Subsoil rhizosphere modification by chickpea under a dry topsoil: implications for phosphorus acquisition
Abstract
Chickpea (Cicer arietinum L.) roots exude carboxylates. While chickpea commonly grows where the topsoil dries out during crop growth, the importance of carboxylate exudation by the roots and mobilization of soil P from below the dry topsoil has not been examined. The study investigates the response of carboxylate exudation and soil P mobilization by this crop to subsoil P fertilizer rate. In constructed soil columns in the glasshouse, the P levels (high, low, and nil P) were varied in the well-watered subsoil (10–30 cm), while a low level of P in the dry topsoil (0–10 cm) was maintained. At flowering, rhizosphere carboxylates and rhizosphere soil from topsoil and subsoil roots were collected separately and analyzed. The concentration of total carboxylates per unit rhizosphere mass in the subsoil was nearly double that of the topsoil. Plants depleted sparingly soluble inorganic P (Pi), NaOH-Pi, and HCl-Pi, along with the labile Pi (water soluble and NaHCO3-Pi). The P depletion by plants was greater from the subsoil than the topsoil. The study concluded that depletion of sparingly soluble P from the chickpea rhizosphere in the subsoil was linked with the greater levels of carboxylates in the rhizosphere. These findings indicate that chickpea, with its deep rooting pattern, can increase its access to subsoil P when the topsoil dries out during crop growth by subsoil rhizosphere modification.
1 Introduction
Phosphorus is applied to agricultural soils in soluble forms, but much of it quickly becomes sorbed to Al and Fe oxyhydroxides in acid soils and on the surfaces of Ca- and Mg-carbonates or precipitated as Ca-phosphates in calcareous soils (Holford, 1997). Hence soluble P fertilizer is converted into less soluble forms over time, leading to a store of P in fertilized agricultural soils (Holford, 1997). In order to acquire this less soluble P, plants use a variety of strategies: increased production and secretion of phosphatases, exudation of organic acids, increased root growth along with modified root architecture, formation of cluster roots, and expansion of root surface area by prolific development of root hairs or mycorrhizal symbioses (Raghothama, 1999).
Carboxylate exudation and the resulting modification of rhizosphere P availability is considered the most efficient process to facilitate uptake of sparingly soluble P forms from soils with low total P (Lambers et al., 2012). The mechanisms involved in improved P availability are ligand exchange between the organic anions and orthophosphate, or the formation of complexes with metal cations (Fe3+, Al3+) and the release of associated anions (orthophosphate) into solution (Jones, 1998). Many species exude carboxylates and mobilize P from low-P soil (Vance et al., 2003). The role of carboxylates from specialized roots (e.g., cluster roots) for the mobilization of soil P of Lupinus albus L. has been investigated comprehensively (Gardner et al., 1983; Shane et al., 2003). A range of other species without specialized roots, including Brassica napus L. (Hoffland et al., 1989), Medicago sativa L. (Lipton et al., 1987), Cajanus cajan L. (Ae et al., 1990), and Cicer arietinum L. (Veneklaas et al., 2003), also release carboxylates from their roots.
Chickpea is an important grain legume crop grown on about 12 million ha from temperate to sub-tropical regions of the world (Siddique et al., 2012). This crop usually faces terminal drought due to rising temperatures towards maturity and lack of, or irregular distribution of, in-season rainfall. As a result, topsoil drying is a common feature of chickpea crops especially during the reproductive growth stage (Toker et al., 2007). Due to topsoil drying, plants face nutritional (N, P) as well as water stress which probably jointly contributes to yield decreases (Johansen and Sahrawat, 1991). Chickpea grown in a progressively drying profile can avoid water stress by enhanced root proliferation in subsurface layers which contain available water. Under this condition, the strategies for adequate P uptake are not clear. Kabir (2012) concluded that chickpea continues P uptake until maturity by absorbing P from the subsoil layer when the topsoil dries out towards maturity. Hence, we hypothesize that chickpeas' continuous P uptake relies on carboxylate exudation (Marschner and Römheld, 1983; Ae et al., 1991) in the subsoil under a dry topsoil condition. While numerous studies examined carboxylate exudation in this crop, they were done in shallow pots under well-watered conditions (e.g., Veneklaas et al., 2003) without considering the subsoil, and the exudates were collected from the whole root system rather from sections of the whole root separated by soil depths. Despite the prevalence of dry land crops, where topsoil drying is common, and the critical importance to crop nutrition of continued P acquisition when the topsoil dries (Johansen and Sahrawat, 1991), only few pot studies have examined carboxylate exudation under dry topsoil conditions without extracting exudation from the root in well-watered subsoil (e.g., Suriyagoda et al., 2010, 2011). Thus, the present glasshouse study was aimed at exploring the relationship between exuded carboxylates from roots at different soil depths (dry topsoil and well-watered subsoil) and P extraction by chickpea plants and to changes in P fractions in the rhizosphere soils.
2 Material and methods
Desi chickpea cv. ‘Genesis 836' was grown in a pot experiment in a naturally-lit glasshouse at Murdoch University, Perth, Western Australia (32°04'S, 115°50'E). The sowing date was September 12, 2012. The maximum and minimum temperatures during plant growth were 20 ± 3 and 14 ± 2°C, respectively with a day length of 12 ± 0.6 h (all values are mean ± standard deviation).
2.1 Soil
The 0–10 cm horizon of a sandy loam soil under natural Eucalyptus woodland was collected from 10 km N of New Norcia, Western Australia (31°03'51”S, 116°12'16”E, 250 m asl) (Mollic Parexeralf, MRA 10 in McArthur, 1991). The air-dried soil was passed through a 4-mm sieve before being used in the experiment. Soil samples were analyzed (following the methods of Rayment and Higginson, 1992) by CSBP FutureFarm analytical laboratories (Bibra Lake, Western Australia) for the properties given in Table 1.
| Properties | Values | Propertiesa | Values |
|---|---|---|---|
| pH (H2O) | 5.5 | DTPA-Mn / mg kg−1 | 4.8 |
| pH (CaCl2) | 4.9 | Colwell-P / mg kg−1 | 3.0 |
| Sand / % | 75.7 | Olsen-P / mg kg−1 | 0.7 |
| Silt / % | 9.1 | Total P / mg kg−1 | 145 |
| Clay / % | 15.2 | BSES P1 / mg kg−1 | 5.0 |
| Organic C / g kg−1 | 11.0 | P retention index (PRI) | 19.0 |
- aBSES P1: 0.005 M H2SO4 extraction procedure for soil P.
2.2 Soil columns
Vertically-split halves of PVC columns (60 cm long, 10 cm diameter) were re-joined with adhesive tape. Each column was free draining with a perforated cap at the base. In each column, two treatment layers—topsoil (0–10 cm) and subsoil (10–30 cm) (using the same soil in both layers)—were constructed and P treatments imposed (Fig. 1). For watering directly to the subsoil while keeping the topsoil dry, a 40 cm-long and 1 cm-diameter plastic tube with holes was inserted in the middle of each column. No barrier was placed between topsoil and subsoil layers in order to mimic the field profile. The holes in the watering tube were positioned at 12.5 cm and deeper in the column-soil that effectively avoided wetting-up the topsoil (in a preliminary and in the present study) by capillarity. In the present study the dryness of the topsoil was verified by opening up four extra columns periodically during experimentation. The topsoil dried progressively and approx. 6 weeks after sowing (about 2 weeks after withdrawing surface watering) it had dried completely.
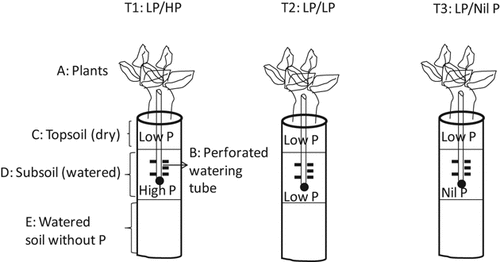
Illustration of P treatments in the dry topsoil (C: 0–10 cm) and well-watered subsoil (D: 10–30 cm). The dry topsoil of every column has low P supply. P levels varied in the well-watered subsoil: T1 has high P supply (LP/HP); T2 has low P supply (LP/LP), and T3 has no added P (LP/Nil P). In the 60 cm-long column, a 2 cm gap from the top of the soil to the top of the column was kept free, 10 cm of topsoil extended to 12 cm below the top of the column, but in the subsequent descriptions it was denoted as 0–10 cm or dry topsoil. Twenty cm of subsoil (12–32 cm) underlies the topsoil (but the subsoil was denoted as 10–30 cm in the text below). In the 32–57 cm of the column (E), soil was supplied with basal nutrients only and remained continuously well-watered. At the bottom, a 3-cm layer of washed river sand was placed. The narrow tube in the centre of the column (B) was a watering tube with perforations in the subsoil from 2.5 cm below the interface with topsoil to allow watering of the subsoil independently of the topsoil. The lower end of the watering tube was closed with a cotton plug that made the watering tube non-permeable to water. Each column had two chickpea plants (A).
2.3 Experimental design and treatments
The experiment consisted of six replications of three treatments with one harvest. Among six replications, three replications were used to measure rhizosphere carboxylates and the other three were for collecting rhizosphere soil. The treatments were arranged in a completely randomized design (18 columns). The experimental soil was very low in P (3 mg kg−1 Colwell-P, which in a preliminary study was too low to allow chickpea to grow to maturity) and was enriched with two P levels. In all treatments, low (L) P (45 mg Colwell-P kg−1 soil, LP) was incorporated in the topsoil layer of the column, while P levels were varied only in the subsoil. The subsoil P levels were designated: high (H) P (90 mg Colwell-P kg−1, HP), LP (45 mg Colwell-P kg−1, LP), and nil P (no P added, Nil P). Thus, the P treatment notations were: LP/HP, LP/LP, and LP/Nil P, and the P levels left and right of the slash (/) represent the P in the topsoil and subsoil, respectively.
2.4 Plant culture and measurement
The soil columns were filled by placing soil with basal nutrients and P treatments according to Fig. 1. Measured amounts of soil required for each layer (0–10, 10–30, 30–57 cm) of a column were kept in different buckets. Basal nutrients were added (mg kg−1 soil) at double the rate used in sandy soil by Bolland and Brennan (2008): K2SO4–131; MgSO4 · 7 H2O–34; CuSO4 · 5 H2O–14; ZnSO4 · 7 H2O–14; CoSO4 · 7 H2O–0.44; Na2MoO4 · 2 H2O–0.44; H3BO3–0.4. Calculated amounts of basal nutrients were pipetted on the surface of the soil (in buckets) and dried for 48 h before adding treatment P. The treatment P, as mono-Ca-phosphate [Ca(H2PO4)2], was applied in powder form (because of its low solubility in water) according to the P treatments. To raise the initial pHH2O of soil from 5.5 to 6.5, Ca(OH)2 was added at 0.8 g kg−1 soil following the method described by Asher et al. (2002). The soil with basal nutrients, Ca(OH)2 and P were then mixed well by vigorous shaking in an electric rotary mixer. In the base (30–57 cm) of the column, soil mixed with basal nutrients only was placed on top of the 3-cm river sand layer followed by the 20-cm subsoil layer with either HP or LP or nil P treatment. Finally, the 10-cm topsoil layer with LP was placed on top of the subsoil layer.
After filling the columns with soil (care was taken to ensure uniform bulk density in layers of a single column and for all columns) and setting the watering tube, the whole column-soil was watered from the surface to 75% of field capacity. Three days after wetting, 6 uniform seeds of chickpea per pot were sown. One mL of 10% peat-Rhizobium inoculum (Group N) was added per seed. Two weeks after sowing, the plants were thinned to two even-sized seedlings per pot. No water stress was applied up to 4 weeks after sowing and the required amounts of water were applied to the surface by weighing the columns every 3 d. After 4 weeks, the roots had reached the subsoil (observed from an extra column with transparent walls covered by black plastic) and watering to subsoil-only was started through the watering tube to keep the soil below top 10 cm at 75% field capacity. At this stage the amount of water required per column (10–57 cm depth) was reset by subtracting the amount of water initially contained in the topsoil layer from the total water initially contained by the whole column-soil at 75% field capacity. Watering to the subsoil only was continued up to flowering of the plants. At flowering, shoots of the plants were cut at soil level and put in a plastic bag and stored in a cold room (4°C) for further processing.
2.5 Rhizosphere chemistry
At the time of harvest (61 d after sowing for rhizosphere carboxylate extraction), the adhesive tape holding the column-halves together was severed and the soil plus root system was exposed and placed horizontally on a bench. Holding the stem base, the whole root system was removed from the soil gently and carefully. The root system was shaken to remove the bulk soil and soil in root clumps. At this stage, the soil adhering to the root was considered rhizosphere soil (approx. 1 mm from the root surface, which is equivalent to the usual size of P depletion zones; Hinsinger, 2001). Despite care, some root breakage was inevitable. Root breakage was less in the topsoil layer due to few live and new roots compared to the subsoil. However, the broken roots were included with the total root of each soil layer when measuring dry weight (DW). For separately collecting rhizosphere extracts from topsoil root and subsoil root, without cutting the root at individual soil-layer length, the whole root system was placed on a long clean tray (40 cm × 20 cm). At 10 and 30 cm from the stem base, a soft round Styrofoam bar (15 mm in diameter) was gently inserted under the root system at a right angle to the root to raise that part of root system from the tray. A small piece of transparent tape (about 1 cm diameter) was used to loosely fasten the root system to it. This was done to prevent the movement of extracting solution between roots of topsoil and subsoil or between subsoil and the rest of root during washing (as the root system was placed horizontally on the tray). A Petri dish (15 cm in diameter) was placed under the 0–10 cm root section. The rhizosphere soil of 0–10 cm root was repeatedly washed with measured volumes of 0.2 mM CaCl2 solution. Rhizosphere soil from subsoil root was washed off in the same way described for topsoil root washing. The root system was then cut at 10 cm (0–10 cm) and 30 cm (10–30 cm). The roots from topsoil, subsoil, and below subsoil were kept separately in water for subsequent washing, cleaning, drying, and weighing.
The extract was filtered first by Whatman #41 into a 50-mL beaker and then by 0.22-µm Pal Gelman Acrodisc® syringe filter in to a 1-mL HPLC vial. In the vial, 10 µL of concentrated orthophosphoric acid was added to adjust the pH to < 4 and then it was kept in a box containing dry ice. In this process of extraction, 2–3 g of rhizosphere soil (dry weight) were recovered from the topsoil and 7–9 g from the subsoil. Similar weights of bulk soil from both layers and soil from the column without plants were extracted using the same amount of solution (0.2 mM CaCl2). All columns (9) were harvested in one day (10.00 am to 3.00 pm). Each of the columns took a similar time period (approx. 30 min for both the topsoil and subsoil) for the final extract (filtering, acidifying, and placing in the dry ice box). However, the mass of the rhizosphere soil was determined by weighing, taking into account the amount of CaCl2 added. For measuring the DW of rhizosphere soil, the soil on the filter paper (Whatman #41) was quantitatively recovered and added back to the sample.
For the fractionation of P in rhizosphere soil, the remaining three replications were harvested (at 62 d after sowing). Each column was cut open longitudinally and the soil was sectioned (with root) into 0–10 cm (topsoil), 10–30 cm (subsoil), and 32–57 cm layers. After removing the bulk soil from roots (as described earlier), rhizosphere soil was collected from the topsoil and subsoil roots separately by mild brushing of the roots with a paint brush.
After collecting rhizosphere soil from three replications and rhizosphere extract from the other three replications, the nodules in the roots of topsoil were ranked according to Rupela (1990). The nodule scoring was as follows: 0 = none, or only a few (< 4) small, ineffective (green interior) nodules on either the tap root or lateral roots; 1 = only one or a few small (< 4) effective (pink interior) nodules on tap root or, rarely, on nearby lateral roots; 2 = a few larger crown nodules but rarely any nodules on lateral roots; 3 = frequent crown nodules, some large, and a few nodules on laterals; 4 = dense crown nodulation and a few nodules on laterals; 5 = dense crown nodule mass and frequent nodules on laterals, including some large ones. However, the roots of the respective layers were washed and cleaned separately on a 1-mm sieve.
The whole shoot of plants in each pot was separated into tip (including the three subsequent leaves) and the rest of the shoot (leaf, stem, and flower). All plant parts were dried in a forced draft oven at 70°C for 72 h. Dried tips and shoots were ground separately in a stainless steel grinder (Coffee n Spices, Breville). For the measurement of P in each part, subsamples (about 0.1 g) were digested in 2 mL of concentrated H2SO4 (mixed with 0.5% selenium). Phosphorus in the extract was determined colorimetrically (O'Neill and Webb, 1970) using a U-1100 spectrophotometer (Hitachi, Tokyo, Japan). Plants' P content was calculated by multiplying the DW of a particular sample with its P concentration. The sum of P content of tip and shoot gave the total P per two plants (per column) of a particular treatment.
2.6 Carboxylates analysis
The rhizosphere extract samples were analyzed by HPLC (following Cawthray, 2003), using a 600E pump, 717 autosampler and 996 photo-diode array detector (Waters, Milford MA, USA).
2.7 Soil analysis
For determination of soil P pools in the rhizosphere, sequential P fractionation was undertaken using a modified scheme of Hedley et al. (1982). Air-dried rhizosphere soil was passed through a 0.5-mm sieve and a subsample of about 0.5 g soil was sequentially extracted by water, 0.5 M NaHCO3, 0.1 M NaOH (for Fe-/Al-P), and then 1 M HCl (for Ca-P).
2.7.1 Residual and total P of unfractionated soil
Residual soil from the sequential extraction was transferred to a long-necked Pyrex tube by repeated washing with DDI water. The residual and unfractionated soil was digested with H2SO4 and H2O2. Phosphorus concentration in the extracts was determined following the molybdo-vanadate method (Murphy and Riley, 1962).
The bulk soils from the topsoil, subsoil, and the virgin soil (without added P) were treated in the same way as rhizosphere soil for P fractions determination (topsoil and subsoil from the column without plant was also treated in the same way). The relative depletion of a fraction was calculated by subtracting the fraction value for rhizosphere soil from its respective bulk value (high P, low P or Nil P).
2.4 Statistical analysis
One-way analyses of variance (ANOVA) for individual soil depths were performed for a completely randomized block design for fractions of rhizosphere carboxylates, P fractions of rhizosphere soil, root DW, and nodule DW using the SPSS version 18. One-way ANOVA was also performed for shoot DW, shoot P, and crown nodule ranking. For measuring significant difference between bulk soil P and rhizosphere soil P fractions of subsoil, paired t-test was done (because the bulk soil P values were different in the subsoil in different P treatments). The treatment means were separated by Duncans' multiple range test (DMRT) at 5% level of significance.
3 Results
3.1 Plant growth and P accumulation
Subsoil P supply (HP or LP) increased dry weight (DW) of total shoot (Fig. 2a) and root (Fig. 2b) significantly (P ≤ 0.01) compared to nil P in the subsoil (LP/Nil P). Root growth mostly occurred within 0–30 cm depth in the column-soil. About 30% of total root DW was in the topsoil (0–10 cm) (Fig. 2c) and about 50% of that (total root DW) was in the subsoil (10–30 cm) in both LP/HP and LP/LP treatments. Subsoil nil P significantly reduced the root DW of topsoil and subsoil compared to that of LP/HP or LP/LP.
Plants accumulated significantly higher (P ≤ 0.01) amounts of P when HP was applied in the subsoil than with subsoil LP (Fig. 2d). Shoot P was lowest in the LP/Nil P which was statistically similar to that of LP/LP treatment. Shoot P concentration and the nodulation followed the similar trend to the plant's total P, i.e., application of HP in the subsoil increased P concentration of shoot and improved crown root nodulation relative to that of subsoil LP or nil P (Table 2).
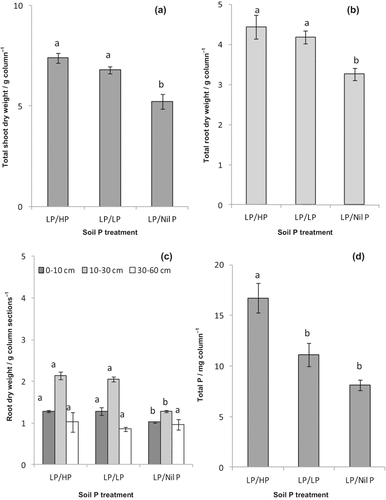
(a) Total shoot dry weight (DW) (g column−1), (b) total root DW, and (c) root dry weights in topsoil (0–10 cm), subsoil (10–30 cm), and below-subsoil (30–60 cm) of column. (d) Total P content column−1 of glasshouse-grown chickpea at flowering at different levels of subsoil P under dry topsoil condition. Treatment notations refer to: low P in the topsoil (LP) and high P in the subsoil (HP), LP in both topsoil and subsoil (LP/LP), LP in the topsoil, and no P in the subsoil (LP/Nil P). Slash (/) divides topsoil P (left side of the slash) and subsoil P (right side of the slash). Vertical bars indicate standard error (n = 6). Means of DW of total shoot and root, roots in column depths, and total plant P followed by the same letter are not significantly different at P < 0.05.
| Treatment | Shoot P concentration / % dry weight | Nodulation rank (highest rank: 5; lowest rank: 0 ) |
|---|---|---|
| LP/HP | 0.31a | 4.2a |
| LP/LP | 0.24b | 3.0b |
| LP/Nil P | 0.24b | 2.7b |
3.2 Rhizosphere carboxylates
In all treatments, rhizosphere carboxylate concentration per unit mass of roots (μmol per g root dry mass, extracted using a 0.2 mM CaCl2 solution) in the well-watered subsoil was much higher (P ≤ 0.001) than that of roots in dry topsoil (Fig. 3a). In both the topsoil and subsoil of LP/Nil P, a greater amount of total carboxylates per unit root mass was indicated than that of subsoil HP or LP. Carboxylates were mainly malonate (57%) followed by malate (25%) and citrate (18%) both in the topsoil and subsoil rhizosphere and irrespective of P treatments (Fig. 3b, c). Carboxylate was not detectable in the bulk soil or in the soil without plants.
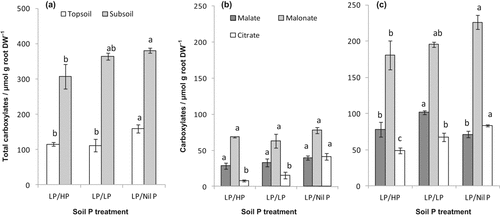
Rhizosphere carboxylates (μmol g−1 root dry weight, extracted using a 0.2 mM CaCl2 solution) in (a) total soil, (b) topsoil, and (c) subsoil of chickpea grown in different subsoil phosphorus levels. Treatment notations are as in Fig. 2. Vertical bars indicate ± standard error (n = 3). Treatment means of topsoil and subsoil carboxylates, and carboxylate fractions (malate, malonate, and citrate) followed by the same letter are not significantly different at P < 0.05.
Total and forms of carboxylate concentration in the rhizosphere soil (μmol per g soil dry mass, extracted using a 0.2 mM CaCl2 solution) in all treatments followed similar trends to that observed for carboxylate concentration on a root mass basis (data not shown). The amount of rhizosphere soil (dry weight basis) from the topsoil and subsoil was 3.2 g and 8.1 g, respectively. The carboxylate concentrations expressed on a dry soil basis correlated with levels expressed on a dry root basis (r2 = 0.73).
3.3 P fractions
Almost all P fractions were increased after P addition (except NaHCO3-Po and HCl-Po) especially those extracted as resin P, NaHCO3-Pi, and NaOH-Pi (Table 3). From topsoil, chickpea did not significantly deplete rhizosphere resin P, NaHCO3-Pi, and NaOH-Pi relative to bulk soil P concentration of LP/HP and LP/LP treatments (Fig. 4a, c, e). However, a significant depletion of those fractions was observed in the subsoil (Fig. 4b, d, f). In the subsoil rhizosphere of LP/Nil P, the value for resin P and NaHCO3-Pi was unchanged (around 3 mg kg−1) relative to the initial bulk soil P. Plants did not deplete HCl-extractable Pi significantly from any soil layers of LP/HP and LP/LP treatments, but it did from the subsoil of LP/Nil ; plants depleted the NaOH-Pi and HCl-Pi fractions by around 50% (Fig. 4f, h). There was no depletion of organic P (Po) (from NaHCO3-Po, NaOH-Po, HCl-Po) and residual P from any treatment (data not shown).
| Soil | Resin P | NaHCO3 Pi | NaOH Pi | HCl Pi | NaOH Po | Residual | Total (t1, sum of all fractions) | Total P (t2, unfractionated soil) | % Recovery (t1/t2) |
|---|---|---|---|---|---|---|---|---|---|
| Virgin soil | 3 ± 0.2 | 3 ± 0.3 | 10 ± 1.5 | 12 ± 1.7 | 36 ± 2.0 | 80 ± 7.2 | 144 ± 9.2 | 149 ± 7.4 | 96 |
| Low P | 23 ± 2.9 | 27 ± 1.4 | 40 ± 3.5 | 13 ± 1.7 | 38 ± 2.1 | 95 ± 4.8 | 236 ± 15.8 | 253 ± 11.3 | 94 |
| High P | 50 ± 3.5 | 62 ± 3.5 | 66 ± 4.1 | 18 ± 1.8 | 38 ± 2.2 | 98 ± 6.2 | 337 ± 14.2 | 354 ± 15.4 | 95 |
- aNaHCO3 Po and HCl Po fractions were uniformly low in virgin soil and soils with added high P or low P and hence not tabulated.
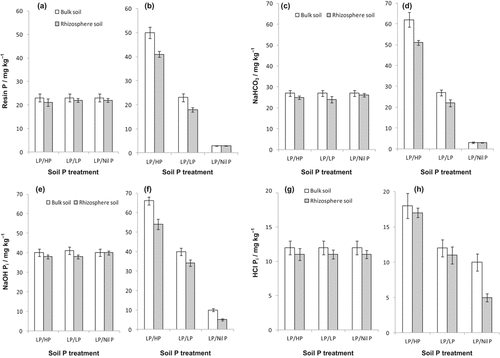
Rhizosphere and bulk soil P: resin P [(a) topsoil, (b) subsoil]; NaHCO3 Pi [(c) topsoil, (d) subsoil]; NaOH Pi [(e) topsoil, (f) subsoil]; HCl Pi [(g) topsoil, (h) subsoil] of glasshouse-grown chickpea. Rhizosphere soil collected from the topsoil (0–10 cm) and subsoil (10–30 cm) of soil column at flowering stage. Rhizosphere soil P (grey bars) of topsoil (low P added to all) or subsoil (high P, low P, or no P added) was compared to the respective bulk soil P (open bars) of each soil layer. The initial P concentration of the virgin soil was 3 mg kg−1 (Colwell) in Fig. 4 d in treatment LP/Nil P. Treatment notations are as in Fig. 2. Vertical bars indicate ± standard error (n = 3).
4 Discussion
While numerous studies examined carboxylate exudation by chickpea in shallow pots under well-watered conditions (e.g., Veneklaas et al., 2003) the present study demonstrated: (1) chickpea exudes higher amounts of carboxylates in the well-watered subsoil than in a dry topsoil; and (2) the depletion of sparingly soluble NaOH-Pi (in the subsoil of all treatments) and HCl-Pi (only in the no-P supplied subsoil) in the rhizosphere of the subsoil roots. Taken together, these findings support our hypothesis that chickpeas' continuous P uptake, despite a dry topsoil, relies on carboxylate exudation (Marschner and Römheld, 1983; Ae et al., 1991) to enhance P acquisition from the subsoil. The weak responses of chickpea to P fertilizer, when grown with a dry topsoil, are also consistent with this species having the capacity to efficiently acquire subsoil P. Given the critical importance to crop nutrition of continued P acquisition when the topsoil dries (Johansen and Sahrawat, 1991), these findings have relevance to the P nutrition of crops on large areas of dry land where topsoil drying is common.
4.1 Carboxylates in topsoil relative to subsoil
Under a dry topsoil condition, excretion of significantly higher amounts of rhizosphere carboxylates in the well-watered subsoil (than that of topsoil), regardless of whether the amount was expressed on a root mass basis or soil mass basis (data on soil mass basis not shown), is an important observation in chickpea.
The lesser amount of carboxylates in the dry topsoil compared to the subsoil might be primarily due to low density of live fine roots, lower numbers of root tips, and presence of more aged roots in that layer. By contrast, a high proportion of live roots (to total root) with root tips in the subsoil might be the principal cause of high concentration of carboxylates in that layer. As active carboxylate exudation only occurs from live roots (Hinsinger, 2001), the lesser live roots (relative to the total) in dry soil might prevent a continuous production of carboxylates inside the root that, in turn, lowered the amounts extracted. Chickpea exudes carboxylate from the entire root system, as a constitutive trait, with lower concentration from the older roots than the growing tips (Wouterlood et al., 2004). In addition, possible death or reduced activity of fine roots and/or microbial degradation of carboxylate might lower the amount of carboxylate in the dry soil as argued by Suriyagoda et al. (2011).
Carboxylates are one of the most labile C sources for microbes in soil for their cell growth and maintenance (Jones and Farrar, 1999). However, little quantitative information is available about the turnover of root exudates at different soil depths. Jones and Darrah (1994) found in a soil decomposition study (without plants using bulk soil rather than rhizosphere soil) that citrate decomposition was faster in the organic topsoil (0–20 cm soil) but was more resistant to degradation in soil collected from subsoil (20–40 cm). The microbial population is expected to be low in the subsoil horizon (Jones and Darrah, 1994) and declines gradually with soil depth (Ekelund et al., 2001; Taylor et al., 2002). Microbial degradation is also related to adsorption (on Al and Fe oxyhydroxides) of carboxylates by soil colloids (Jones, 1998) which provides the greatest protective effect from degradation (Jones and Edwards, 1998) as shown by the increasing half-life of carboxylates (Oburger et al., 2009). As the same soil was placed in both soil layers in the current study, differences in microbial activity and carboxylate adsorption were not expected to alter the carboxylate concentration between topsoil and subsoil. However, microbial degradation rate and adsorption might be changed with soil moisture content and soil P concentration, which were not investigated in our study.
Carboxylate degradation could occur during extraction from the root. In the current study, the extraction period (about 30 min) of carboxylate was less than the known half-life of malate (1.7 h, Jones et al., 1996; 1.9 h, Oburger et al., 2009), citrate (2–3 h, Jones and Darrah, 1994; 7.7 h, Oburger et al., 2009), and malonate (3.3. h, Oburger et al., 2009), suggesting that the degradation of carboxylates during extraction was likely to be minimal. Moreover, the time of the day and transpiration has an influence on the exudates released from the root. In the current study, the extraction from both layers of soil of a column was done at a same time of the day. Hence, it can be concluded that the higher root activity in the subsoil was the principal cause of higher levels of carboxylates extracted from this layer.
4.2 Total carboxylate, their composition, and P mobilization
The rates of exudation of carboxylates from non-specialized root per unit mass or area need to be faster in order to reach local concentrations that are as high as those in the rhizosphere of cluster roots (Shane and Lambers, 2005). In the subsoil of the current study, total carboxylate concentrations (across the treatments) were 346 µmol g−1 root DW and 81 µmol g−1 dry soil, which were high compared to the other studies in chickpea. For example, Veneklaas et al. (2003) reported an average of 174 µmol total carboxylates g−1 root DW or 8.5 µmol g−1 dry soil from whole root systems under well-watered conditions when extracted in 0.2 mM CaCl2. These concentrations were efficient in mobilizing P from strongly P-sorbing soils. In the subsoil of the current study, the NaOH-Pi (from all P treatments) and HCl-Pi fractions (only when no P supplied in the subsoil) were depleted in the rhizosphere, suggesting that the total carboxylate concentration was sufficient to mobilize soil P (discussed below; cf section 4.2.1). It is unclear whether the concentration of a mixture (total) or of individual carboxylates in the rhizosphere mobilizes soil P. Since carboxylates are indeed exuded as a mixture that implies the importance of total concentration to mobilize P (Oburger et al., 2009).
The most abundant carboxylate in both soil layers in the current study was malonate followed by malate and citrate, as found in other studies with chickpea under well-watered conditions (Veneklaas et al., 2003; Wouterlood et al., 2004). In chickpea, the individual concentrations of each carboxylate required to mobilize P are not known. However, in a model calculation based on the citrate concentration sorbed to the soil solid phase at least 10 µmol citrate g−1 dry soil was required to mobilize P (Gerke et al., 2000). The citrate concentration in the subsoil (about 15 µmol g−1 soil, data not shown) of the present study could therefore be sufficient to mobilize P notwithstanding the fact that citrate was underestimated due to decomposition. Malate also has the capacity to mobilize P but to a lesser extent than citrate (Jones, 1998; Gerke et al., 2000; Veneklaas et al., 2003).
Compared to the least required concentration of citrate to mobilize P (10 µmol g−1 dry soil, Gerke et al., 2000), malonate concentration of this study in the subsoil was about 4–5 times higher (40–48 µmol g−1 dry soil). This concentration might be sufficient to mobilize P.
4.2.1 P fractions and P mobilization
In the rhizhosphere of all P treatments, both labile P (resin P and NaHCO3-Pi) and sparingly soluble P (NaOH-Pi) were depleted, while in the subsoil of LP/Nil P the depletion of HCl-Pi fraction also occurred. For a legume like chickpea, two mechanisms may be important for P mobilization: (1) Rhizosphere acidification in calcareous soils and release of Ca-bound P (Jones, 1998), and (2) ligand exchange in acid soil and release of Fe-/Al-bound P (Gerke, 1992). Nitrogen fixing legumes including chickpea take up more cations than the anions and extrude protons (H+) from their roots (Marschner et al., 1986; Tang et al., 1998; Lambers et al., 2013), thereby acidifying the rhizosphere (Dinkelaker et al., 1989; Tang et al., 1998). The plants in the present study had 50% of total nodules (DW basis) in the subsoil (data not shown). Hence, excess cation over anion uptake presumably made the subsoil acidic compared to the dry topsoil (from which limited nutrient uptake, cations or anions, would be expected). In slightly acidic to neutral soil, low pH contributes to greater P availability from Ca-bound P (Hinsinger, 2001). Thus, the depletion of Ca-bound P (HCl-extractable) from the rhizosphere of subsoil with no P supplied might be linked to the rhizosphere acidification. Depletion of Ca-P was previously reported in chickpea but in an Indian Vertisol (Ae et al., 1991) under well-watered conditions.
Depletion of the NaOH-Pi (Fe- and Al-bound P) fraction in the rhizosphere of the subsoil might be due to the ligand exchange and displacement of phosphate from Fe- and Al-oxyhydroxides by carboxylates in this study. Other studies have reported that the NaOH-Pi fraction is readily depleted by crops (George et al., 2002; Nuruzzaman et al., 2006; Vu et al., 2008), including highly weathered acid soils (Hedley et al., 1994; Buehler et al., 2002). On the contrary, Rose et al. (2010) did not find any depletion of this fraction in the chickpea rhizosphere in acid sand and attributed their result to the presence of very low amounts of NaOH-Pi (< 2 mg kg−1). Surprisingly, with P added to subsoil there was no depletion of HCl-Pi possibly because the plants acquired a substantial amount of P from the easily extractable P (resin P and NaHCO3-Pi) and relatively labile NaOH-Pi fraction. However, the apparent mobilization of Fe-/Al- and Ca-P forms (in the subsoil) in the present study warrants further investigation into how these two different mechanisms of P mobilization could co-exist in the rhizosphere soil.
No reports of hydraulic redistribution by chickpea plants could be located although this phenomenon is found in some other crops. Rose et al. (2008), studying canola, and Wang et al. (2009), studying cotton, found in sandy soil that P uptake from dry topsoil was aided by hydraulically-lifted water. By contrast, in clay soil, cotton plants lifted water hydraulically but this made no substantial contribution to P uptake suggesting that the benefit of hydraulically lifted water on P uptake may depend on soil texture (Wang et al., 2009). Given that the topsoil around the roots appeared dry and the predominance of dead and dry roots in the current and a previous pot study of Kabir (2012) in the sandy loam topsoil cast doubt on the occurrence of hydraulic redistribution in chickpea.
5 Conclusion
The present study revealed a substantially higher amount of carboxylate exudation by chickpea in the subsoil than in the dry topsoil. The amount of carboxylate was increased to a limited extent by low P levels in the subsoil. The depletion of sparingly soluble Fe- and Al-P (NaOH-Pi) and Ca-P (HCl-Pi) was correlated with the exudation of higher quantities of carboxylates in the subsoil, suggesting a causal link. Exudation by the subsoil root and the depletion of P from the subsoil helps explain why chickpea only marginally responds to surface applied P fertilizer under a progressively drying profile. The present results are relevant to chickpea and other dryland crops grown on stored soil profile water where the roots have access to and depend on subsoil water. Moreover, subsoil rhizosphere modification might also contribute to P acquisition by chickpea when grown on seasonal rainfall that is erratic and allows topsoil to dry out between rain events.
Acknowledgements
This work was supported by the Australian Centre for International Agricultural Research under Project LWR/2005/001, and Murdoch University, Australia.




