50% of the loaded drug. Interestingly, at 18–20 h, PTX was completely released to the medium in all the assayed formulations.
In Vitro Ussing Chambers
The transport and permeability of PTX when loaded in nanoparticles, through the intestinal mucosa of rats was investigated in vitro by the Ussing chamber technique.
Figure 3 represents the percentage of the dose of PTX absorbed through the jejunum portions in the Ussing chambers plotted against time for the absorptive direction (M–S). When the experiments were carried out with the nanoparticle formulations, there was a prolonged increase of the amount of PTX absorbed through the intestine for the first 30 min. In contrast, after 90 min a slower absorption profile was observed, becoming rather stable. At the end of the study, the amount of PTX in the receptor chamber for the nanoparticle formulations was five to eight times higher than for the commercial Taxol®. Thus, significantly higher levels of PTX present in the receptor chamber were observed for pegylated containing formulations than for PTX–CD–NP (p < 0.05).
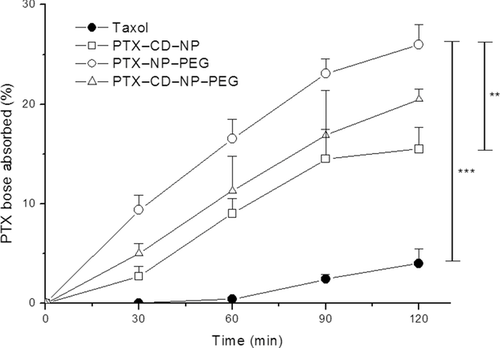
In M–S direction, for Taxol®, the apparent permeability coefficient (Papp) was very low (1.77 × 10−6 cm/s). However, when PTX was encapsulated in nanoparticles, the intestinal permeability increased almost sevenfold, ninefold, and 12-fold for PTX–CD–NP, PTX–CD–NP–PEG, and PTX–NP–PEG, respectively. Comparing between nanoparticles, the highest permeability values were obtained for the PEG2000 containing formulation (PTX–NP–PEG) (Papp = 20.6 × 10−6 cm/s). For the two formulations containing the PTX–CD complexes, there were different values of permeability (Papp PTX–CD–NP = 11.4 × 10−6 cm/s versus Papp PTX–CD–NP–PEG = 15.1 × 10−6 cm/s) (p < 0.05).
The absorption enhancement ratios (R) are summarized in Figure 4 where the variation in absorption for the different formulations is plotted after a 2-h experiment in the Ussing chambers for all the experimental conditions evaluated.
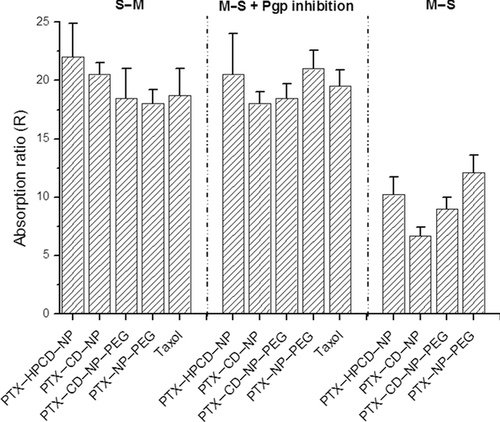
When the apparent intestinal permeability was evaluated on the absorptive direction (M–S), differences were observed between the poly(anhydride) nanoparticle formulations. PTX–HPCD–NP presented a higher absorption ratio than PTX–CD–NP, around 1.5 times higher. Interestingly, when the PTX–CD complex was encapsulated in pegylated nanoparticles (PTX–CD–NP–PEG), a rise in the absorption ratio was evidenced, from an R of 6.7–9. On the other hand, PTX–NP–PEG presented the highest value of absorption ratio (R = 12.1). In addition, statistical differences were found between PTX–CD–NP formulation and the rest of poly(anhydride) nanoparticles (PTX–HPCD–NP, PTX–CD–NP–PEG, and PTX–PEG–NP) (p < 0.01).
In contrast, when the permeability was evaluated in the excretory direction (S–M), no statistical differences were observed between the commercial Taxol® and the poly(anhydride) nanoparticles with similar values of absorption ratio (R ≈ 18–20). On the other hand, when the intestinal permeability of PTX was assessed in the presence of verapamil in the medium, the values of Papp increased compared with the mucosal-to-serosal transport. In these circumstances, the absorption ratio for commercial Taxol® and for the different formulations rose to 20 approximately, in all cases (Fig. 4) with no statistical differences.
Pharmacokinetic Studies in C57BL/6J Mice
When Taxol® was administered as a single i.v. injection (dose 10 mg/kg) to laboratory animals, the PTX plasma concentration decreased rapidly in a biphasic way and the data were adjusted to a non-compartmental model (data not shown). The peak plasma concentration (Cmax) of PTX was about 65 μg/mL. The values obtained for AUC and half-life (t1/2) were 71 μg h/mL and 3.1 h, respectively. The MRT was 2.22 h (Table 2).
| Route | AUC (μg h/mL) | Cmax (μg/mL) | Tmax (h) | t½z (h) | Cl (L/h) | V (L) | MRT (h) | Fr (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Taxol® | i.v. | 71.1 ± 2.1 | 64.7 ± 1 | 0.03 | 3.1 ± 0.3 | 2.7 ± 0.8 | 6.9 ± 1 | 2.2 ± 0.2 | 100 |
| Taxol® | p.o. | ND | ND | ND | ND | ND | ND | ND | ND |
| PTX–HPCD–NP | p.o. | 51.1 ± 1.7 | 3.1 ± 1 | 1.0 | 10.1 ± 1 | 0.7 ± 0.2 | 8.1 ± 0.3 | 21 ± 0.3 | 72 |
| PTX–CD–NP | p.o. | 43.7 ± 2.1 | 2.4 ± 1 | 1.0 | 9.7 ± 0.3 | 0.5 ± 0.4 | 8.9 ± 0.3 | 22 ± 1.2 | 61 |
| PTX–CD–NP–PEG | p.o. | 49.4 ± 2.8 | 3.1 ± 0.7 | 1.5 | 8.5 ± 1.1 | 0.4 ± 0.2 | 7.3 ± 0.4 | 21 ±0.5 | 68 |
| PTX–NP–PEG | p.o. | 55.9 ± 3.1 | 4.0 ± 0.9 | 1.5 | 10.3 ± 0.5 | 0.5 ± 0.2 | 8.5 ± 0.1 | 26 ± 0.5 | 79 |
- ND, not determined, below limit of quantification.
- PTX–HPCD–NP, paclitaxel complexed with 2-hydroxylpropyl-β-cyclodextrin and loaded in poly(anhydride) nanoparticles; PTX–CD–NP, paclitaxel complexed with β-cyclodextrin and loaded in poly(anhydride)nanoparticles; PTX–NP–PEG, paclitaxel loaded in poly(anhydride) nanoparticles combined with PEG2000; PTX–CD–NP–PEG, paclitaxel complexed with β-cyclodextrin and loaded in poly(anhydride)nanoparticles combined with PEG2000; i.v., intravenous; p.o., per oral; AUC, area under the concentration–time curve from time 0 to ∞; Cmax, peak plasma concentration; Tmax, time to peak plasma concentration; t1/2z, half-life of the terminal phase; Cl, clearance; V, volume of distribution; MRT, mean residence time; Fr, relative oral bioavailability.
Figure 5 shows the plasma concentration versus time profile after oral administration of PTX (single dose of 10 mg/kg) included in the different nanoparticle formulations. Although orally administered Taxol® showed no detectable plasma levels in mice, the poly(anhydride) formulations offered sustained plasma curves characterized by increasing amounts of PTX for the first 1–2 h (Tmax) reaching the maximum concentration (Cmax) followed by another phase of slow and prolonged decline of the plasma levels until at least 24 h.
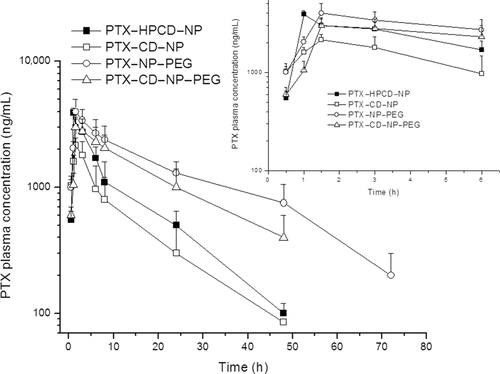
Comparing the different nanoparticle formulations, the PTX plasma levels reached with either PTX–NP–PEG or PTX–HPCD–NP or PTX–CD–NP–PEG were found to be between 1.5-fold and twofold higher than for PTX–CD–NP. In fact, PTX–NP–PEG displayed the highest plasma levels and, more importantly, remained within the detection range for at least 72 h postadministration. On the other hand, both PTX–HPCD–NP and PTX–CD–NP displayed PTX plasma levels for at least 24 h, whereas the pegylation of nanoparticles loaded with PTX–CD complexes offered plasma levels for at least 48 h. In addition, for this formulation (PTX–CD–NP–PEG), the second phase of the plasma curve followed a similar trend than the one obtained for the PTX–NP–PEG (see Fig. 5).
Table 2 summarizes the pharmacokinetic parameters estimated for a non-compartmental analysis of the experimental data obtained after the administration of the different PTX formulations to mice. The AUC of PTX in nanoparticles varied depending on the formulation. Thus, for PTX–HPCD–NP and PTX–NP–PEG, AUC was around 52–56 μg h/mL. For the nanoparticles containing just the PTX–CD complexes (PTX–CD–NP), AUC was around 44 μg h/mL. In contrast, when PEG was added to this formulation (PTX–CD–NP–PEG), the AUC increased (around 49 μg h/mL). Within the different nanoparticle formulations, the rank order of the mean AUC of plasma drug concentration versus time was as follows: PTX–NP–PEG > PTX–HPCD–NP = PTX–CD–NP–PEG > PTX–CD–NP (p < 0.05). In addition, the AUC values of poly(anhydride) nanoparticles were always significantly higher than those of orally administered Taxol® as no detectable levels were obtained. The peak plasma concentration (Cmax) of PTX in the poly(anhydride) nanoparticles was between 2.4 and 4 μg/mL. These maximum plasma concentrations for the poly(anhydride) nanoparticles were around 16–35 times lower than that of the i.v. Taxol®.
In addition, MRT of the drug in plasma and the t1/2 were found around 10 times higher when PTX was administered in the nanoparticle formulations by the oral route than when i.v. administered as Taxol®.
When Taxol® was i.v. administered, the Cl of the drug was 2.7 L/h and the V was 7 L, approximately. Interestingly, the Cl values of the anticancer drug when loaded in nanoparticles were always relatively lower than for the commercial Taxol®.
Finally, the relative oral bioavailability of PTX when incorporated to poly(anhydride) nanoparticles varied from 60% to 80% depending on the formulation. The highest relative oral bioavailability of the drug was obtained by the pegylated nanoparticles PTX–NP–PEG (79%), followed by the PTX–HPCD–NP (72%), PTX–CD–NP–PEG (68%), and finally, the lowest achieved by PTX–CD–NP (61%).
DISCUSSION
The aim of this work was to explore the potential of combining PTX cyclodextrin complexes and PEG with poly(anhydride) nanoparticles and, thus, to determine if the incorporation of PTX as inclusion complex with CD in pegylated nanoparticles would also modify the intestinal permeability and oral bioavailability of this anticancer drug in C57BL/6J mice.
Overall, when PTX was encapsulated in the form of inclusion complexes with cyclodextrins, the resulting nanoparticles displayed a mean size higher than those in which PEG was used (Table 1). In this way, it was clear that the presence of PEG reduced the mean size of the resulting carriers. Concerning the drug loading, the nature of the cyclodextrin selected to prepare the inclusion complex with PTX clearly influenced this parameter. Thus, the use of HPCD increased three times the PTX loading compared with CD. In both cases, the amount of drug associated with the resulting nanoparticles was found similar to values previously reported.23 For pegylated nanoparticles, the drug loading was high (about 11% by weight) but lower than the data previously reported.31 This difference could be related to a lower incubation time between the drug, PEG and the poly(anhydride) before the formation of nanoparticles (30 min vs. 1 h). Encouragingly, when nanoparticles were prepared with PTX–CD complexes in the presence of PEG2000 (PTX–CD–NP–PEG), the amount of drug loaded in the nanoparticles increased from 45 μg/mg in the absence of PEG, to 65 μg/mg nanoparticles with PEG.
The release profile of PTX from the different poly(anhydride) nanoparticles was clearly dependent on the pH conditions. Under acid pH conditions, no release of drug was observed. This fact can be explained by the low tendency of the poly(anhydride) polymer to undergo hydrolysis in an acid medium (SGF)35 preventing the erosion of the nanoparticles. On the contrary, when the experiment was carried out in simulated intestinal fluid (pH 6.8), the release of the drug occurred and, surprisingly, it was found to be dependent on the composition of the nanoparticles. In fact, under these conditions, the hydrolysis of anhydride groups would yield two carboxylic acids groups, which would cause the swelling of the nanoparticles. In fact, pegylation of nanoparticles induced a much more rapid release of PTX from nanoparticles in simulated intestinal fluid than non-pegylated ones. These results agree well with the observation reported by Yu et al.36 who demonstrated that pegylation of poly(anhydride) microparticles (0.8–10 μm) accelerated the release rate of bovine serum albumin. It is also noteworthy that, for PTX–NP–PEG, after a quite rapid initial release of 50%–60% of the loaded drug, the release profile of the remained drug followed a similar trend to that observed for PTX–CD–NP.
Concerning the permeability studies of PTX in Ussing chambers, the asymmetric permeation found for free PTX (Taxol®) when both absorptive and excretory directions were assessed with an absorption enhancement ratio (S–M) (calculated by Eq. 2) of about 20, confirmed the presence of an active mechanism for which PTX is a good substrate.37, 38 Similarly, when verapamil, a selective Pgp inhibitor, was added to the donor chamber, the amount of PTX absorbed through the intestinal membrane increased in all cases, with no differences between the commercial Taxol® and the poly(anyhydride) nanoparticles (Papp values around 32 × 10−6 cm/s). These results are in agreement with previous studies with pegylated nanoparticles31 or nanoparticles loaded with PTX–HPCD inclusion complex.27
In our work, when PTX was included in the different nanoparticles, the permeability and the absorption ratios of the anticancer agent increased significantly compared to the control (Taxol®), confirming a significant decrease in the efflux transport of PTX which can be considered as a clear evidence of the inhibition of the active pump by both cyclodextrins and PEG. However, this effect seemed to be more intense when nanoparticle formulations were prepared in the presence of PEG. This fact again can be directly related to the ability of PEG to yield nanoparticles with a more slippery surface that could permit an easier penetration through the mucus layer of the gut than nanoparticles containing cyclodextrins. Another interesting thing to highlight would be the good correlation between the in vitro release profiles of PTX from nanoparticles in SIF and the Papp of the anticancer drug obtained by the Ussing chamber technique, confirming the inhibitory effect on Pgp and cythocrome P450 for all the nanoparticle formulations tested.
For the in vivo studies in C57BL/6J mice, a single-dose of 10 mg/kg was selected. When Taxol® was administered i.v., the profile of the curve was biphasic and similar to that published previously.11 In contrast, after the oral administration of Taxol®, no drug plasma levels could be estimated as they were not detectable. So, the oral bioavailability of Taxol® could not be estimated. In any case, these values have been previously reported to be between 2% and 10.5%.12, 39
When PTX was loaded in nanoparticles and orally administered to C57BL/6J mice, the plasma levels of the drug were high and prolonged in time (Fig. 5). However, some differences in the profile of the curves were found. Thus, pegylated nanoparticles demonstrated the longest plasma levels in mice as previously described in rat,31 reaching 72 h postadministration with the highest Cmax. Nanoparticles loaded with either PTX–HPCD or PTX–CD induced PTX plasma levels for at least 24 h, whereas when the PTX–CD inclusion complex was loaded in nanoparticles in the presence of PEG, therapeutic plasma levels were maintained for at least 48 h (Fig. 5). In any case, these prolonged plasma levels can also be explained by an increased residence time of the pharmaceutical dosage form (nanoparticles) in close contact with the absorption site. As a consequence, in all cases, the calculated relative oral bioavailability of PTX when formulated in the nanoparticles was high and up to 60%–80%.
For all the formulations tested (Taxol® and nanoparticles) the V was calculated to be around 7–8 L. These values would be an indirect proof that PTX was absorbed in its molecular form (and not associated with the nanoparticles) leading then, to an unchanged PTX distribution in the body of the animal, which could have been different in the hypothesis of an absorption of PTX-loaded nanoparticles. In addition, these values are much higher than the total body water in mice (around 14 g or 69% of body weight40), suggesting that the drug is extensively distributed to tissues. In contrast, the Cl of PTX when administered i.v. (Taxol®) was higher (2.7 L/h) than when formulated in nanoparticles and orally administered (around 0.4–0.7 L/h). This noticeable lower Cl following the oral administration could be due to the time-controlled release of the drug from the delivery system and as a consequence, to a delayed appearance of PTX in plasma and at lower concentrations compared to the i.v. administration of a PTX solution.
Taken all together, a certain degree of correlation between the permeability parameters obtained from Ussing chambers experiments in rats and the AUC values obtained from the plasma curves after the oral delivery of nanoparticles formulations from mice appears to exist. As observed, for the formulation leading to the lowest apparent permeability (PTX–CD–NP), the AUC was the smallest and consequently, the relative oral bioavailability Fr (61%). In contrast, when the permeability of the drug through the intestinal membrane of the rats increased, such rise was also obtained for the AUC and subsequently for the bioavailable fraction Fr. In any case, further work may be required to confirm this plausible correlation between in vitro permeability and in vivo plasma levels.
CONCLUSIONS
The incorporation of cyclodextrins and/or PEG to poly(anhydride) nanoparticles appeared to be an adequate strategy to promote the oral bioavailability of PTX and maintain drug plasma levels for at least 24 h in C57BL/6J mice. The addition of PEG to the poly(anhydride) nanoparticles containing the PTX–CD increased the drug loading of the anticancer agent and favored its absorption at the intestinal level as evidenced by an increase in the absorption ratio (R) in the Ussing chambers technique. In addition, significant plasma levels of the drug were found after the oral administration of the different poly(anhydride) nanoparticles. These results were especially remarkable in the cases of PTX–CD–NP–PEG and PTX–NP–PEG which provided a relative oral bioavailability of PTX in mice of about 68% and 79%, respectively.
ACKNOWLEDGMENTS
This work was supported by a collaborative French-Spanish project (PICASSO grant, number HF2007–0061), by CAN project “Nanotecnología y medicamentos” (ref 10828) and by the (project SAF2008–02538) from the Spanish Ministry of Science and Innovation. P.C. acknowledges Instituto de Salud Carlos III (PFIS fellowship FI08/00527) Ministry of Science and Innovation in Spain for the financial support.




