Efficient and High-Speed Transduction of an Antibody into Living Cells Using a Multifunctional Nanocarrier System to Control Intracellular Trafficking
Abstract
The transduction of antibodies into living cells would represent a major contribution to both basic and applied biomedical fields, as currently available methods suffer from limitations such as low-uptake efficiency and endosomal entrapment. In this study, a liposome-based carrier was designed to overcome these issues. Liposomes were modified with octaarginine (R8), a cell penetrating peptide and GALA, a pH-sensitive fusogenic peptide. The presence of R8 enhanced the cellular uptake of antibodies, whereas GALA reduced endosomal entrapment, resulting in antibodies being released into the cytosol within 30 min. Moreover, compared with commercially available reagents for delivering antibodies, our system was superior in both cellular uptake and endosomal escape. In addition, specific antibodies delivered by R8-GALA liposomes were found to be associated with their epitope, confirming the preservation of functionality. This system for the efficient and high-speed cytosolic delivery of an antibody provides a valuable tool that can be useful in basic and applied research for analyzing the expression and function of intracellular molecules. © 2014 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2845–2854, 2015
INTRODUCTION
The high specificity of antibodies explains their extensive use in both basic and applied research for analyzing the expression and function of proteins. However, their use for studying intracellular phenomena in living cells requires a delivery system that can overcome the cell membrane barrier and release functional antibodies into the cytosol. To date, several attempts to transduce antibodies into intact cells have been reported, including mechanical approaches such as electroporation1 and microinjection,2 or the use of cellular uptake mediators such as cationic liposomes,3, 4 amphiphilic peptides,5-7 polymeric carriers,8, 9 and recombinant viruses.10 Nevertheless, these strategies remain problematic, and show low-transduction efficiencies, long periods for the effective dissociation and release of the antibody from the carrier, or time-consuming preparation methods.
Octaarginine (R8), a cell-penetrating peptide that is similar to other arginine-rich peptides such as HIV-1 TAT11 and oligoarginines,12 has been shown to be translocated across the cytoplasmic membrane. In addition, these arginine-rich peptides are able to mediate the membrane translocation of macromolecules such as peptides, proteins, and oligonucleotides.13-15 In a previous study, R8 was incorporated into liposomes through a stearyl moiety that firmly anchors the cell-penetrating peptide to the liposomal surface while allowing the polyarginine component to interact with the cytoplasmic membrane.16 The inclusion of R8 on the surface of the liposomes promoted the cellular uptake of carriers. Moreover, high contents of R8 resulted in stimulation of macropinocytosis compared with clathrin-mediated endocytosis promoted by low-density R8 modification.16 So far, liposomes composed of fusogenic lipids and modified with R8 have been successfully used for the delivery of several macromolecules including pDNA,17 oligoDNA,18 siRNA,19 the green fluorescence protein,20 superoxide dismutase,21 and DNase I protein.22
On the contrary, a common problem associated with the efficient cellular delivery of macromolecules using nonviral delivery systems is that the carriers accumulate in the endosomes/lysosomes.23-25 Endosomal escape of the macromolecules is a critical step for obtaining the desired biological function. Therefore, the inclusion of an endosomal release device is useful in the design of delivery systems. GALA (WEAALAEALAEALAEHLAEALAEALEALAA) is a synthetic peptide that at neutral pH displays a random coil but under acidic conditions adopts an amphipathic α-helical structure.26 At pH values lower than 6.0, GALA has a high affinity for neutral and negatively charged membranes, inducing fusion and fragmentation of model membranes via pore formation.27, 28 Therefore, the GALA peptide has been extensively used as an endosomal disrupting peptide in a number of delivery systems.29, 30 Previously, GALA was successfully integrated to liposomes via N-terminal modification with cholesteryl moiety.31 The inclusion of GALA in the surface of liposomes promoted the destabilization of both the endosomal and liposomal membranes producing the cytosolic delivery of the encapsulated molecules. Moreover, the synergistic combination of R8 and GALA in liposomes was showed to be effective for siRNA delivery.32
In this study, we report on an alternate method for antibody delivery based on liposomes that contain functional devices that permit the major barriers to antibody delivery to be overcome. We prepared liposomes modified with R8, a cell-penetrating peptide, and GALA, a pH-sensitive fusogenic peptide. The cellular uptake of the antibody by the carrier was evaluated by flow cytometry. We also observed intracellular trafficking of the antibody using confocal laser scanning microscopy (CLSM). Finally, the R8-GALA liposomes were used to deliver specific antibodies in order to confirm the integrity of the antibodies released into the cytosol.
EXPERIMENTAL
Materials
Cholesteryl hemisuccinate (CHEMS), amiloride, and Fillipin III were purchased from Sigma (St. Louis, Missouri). 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and rhodamine–DOPE were purchased from Avanti Polar Lipids Inc. (Alabaster, Alaska). Stearyl octaarginine12, 33 and cholesteryl–GALA29, 31 were obtained from Kurabo Industries Ltd. (Osaka, Japan). Goat IgG was purchased from Rockland (Gilbertsville, Pennsylvania). HeLa human cervix carcinoma cells were obtained from RIKEN Cell Bank (Tsukuba, Japan). Dulbecco's modified Eagle medium (D'MEM) and fetal bovine serum (FBS) were purchased from Invitrogen Corporation (Carlsbad, California). All other chemicals were commercially available reagent-grade products.
Preparation of Carriers
In a typical preparation, DOPE and CHEMS were dispersed in the bottom of a glass tube, with or without cholesteryl–GALA [DOPE/CHEMS/(+/−)cholesteryl–GALA, in a molar ratio of 9/2/0.22]. The solvent was removed by vacuum treatment and the lipid film was hydrated with a solution of Goat IgG or Goat IgGAlexa488 at 0.125 mg/mL in 10 mM HEPES buffer (pH 7.4). IgGAlexa488 was prepared with an AlexaFluor®488 Protein Labeling Kit (Invitrogen Corporation). After a 10-min incubation at room temperature, the lipid film was sonicated for 2 min in a bath-type sonicator (Aiwa, Tokyo, Japan) to give Lip(IgG) or GALA-Lip(IgG) (final composition 550 μM lipid, 0.125 mg/mL IgG). Subsequent modification with R8, to give R8-Lip(IgG) or R8-GALA-Lip(IgG), involved incubating liposomes in the presence of stearyl-R8, for 30 min at room temperature (11 μM final concentration). To prepare lipoplex-style carriers (R8-Lip—IgG and R8-GALA-Lip—IgG), the lipid film was hydrated with HEPES buffer, modified with stearyl-R8 and then incubated with an antibody solution for 15 min at room temperature, in a volume ratio of empty liposomes to antibody solution of 1:1 (final composition 275 μM lipid, 0.0625 mg/mL IgG). For the observation of the lipid phase of liposomes, 1% rhodamine-DOPE was introduced in the lipid film. The average diameter and the ζ-potential of liposomes were determined through dynamic light scattering and Laser Doppler Electrophoresis (Zetasizer Nano ZS ZEN3600; Malvern Instruments, Worcestershire, UK).
Cellular Uptake Assay
HeLa cells (2 × 105 cells) were seeded in six-well plates 24 h before the experiment. Cells were incubated for 1 h with carriers [27.5 μM lipid, 6.25 μg/mL IgG (15% IgGAlexa488), D'MEM, FBS(−)], or 10, 15, 30, 45, 60, or 120 min in the experiment for cellular uptake as a function of incubation time. The cells were then washed twice with a heparin solution (20 U/mL) and trypsinized. After adding complete medium [D'MEM, FBS(+)], the cells were precipitated and the pellet suspended in phosphate-buffer saline [0.5% bovine serum albumin (BSA), 0.1% sodium azide]. The fluorescence of samples was registered in a FACScan instrument and analyzed with the CellQuest software (Becton Dickinson, Franklin Lakes, New Jersey). A total of 10,000 cells were analyzed in each sample.
To investigate the mechanism responsible for the cellular uptake of carriers, the cells were incubated with rhodamine-DOPE-labeled R8-GALA-Lip-IgG, R8-GALA liposomes and R8-liposomes. The cells were treated with inhibitors (5 mM amiloride, 0.5 M sucrose, or 1 μg/mL Fillipin III) for 30 min before adding the carriers to the cells. The relative cellular uptake when the cells were treated with inhibitors was calculated as follows:
Relative cellular uptake value (%) = UP/UA × 100 where UP and UA represent the cellular uptake of the rhodamine-DOPE-labeled carriers when cells were treated with carriers in the presence and absence of inhibitors, respectively.
CLSM Observations
HeLa cells (5 × 104 cells) were seeded in 35-mm glass-bottom dishes 24 h before the experiment. Cells were incubated in the presence of carriers [13.75 μM lipid, 3.125 μg/mL IgGAlexa488, D'MEM, FBS(−)] for 1 h. After washing with heparin (40 U/mL), complete medium [D'MEM, FBS(+)] was added and the solution incubated for 1 h. For the time course analysis of antibody delivery, the cells were incubated in the presence of carriers for 15, 30, 60, or 120 min. After washing (heparin 40 U/mL), complete medium was added and CLSM observations were performed immediately. Nuclei were stained with Hoechst 33342 (Dojindo Laboratories, Kumamoto, Japan). For the analysis of colocalization between antibodies and endosomes, acidic compartments were stained with LysoTracker Red DND-99 (Invitrogen Corporation). CLSM images were obtained using a Nikon A1 confocal imaging system (Nikon, Tokyo, Japan) equipped with the objective lens Plan Apo 60x/1.20 PFS WI and Plan Apo 20x/0.75 PFS dry, and a first dichroic mirror (405/488/561/640).
Comparison of R8-GALA Liposome with Commercially Available Reagents
Complexation of antibodies with the delivery reagents ChariotTM (Active Motif, Carlsbad, California) and Pro-JectTM (Thermo Scientific, Waltham, Massachusetts) was carried out according to manufacturer's recommendations (see Supporting Information for details). The cellular uptake of antibodies was determined as described above with HeLa cells incubated in the presence of carriers corresponding to 2 μg of IgG (40% IgGAlexa488). In the case of CLSM observations, cells were incubated with carriers corresponding to 3 μg/mL IgGAlexa488 and treated as described above.
Delivery of Specific Antibodies
HeLa cells (5 × 104 cells) were seeded in 35-mm glass-bottom dishes (precoated with gelatin) 48 ± 4 h before the experiment. Cells were incubated in the presence of free antinuclear pore complex (NPC) antibody (Mouse monoclonal anti-NPC antibody; Abcam, Cambridge, UK), R8-GALA-Lip—IgGAlexa488 or R8-GALA-Lip—anti-NPC for 1 h [13.75 μM lipid, 3.125 μg/mL antibody, D'MEM, FBS(−)]. After washing with a heparin solution (40 U/mL), incubation with complete medium [D'MEM, FBS(+)] was performed for 4 h. Cells were fixed with ice-cold methanol (7 min, −20°C) and incubated with 1% BSA (30 min, 37−C). The anti-NPC antibody was detected by incubating the cells with goat antimouse IgGAlexa488 (Invitrogen Corporation) for 1 h at 37°C. In direct immunostaining, the cells were fixed with methanol, blocked with 1% BSA, and incubated with mouse anti-NPC antibody (1 h, 37°C). Detection with goat antimouse IgGAlexa488 was then carried out. Cells were analyzed by CLSM.
RESULTS
Construction of R8-Modified Liposomes Loaded with Antibody and the Cellular Uptake Evaluation
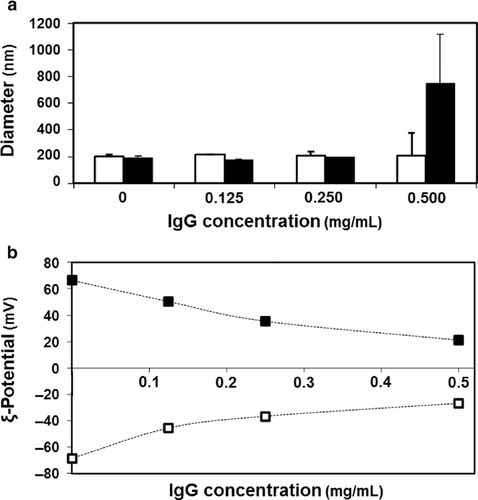
Liposomes (DOPE/CHEMS, 9/2 molar ratio) loaded with nonspecific antibodies [Lip(IgG)] were prepared by the lipid film hydration method. Stable liposomes, approximately 200 nm in diameter, were obtained using antibody solutions up to 0.5 mg/mL (Fig. 1a, open bars). The ζ-potential of the carriers was highly dependent on the antibody concentration (Fig. 1b, open symbols). The modification of Lip(IgG) with R8 resulted in an inversion in the ζ-potential from negative to positive values, because the positive moiety was anchored (Fig. 1b). The final surface potential of R8-Lip(IgG) was also dependent on the concentration of the antibody solution in the preparation of liposomes, with values close to neutrality for high concentrations (Fig. 1b, closed symbols). In this study, the antibody concentration for preparing a stable R8-Lip(IgG) was used to be 0.125 mg/mL (Table 1).
| Type of Carrier | Diameter (nm) | ζ-Potential (mV) |
|---|---|---|
| Lip(IgG) | 205 ± 19 | −51 ± 3 |
| R8-Lip(IgG) | 185 ± 10 | 49 ± 3 |
| R8-Lip—IgG | 208 ± 15 | 44 ± 8 |
- Data denote the mean ± SD (n = 4).
The cellular uptake of antibodies was evaluated by flow cytometry (Fig. 2a). As expected, no detectable IgG was internalized without a carrier (free IgG), revealing the necessity for a system that mediates antibody internalization. The use of unmodified liposomes [Lip(IgG)] did not enhance the internalization of the antibodies compared with free IgG. However, when the surface of the liposomes was modified with R8 [R8-Lip(IgG)], antibody uptake was significantly increased.
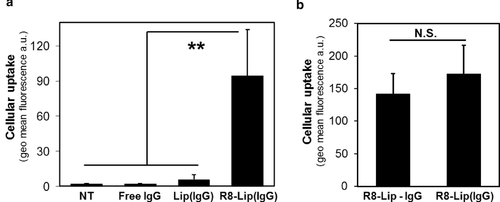
The dependence of the ζ-potential of liposomes on the concentration of the antibody solution (Fig. 1b) suggests that the antibodies interacted strongly with the surface of the liposomes. To clarify this possibility, R8-Lip(IgG) was compared with carriers prepared in a lipoplex-style (R8-Lip—IgG), defined as the result of the complexation of empty R8-liposomes (hydrated with only HEPES buffer) with an antibody solution. In lipoplex-style carriers, the antibodies can only be associated with the surface of liposomes. The size and ζ-potential of R8-Lip(IgG) and R8-Lip—IgG remained comparable (Table 1). Moreover, the uptake of antibodies mediated by the two systems was statistically equivalent (Fig. 2b). This indicates that antibodies are carried by liposomes mainly via a surface adsorption mechanism, rather than in an encapsulated form in the aqueous phase.
Intracellular Observation of R8-Lip(IgG) Using CLSM
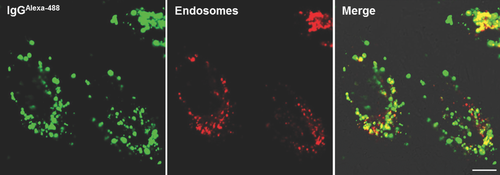
The intracellular trafficking of antibodies delivered by R8-Lip(IgG) was observed by CLSM. When HeLa cells were incubated in the presence of R8-Lip(IgG), the antibodies were effectively internalized and strong fluorescent dots were detected in the cells (left panel, Fig. 3). In contrast, only a few weak fluorescent dots were observed when the cells were incubated in the presence of Lip(IgG), and no detectable IgG was internalized without a carrier (free IgG) (Fig. S1). The cellular uptake of positive nanoparticles is often associated with endocytosis.23-25 Therefore, the possible entrapment of antibodies in endosomes was investigated. Acidic organelles were stained and examined for colocalization with the antibodies (right panel, Fig. 3). Although some free IgG was found in the cytosol, the majority of the antibodies were colocalized with endosomes.
Cytosolic Delivery of Antibody by R8 and GALA-Modified Liposomes
To overcome endosomal entrapment, liposomes were modified with an endosomal release device, GALA, a pH-sensitive fusogenic peptide.26-28 Previous study showed that the inclusion of GALA on the surface of liposomes promoted the destabilization of both the endosomal and liposomal membranes, resulting in the cytosolic delivery of the encapsulated molecules.31 The presence of GALA resulted in a decrease in the diameter (124 ± 3 nm) of R8-GALA-Lip(IgG) compared with R8-Lip(IgG) (185 ± 10 nm), whereas the ζ-potential remained unchanged (48 ± 6 mV). Importantly, a flow cytometry analysis showed that the cellular uptake of antibodies mediated by R8-GALA-Lip(IgG) did not vary significantly compared with R8-Lip(IgG) (Fig. S2).
We then evaluated the ability of the R8-GALA-Lip(IgG) to escape from endosomes and release antibodies into the cytosol. Cells incubated in the presence of R8-GALA-Lip(IgG) displayed a strong fluorescence that was widely distributed in the cytosol, as evidenced by CLSM images (Fig. 4a). Although some antibodies were still colocalized with endosomes, the high amount of antibodies was detected in the cytosol. It is noteworthy that the increased level of antibody in the cytosol can be attributed to the presence of both R8 and GALA, as liposomes containing only GALA [GALA-Lip(IgG)] did not mediate the delivery of antibodies (right panel, Fig. S1). These results suggest that R8 first interacted with the cellular membrane promoting carrier uptake, and the pH-dependent fusogenic peptide GALA then induced the release of antibodies from endosomes.
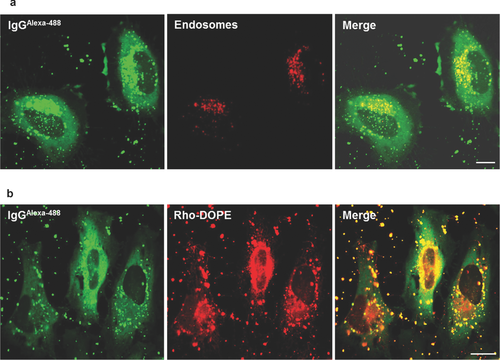
As discussed above, the antibodies tended to become adsorbed to the surface of liposomes. The above strategy permitted the effective dissociation of antibodies from liposomes after endosomal escape. The lipid phase of R8-GALA-Lip(IgG) was labeled with rhodamine-DOPE and analyzed for colocalization with antibodies (Fig. 4b). CLSM images showed some colocalization of antibodies with the lipid, as evidenced by punctuate dots, probably corresponding to carriers still entrapped inside endosomes. Intracellular observations of the lipid phase also showed some degree of colocalization of rhodamine-DOPE and endosomes (Fig. S3). However, antibodies that had diffused into the cytosol appeared to be liposome free. This suggests that antibodies are released from the liposome surface during the fusion process of liposomes and endosomes triggered by GALA. Importantly, cytotoxicity analyses showed that R8-GALA-Lip(IgG) had a negligible effect on cell viability (Fig. S4).
Time Course Analysis of Antibody Delivery by R8-GALA Liposomes
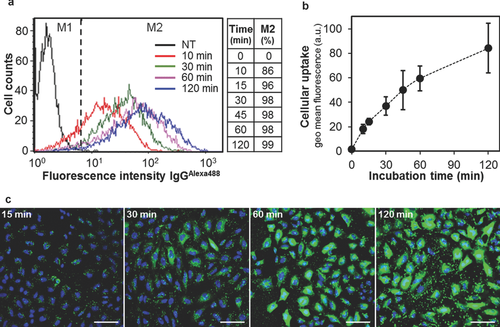
Currently used antibody delivery systems typically require long incubation times (4–24 h) for cellular uptake and the cytosolic release of the cargo.3, 8, 9 In contrast, the delivery of antibodies by R8-GALA-Lip(IgG) was both efficient and rapid. Flow cytometry analyses showed that after only 10 min of incubation in the presence of R8-GALA-Lip(IgG), 86% of cells registered some grade of antibody internalization, and after 5 min, this percentage increased to 96% (Fig. 5a). The levels of cellular uptake of the antibodies increased with incubation time, with a high accumulation of antibodies in the first hour of incubation, whereas for extended incubation times, the speed of uptake decreased as saturation was approached (Fig. 5b). The release of the antibody to the cytosol was also remarkably rapid (Fig. 5c). CLSM images revealed that, after 15 min of incubation in the presence of R8-GALA-Lip(IgG), the majority of cells contained strong fluorescence dots. Moreover, after only 30 min in the presence of carriers, in most of the cells, antibodies had already been released into the cytosol, and after 60 min, a much stronger fluorescence were widely distributed in the cytosol. Collectively, these results indicate that R8-GALA-Lip(IgG) mediates the cytosolic delivery of antibodies in less than 1 h after transduction.
Comparison of R8-GALA-Lip(IgG) and Commercially Available Reagents for Protein Delivery
The performance of the R8-GALA liposomes was compared with two commercially available reagents, to identify whether this new delivery system offers any improvement. The efficiency in the cellular uptake of antibodies mediated by the peptide-based carrier ChariotTM (or PEP-1), the cationic lipid-based carrier Pro-JectTM, and R8-GALA liposomes was analyzed by flow cytometry. The histogram profiles showed that the three carriers all mediate antibody internalization (Fig. 6a). The cellular accumulation of antibodies mediated by R8-GALA-Lip(IgG) was significantly higher compared with the two commercial regents, with antibody uptake levels in excess of two and four times the levels for Pro-JectTM and ChariotTM, respectively (Fig. 6b). The trafficking of the antibodies delivered by the three systems was then observed by CLSM (Fig. 6c; Fig. S5). Interestingly, antibodies delivered by ChariotTM and Pro-JectTM appeared to be concentrated in small dots that were mainly colocalized with endosomes, whereas antibodies delivered by R8-GALA liposomes were diffused in the cytosol. These results indicate that the R8-GALA liposome system is highly effective not only in mediating the cellular uptake of antibodies, but also for overcoming endosomal entrapment compared with commercially available carriers.

Analysis of the Ability of R8-GALA Liposomes to Deliver Specific Antibodies
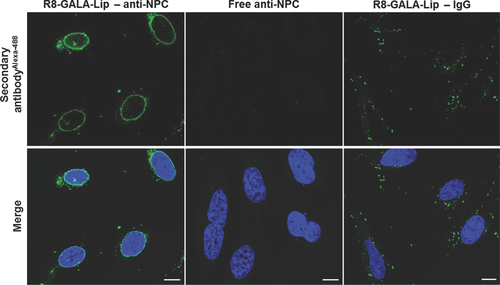
R8-GALA liposomes were used to deliver specific antibodies in order to confirm the integrity of the antibodies released into the cytosol. For this purpose, the NPC, a protein complex responsible for the exchange of components between the nucleus and the cytoplasm,34 was targeted. HeLa cells were incubated in the presence of R8-GALA liposomes complexed with anti-NPC antibody (R8-GALA-Lip—anti-NPC). The primary antibody (anti-NPC antibody) was detected by secondary antibodies (IgGAlexa488) after fixing the cells. Anti-NPC antibody delivered by R8-GALA liposomes was bound to nuclei (left panels, Fig. 7) in similar fashion to direct immunostaining (Fig. S6). This indicates that, after cellular uptake and the escape from endosomes, the antibodies retained their specific function. As a control, cells were incubated in the presence of free anti-NPC antibody. As expected, none of the antibodies interacted with the nuclei, with any artificial import of anti-NPC antibody during the fixation process discarded (center panels, Fig. 7). In the case of cells incubated with R8-GALA liposomes complexed with nonspecific antibodies (R8-GALA-Lip—IgG), the fixation process removed most of the antibodies and only a few random dots were detected, probably corresponding to some antibodies that were still entrapped in the endosomes (right panels, Fig. 7).
Analysis for the Cellular Uptake Route of R8-GALA Liposomes
To investigate the contribution of the cellular uptake pathway to the internalization of the carriers, HeLa cells were incubated with rhodamine-DOPE-labeled carriers in the presence and absence of each type of specific inhibitor, followed by measuring the amount of carriers that had been internalized by flow cytometry (Fig. 8). We examined the effects of the following inhibitors: amiloride, a specific inhibitor of macropinocytosis by inhibiting the Na/H exchange required for macropinocytosis, a hypertonic medium containing sucrose to specifically inhibit clathrin-mediated endocytosis through the dissociation of the clathrin lattice, and Fillipin III to specifically inhibit caveolar uptake via the depletion of cholesterol.35-37
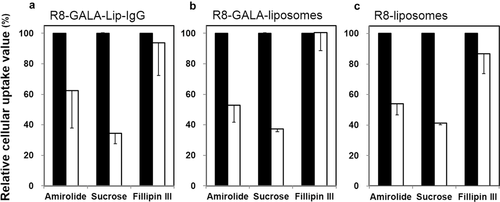
As shown in Figure 8, the use of the macropinocytosis inhibitor (amiloride) or the clathrin-mediated endocytosis inhibitor (a hypertonic medium containing sucrose) largely inhibited the cellular uptake of R8-GALA-Lip-IgG, whereas no inhibition of cellular uptake was observed in the presence of the inhibitor of caveolar uptake (Fillipin III) (Fig. 8a). We also observed the same tendency for cellular uptake, when both R8-GALA liposomes and R8-liposomes without an antibody were used (Figs. 8b and 8c). Taken together, these results indicate that the R8-GALA-Lip-IgG involves both the macropinocytosis pathway and classic clathrin-mediated endocytosis. Moreover, it appears that IgG loading has no effect on the cellular uptake route of carriers in all of the uptake pathways examined in this experiment.
DISCUSSION
In the analysis of the ζ-potential of liposomes after loading of the antibody solution (Fig. 1b), it was assumed that the antibodies were absorbed on the surface of the liposomes. Empty liposomes (hydrated with HEPES buffer) were highly negative particles (∼ −70 mV). On the contrary, after the loading of almost neutral antibodies, the apparent surface charge of the liposomes quickly became neutral (Fig. 1b, open symbols). A similar tendency was observed in the case of R8-modified liposomes (Fig. 1b, closed symbols). The ζ-potential values of both Lip(IgG) and R8-Lip(IgG) tended to reach a plateau at high IgG concentrations reaching a saturation point at which no more antibodies could associate with the liposomes (Fig. 1b). If liposomes were simply encapsulating an antibody solution in the aqueous phase, the apparent surface charge should not be expected to change so drastically. In contrast, if antibodies were adsorbed on the liposome surface, a drastic change in the ζ-potential would be expected. To clarify this phenomenon, R8-Lip(IgG) were compared with carriers prepared in a lipoplex style (R8-Lip—IgG). The data shown in Table 1 indicated that the size and ζ-potential remained comparable in the two systems. This finding suggests that the two systems are equivalent in physical properties and antibodies can be adsorbed on the surface of the liposomes.
The cellular internalization of antibodies using carriers would be influenced by their cellular uptake activity and the association of an antibody with the lipid surface in each of these carriers. Thus, we estimated the IgG absorption to each type of liposome, including plane liposomes, R8-liposomes, GALA liposomes, and R8-GALA liposomes (Table S1). As a result, the absorption of IgG to liposome was confirmed in all cases, indicating that liposomes composed of DOPE/CHEMS (9/2, molar ratio) are capable of carrying IgG on the liposomal surface. Moreover, the values for the R8-modified liposome (more than 50%) were much higher than that for R8-unmodified liposomes (less than 10%) regardless of GALA modification, suggesting that modification with R8 would strongly assist in the absorption of IgG to the liposomal surface. Although the effect of IgG absorption on the ζ-potentials of liposomes showed a similar tendency in the presence/absence of R8 (Fig. 1b), probably because the ζ-potential likely reached a plateau when a certain amount of IgG became bound to the liposomal surface. Furthermore, a previous report showed that the cellular uptake of R8-modified liposome itself was much higher than that of R8-unmodified liposomes in the case of HeLa cells.37 On the basis of our results and findings of previous reports, it can be concluded that R8 assists both the loading of IgG on the liposomal surface and the cellular uptake of the carriers, thus resulting in the efficient cellular internalization of antibodies.
Previous studies showed that liposomes (DOPE/CHEMS) modified with a high density of R8 promoted the cellular uptake of carriers via macropinocytosis, resulting in the release of the cargo to cytosol.16 Therefore, the R8-modified liposomes were proposed for the intracellular delivery of antibodies (Fig. 2). However, the CLSM studies showed that, despite the antibodies are efficiently internalized by cells, they remained entrapped in acidic compartments (Fig. 3). The presence of IgG on the liposomal surface might have disturbed the endosomal escape of the liposomes. It is also possible that the adsorption of IgG on the surface of liposomes changes the property of the carriers, thus interfering with the expected internalization mechanism, favoring a pathway different to macropinocytosis. To investigate this in more detail, the cellular uptake pathway of the carriers was investigated (Fig. 8). The findings show that both R8-GALA-Lip-IgG and R8-GALA liposomes were internalized by cells via a similar cellular uptake route (the macropinocytosis pathway and classic clathrin-mediated endocytosis). Collectively, these findings suggest that IgG loading has no effect on the cellular uptake route of the carriers but might inhibit endosomal escape.
Intracellular observation by CLSM showed that antibodies could be delivered to the cytosol mediated by R8-GALA liposomes (Fig. 4a); however, as it was discussed above, antibodies would be carried on the surface of liposomes. Therefore, it is important to evaluate whether antibodies detected in the cytosol are still associated with the lipid phase of liposomes. CLSM images in Figure 4b, where the lipid phase of R8-GALA-Lip(IgG) with rhodamine-DOPE and antibodies (IgGAlexa488) were observed, showed that much of antibodies that had diffused into the cytosol appeared to be liposome free. To clarify this point, after transduction of antibody by carrier, cells were permeabilized with digitonine and the free solutes were washed out of the cytosol.38 When cells were permeabilized with digitonine for 10 min, the high amount of antibodies were able to escape from the cells but some antibodies still remained inside the cells (Fig. S7). This result suggests that the antibody is dissociated from the lipid phase of liposomes, and the free IgG in the cytosol then would move to the extracellular space.
The mechanism of the release of cargoes encapsulated in the aqueous phase of GALA liposomes appeared to be mainly via membrane fusion with endosomes.31 However, antibodies are mainly carried on the surface of liposomes. Therefore, a simple membrane fusion of liposomes with endosomes would not explain the massive release of antibodies into the cytosol. On the contrary, the GALA peptide causes pore formation in lipid membranes at low pH conditions.28 We hypothesize that, after the fusion of liposomes and endosomes, the GALA in the fused membrane could have clustered and formed pores in the endosomal membrane. This formation of pores might trigger the disruption of the endosomal membrane, resulting in a massive release of antibodies from the endosomes. A detailed study of this issue will be needed in order to understand the precise mechanism by which an antibody is released from endosomes. This issue will be investigated in the future.
The findings reported herein confirm that an anti-NPC antibody delivered by R8-GALA liposomes was bound to nuclei (left panels, Fig. 7), indicating that the antibodies retained their specific function after their release from R8-GALA liposomes. A previous report concerning a detailed investigation of antibody-coated liposomes also showed that the antigen properties of IgG are retained when it is absorbed on the liposomal surface.39 The focus of that study was on the existence of a “hinge” region, located between the Fab and Fc portions of the IgG molecule. They concluded that “this region has a U-shaped conformation which is expected to promote the incorporation of the hydrophobic hinge region into the liposomal bilayer thus externally exposing the Fab and Fc portions.” Thus, the Fab of the antibodies after their release from R8-GALA liposomes might retain their antigenic activity and be able to bind specific proteins, although the details for this remain unknown.
More recently, Erazo–Oliveras et al.40 reported that a tetramethylrhodamine-labeled dimer of dfTAT penetrates live cells by escaping from endosomes with a high efficiency. By mediating the endosomal leakage, dfTAT also delivers various proteins including antibodies into cultured cells after a simple coincubation procedure. They concluded that a strategy for achieving such a delivery should combine efficient endosomal escape, a low cell toxicity, and convenience. We concluded that their proposed strategy is a key factor, in terms of achieving the efficient cytosolic delivery of an antibody. Our carrier-type system equipped with R8 and GALA achieved this with a low cell toxicity. Our approach is likely more complicated compared with a simple coincubation procedure using dfTAT, because antibodies need to be first loaded on R8-GALA liposomes for the transduction. However, a carrier-type system, such as liposomes, can be applied for in vivo delivery. Thus, we plan to optimize the R8-GALA liposomes for use in in vivo delivery to develop a more efficient therapeutic antibody delivery system.
CONCLUSION
Our findings demonstrate that R8-GALA liposomes represent a potentially useful system for the efficient cytosolic delivery of antibodies. The synergistic combination of R8 and GALA overcomes the major barriers to the transduction of antibodies, namely, cellular uptake and endosomal entrapment. This system, characterized by its high speed, ease of preparation, and high performance, provides an alternative tool to continue exploring possibilities in the fields of research with antibodies. Future studies will involve attempts to optimize R8-GALA liposomes in terms of in vivo delivery for antibody therapy. Studies directed toward this goal are currently underway.
ACKNOWLEDGMENTS
This work was supported, in part, by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, Japan (NIBIO), a Grant-in-Aid for Young Scientists (A) and a Grant-in-Aid for Scientific Research (S) from the Ministry of Education, Culture, Sports, Science and Technology of Japanese Government (MEXT). We also thank Dr. Milton Feather for his helpful advice in writing the manuscript.




