Phosphorus buffering capacity of substrate clays and its significance for plant cultivation
Abstract
Peat is commonly used as the main component of horticultural substrates, but it has a very low buffering capacity for the anionic macronutrient phosphorus (P), which can be increased by the addition of clays. The aim of this study was to characterize the P adsorption capacity of different substrate clays and to evaluate its significance for plant P uptake. Substrate clays were characterized with a single-point batch experiment and adsorption and desorption isotherms. The data were fitted to the Langmuir equation for a calculation of the maximum adsorption capacity. Additionally, the contents of oxalate extractable Fe and Al (ΣFeox + Alox) were determined. The influence of a varying P adsorption capacity of the clays on the P availability to plants in the respective peat–clay substrates and pure peat was investigated in a growth experiment with Impatiens walleriana fertigated with 0, 17, and 35 mg P L−1 solution, respectively.
The observed and calculated (Langmuir) P adsorption capacity of the clays could be well-characterized by both the batch experiment and the adsorption isotherms and was highly correlated with the ΣFeox+Alox. A higher P adsorption capacity of the clay amendment in mixed substrates resulted in a lower P concentration in the substrate solution, while the CAT extractable P concentration (PCAT) was the same. Plant growth and shoot P concentrations were enhanced in the substrates, showing a higher P adsorption capacity, since plants were able to take up the whole amount of PCAT, and also part of the non-CAT extractable P. However, the release rate was too low to ensure optimal plant growth, which was in accordance with the result of the desorption experiment. The absolute extent of P release was increased with the increasing P adsorption capacity of the clays and higher degree of P saturation (DPS).
1 Introduction
Phosphorus (P) is a plant nutrient and plays a major role as a structural element, in photosynthesis, respiration and energy transfer. Well-supplied plants contain about 3–5 mg P g−1 shoot dry matter (DM; Hecht-Buchholz, 1967). Phosphorus in soils occurs in both organic and inorganic forms, but only a small amount is present in the soil solution (Holford, 1997). It can form solid phases of low solubility with Ca2+ and Mg2+ in alkaline soils and with Fe3+ and Al3+ in acid soils (Holford, 1997). Furthermore, P is preferentially adsorbed to Fe and Al oxides, oxyhydroxides, hydroxides and to the surface of layered clay minerals. The adsorption capacity for soils varies widely depending on the content and specific surface area of the strongly adsorbing phases as well as the solution chemistry (Nye, 1979). A good correlation between the P adsorption capacity and contents of amorphous Fe and Al oxides is well-known (e.g., Börling et al., 2001; Memon, 2008). The phosphate anion can bind to the functional groups of the oxides by ligand exchange reactions and interact with the charged oxide surface by electrostatic bonding (Antelo et al., 2005; 2007). The electrostatic interaction between phosphate and the oxide surface is highly dependent on the solution pH. Thus, the P adsorption generally decreases with increasing pH values, because of increasing deprotonation of the solid phase and a competition for binding sites with OH− ions (Gao and Mucci, 2001; Antelo et al., 2005). At high concentrations of organic matter P adsorption may be reduced due to a competition for adsorption sites (Antelo et al., 2007). On the other hand, humic acids can inhibit the crystallization of Fe- and Al-oxides (Schwertmann, 1966) and can complex Fe and Al. The humic-Fe and -Al complexes can show a much higher phosphate adsorption maximum compared to Fe and Al oxides (Gerke, 1993).
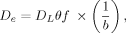 (1)
(1)where DL is the diffusion coefficient of the solute in water. The buffer power denotes the ratio between the change in available P from the solid phase and the corresponding change in the P concentration in the soil solution (Nye, 1979).
The availability of P is not only dependent on the kind and content of clay minerals and Fe and Al oxides in the soil, but also on plant factors. Plants can adapt to low P levels by changes in root morphology, such as an enhanced root length density (RLD) and increased root-hair length; additionally, they have the ability to mobilize bound P by mechanisms such as the release of H+, organic anions or acid phosphatases (Raghothama, 1999). The significance of plant and soil factors affecting the P availability is different between mineral soils and horticultural growth media. The P buffer power is very low in peat-based substrates compared to mineral soils leading to a high P mobility (Khandan-Mirkohi and Schenk, 2008). Furthermore, the RLD is a magnitude higher than under field conditions. This leads to an exploitation of the whole substrate volume, since P depletion zones around roots overlap (Khandan-Mirkohi and Schenk, 2009), which contrasts completely to field conditions, in which plants only use about 20% of the soil volume around roots for P acquisition (Jungk and Claassen, 1997; Claassen and Steingrobe, 1999). The P utilization per unit substrate volume is increased for pot plants compared to field conditions, since the substrate volume is limited and the growth rates of plants are often enhanced under greenhouse conditions.
Peat is commonly used as the main component of substrates in horticultural practice but it has a very low buffering capacity for nutrients such as anionic P. Its buffering capacity can be increased by the addition of clays. The physical and chemical properties of the clay minerals may vary widely depending on their mineralogical composition and surface properties. Up to now, the selection of suitable clays for the production of growth media has mostly been handled empirically. In order to get a first overview of the P adsorption capacity of a clay, Bache and Williams (1971) proposed the application of a single high dose of P and the subsequent measurement of the amount of P adsorbed by the sample. A more detailed characterization of the adsorption behavior can be obtained by adsorption isotherms, allowing the estimation of the maximum adsorption capacity and the buffering capacity of the material analyzed as indicated by the slope of the curve (Bache and Williams, 1971). The process of P replenishment in the soil solution can be evaluated by desorption isotherms (Raven and Hossner, 1994; Sato and Comerford, 2006).
The P pool of a soil or substrate available to plants can be determined by chemical extraction or ion-sink methods using anion exchange resins or membranes (Abdu, 2006; Sato and Comerford, 2006). Several studies have shown that the isotopically exchangeable P is the main source of P available to plants (Frossard et al., 1994; Hamon et al., 2002), but the method is not applicable for standard use. P which is available to plants in agricultural soils in Germany is commonly extracted with Ca-acetate-lactate (CAL) at pH ≈ 4.1 (Schüller, 1969). Furthermore, CAT (CaCl2 + DTPA) is mainly used for horticultural substrates and extracts at the pH of the substrate (Alt and Peters, 1992). It normally has a lower extraction power than CAL. Generally, substrates are supplied with basic fertilization in order to obtain targeted CAT-extractable nutrient concentrations. Thus, a substrate with a higher P buffering capacity needs a higher amount of fertilizer than a substrate with a low buffering capacity in order to reach the same CAT-extractable P concentration. Up to now, it has not been investigated to which extent plants can also use P from fertilization which is non extractable with CAT (non-CAT extractable P) in clay-containing substrates. This P release from non-CAT-extractable P pools could contribute to the reliability of plant cultivation especially with erraneous P fertigation.
The aims of this study were (1) to characterize clay properties describing the P buffering capacity, (2) to identify methods for clay selection regarding the P adsorption capacity, and (3) to evaluate the significance of non-CAT-soluble P for the P supply of plants in peat–clay substrates and its contribution to the reliability of plant cultivation.
2 Material and methods
2.1 Selection and characterization of clays
Fourteen clays were preselected for the characterization of P adsorption capacity (Table 1). Thirteen clays originated from primary and secondary types of deposits in the Mesozoic–Tertiary weathering mantle of the Rhenish Massif, Westerwald area, including two technical mixtures of different clays. The clays were obtained from Stephan Schmidt KG (Langendernbach, Germany). Three different main sources of clays in the Westerwald area are distinguished. There are (autochthonic) bentonites and saprolitic clays (primary deposits) formed in situ and relocated clays (secondary deposits) formed mainly by saprolitic clays being washed out, transported by water and finally settling down in basins in the Tertiary approx. 30 million years ago. The mineral composition of these clays is based mainly on only four types of minerals, namely illite, kaolinite, quartz, and smectite. Additionally, a clay from Sinntal-Jossa near Fulda (Germany), which is used in the substrate industry, was also included for comparison (Table 1). The main component of the clay is smectite, indicating that this clay most probably contains weathering products from volcanic rocks in the Vogelsberg area which were relocated. Elevated contents of SO_4^{2 - } in this clay point to a formation in a marine environment.
| Claya | Textureb | SSAc,d | Ptotd | Pinitd | DPS | Feoxd | Aloxd | Feox : Alox |
|---|---|---|---|---|---|---|---|---|
| Unit | sand/silt/clay / wt % | / m2 g−1 | / mg kg−1 | / mg kg−1 | / % | / mg kg−1 | / mg kg−1 | |
| 39W | 12/18/70 | 74 ± 1.0 | 2374 ± 34.1 | 270 ± 9.4 | 11 | 5629 ± 158.0 | 529 ± 5.5 | 10.6 |
| 07B | 46/41/13 | 106 ±10.7 | 2149 ± 29.9 | 616 ± 12.2 | 29 | 1088 ± 6.4 | 1314 ± 12.8 | 0.8 |
| 06B | 41/38/21 | 110 ± 5.1 | 2071 ± 33.0 | 674 ± 2.0 | 33 | 2061 ± 9.0 | 1658 ± 12.9 | 1.2 |
| 38M | 25/40/35 | 67 ± 6.0 | 1712 ± 13.4 | 353 ± 9.0 | 21 | 771 ± 10.9 | 992 ± 22.0 | 0.8 |
| 14B | 15/45/40 | 60 ± 1.5 | 728 ± 22.9 | 132 ± 0.6 | 18 | 319 ± 4.8 | 595 ± 14.1 | 0.5 |
| 15B | 14/51/35 | 66 ± 2.6 | 718 ± 16.7 | 138 ± 0.9 | 19 | 568 ± 6.6 | 1194 ± 8.6 | 0.5 |
| 12B | 16/45/38 | 64 ± 0.6 | 523 ± 4.2 | 62 ± 0.3 | 12 | 110 ± 1.2 | 316 ± 5.7 | 0.3 |
| 29U | 2/28/70 | 63 ± 1.9 | 468 ± 10.7 | 1 ± 0.0 | 0 | 115 ± 3.5 | 209 ± 4.0 | 0.6 |
| 01S | 42/45/13 | 14 ± 1.7 | 316 ± 4.9 | 81 ± 0.9 | 25 | 381 ± 8.7 | 258 ± 1.0 | 1.5 |
| 03S | 26/53/21 | 18 ± 0.1 | 313 ± 3.5 | 29 ± 0.3 | 9 | 257 ± 2.0 | 120 ± 0.3 | 2.2 |
| 28U | 2/18/80 | 52 ± 6.2 | 282 ± 3.8 | 2 ± 0.1 | 1 | 53 ± 0.8 | 180 ± 1.5 | 0.3 |
| 33U | 8/41/41 | 16 ± 0.8 | 253 ± 4.8 | 20 ± 0.6 | 8 | 55 ± 7.4 | 53 ± 0.5 | 1.0 |
| 36M | 2/35/63 | 27 ± 3.1 | 162 ± 5.6 | 22 ± 0.3 | 14 | 224 ± 14.5 | 96 ± 1.8 | 2.4 |
| 04S | 26/53/21 | 8 ± 0.4 | 47 ± 9.6 | 2 ± 0.1 | 5 | 64 ± 5.1 | 41 ± 4.9 | 1.6 |
| Pure peat | – | – | 133 ± 29.5 | 2 ± 0.3 | 2 | 206 ± 1.3 | 93 ± 1.2 | 2.2 |
- aClay denotations: B = bentonitic clay; M = clay mixture; S = saprolitic clay; U = relocated clay; W = clay from Sinntal/Vogelsberg.
- bParticle size distribution according to DIN ISO 11277:2002-08.
- cSpecific surface area determined by N2-adsorption and multipoint BET.
- dMean of three replicates ± standard error.
In a first screening, the clays were shaken overhead in a batch experiment at pH ≈ 5.5 in a 0.01 M CaCL2 solution with 500 mg P L−1 as KH2PO4 at a ratio of 1 : 10 (w/v) for 24 h in three replicates in order to characterize differences in their P adsorption. The adsorbed amounts at equilibrium conditions, Pnew (mg kg−1), were calculated from the change of the P concentration in the solution. The same procedure was done for a pure peat sample with a water content of 60% (w/w). Additionally, the amount of P initially adsorbed onto the Fe and Al oxides (Pinit) was determined by the extraction with 10 mL 0.2 M ammonium-oxalate and 0.2 M oxalic acid at pH 3.25 at the ratio 1 : 10 (w/v) (Schwertmann, 1964). The total P adsorption capacity (Ptot) was calculated as the sum of Pinit and Pnew (Freese et al., 1992), and the degree of P saturation (DPS) was calculated as the ratio of Pinit in Ptot (Börling et al., 2004).
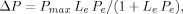 (2)
(2)where ΔP is the amount adsorbed (mg kg−1), Pe the equilibrium concentration (mg L−1), Pmax the adsorption capacity (mg kg−1), and Le the equilibrium constant of adsorption (Langmuir constant).
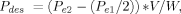 (3)
(3)where Pe2 and Pe1 are the recent and the previous equilibrium concentrations (mg L−1), respectively, and V and W are the liquid volume (L) and the weight of the dried clay sample (kg), respectively.
2.2 Preparation of substrates
The four clays were mixed with white peat at a ratio of 200 kg clay per m3 peat and pure peat was taken as a control. First, P adsorption curves were prepared for each substrate to determine the amount of fertilizer P necessary to achieve the targeted CAT-extractable P concentration (PCAT) in each substrate. The substrates were fertilized with 1.5 g L−1 of the P free compound fertilizer Ferti® 8 (N : P : K = 20 : 0 : 13 + micronutrients; Planta Düngemittel GmbH, Germany) and the pH was adjusted to pH 6 with CaCO3. Phosphorus was added with increasing concentrations from 0 to 150 mg P L−1 substrate as Ca(H2PO4)2 · H2O. The substrates were then equilibrated in an oven at 50°C for 48 h in order to accelerate the P binding and, afterwards, stored at room temperature for another 72 h prior to determining the PCAT. The following P rates were fertilized to reach a PCAT of 25 mg P L−1 substrate, which is a usual concentration in horticultural practice (in mg P L−1 substrate): peat = 30; peat + clay 04S = 38; peat + clay 01S = 65; peat + clay 06B = 72; peat + clay 39W = 100.
2.3 Plant cultivation
Seedlings of Impatiens walleriana F1 “Candy® Coral Bee” were planted in plastic pots (diameter 12 cm), each filled with 600 mL of the different peat–clay substrates and only peat as a control. The plants were grown in a greenhouse at day/night heating temperatures of 22°C/18°C, respectively. Natural radiation was supplemented with 80 µmol m−2 s−1 photosynthetic photon-flux density (PAR) when the radiation was lower than 100 µmol m−2 s−1 to extend the photoperiod to 16 h. Fertigation started immediately after planting on top of the substrate surface with a solution containing (in mg L−1): 120 N as KNO3 and NH4HO3, 130 K as KNO3, K2SO4 and KH2PO4, 10 Mg as MgSO4 · 7 H2O, and 200 Ferty® 10 (micronutrients) (Planta Düngemittel GmbH, Germany). The P concentration in the fertigation solution was varied at three levels (in mg P L−1): 0 (= no P), 17 (= medium P) and 35 (= high P). P was applied as KH2PO4. Plants were harvested after cultivation for 10 weeks.
2.4 Physical and chemical analyses
The texture of clays was determined by sieving and sedimentation after the removal of organic matter by wet oxidation with H2O2, according to DIN ISO 11277: 2002-08. The contents of amorphous Fe and Al oxides (ΣFeox + Alox) were determined by the extraction of 1 g clay with 10 mL 0.2 M ammonium-oxalate and 0.2 M oxalic acid at pH 3.25 (Schwertmann, 1964). The specific surface area (SSA) of the clays was quantified by N2 adsorption with a Nova 4200 analyzer (Quantachrome).
The pH of the substrates was measured in 0.01 M CaCl2 at a substrate : solution ratio of 1.0 : 2.5 ((v/v)). The determination of the bulk density was conducted by a defined compaction of the samples by repeated tamping, according to VDLUFA (1991). Phosphorus, which was available to plants, was determined according to Alt and Peters (1992) using the CAT extraction procedure: 20 g fresh substrate were shaken in 160 mL CAT solution (0.01 M CaCl2 + 0.002 M DTPA) for 1 h. The separation of the substrate solution took place by centrifugation at 3000 g for 20 min in order to measure the P concentration (Psol). All substrates were watered or dried to the same volumetric water content of 500 ml L−1 substrate 48 h before centrifugation. After filtering (cellulose filter paper; 2–3 µm pore size), the P concentration in solutions was determined photometrically (BioTek Instruments, µQuant), according to Schüller (1969) with ammonium-vanadate-molybdate, and according to Murphy and Riley (1962) with molybdenum blue, respectively. Due to the relatively large pore size used for filtration, it can generally not be excluded that colloidal P and some suspended P are also determined in the filtrates. Test measurements with pore sizes of 0.45 µm revealed that this did not occur in our samples.
Buffer power (b) was calculated as the ratio of PCAT : Psol (Khandan-Mirkohi and Schenk, 2009).
The plant material was dried at 70°C for 5 d and ground. P in plant tissue was determined after dry-ashing at 480°C for 8 h, dissolving the ash in 1 : 3 diluted HNO3 at a ratio of 1 : 20 (w/v) and then diluting (1 : 10 (v/v)) with demineralized water. The measurement of the P concentrations in the digestion solution occurred photometrically with ammonium-vanadate-molybdate at 450 nm wavelength according to Gerike and Kurmies (1952).
2.5 Statistics
Analyses of the clays and mixed substrates were performed with three replicates. The treatments in the plant experiment were repeated five times, whereby each replicate consisted of five plants in a randomized block design. Statistical analysis was performed with the program R 2.13.2. Means of treatments were compared by analysis of variance and Tukey's test at P < 5%.
3 Results
3.1 P adsorption and desorption of clays
The 14 preselected clays and the peat sample differed clearly in their total P adsorption capacity (Ptot = ΣPnew + Pinit) and also oxalate-soluble Fe and Al contents (Feox + Alox; Table 1). Most of the clays and the peat contained comparable amounts of Feox and Alox with the exception of 39W, in which Feox clearly dominated. The degree of P saturation of the samples varied between 0 and 33% (Table 1).
The P adsorption (Pnew) of the clays measured was positively correlated with the ΣFeox+Alox (Fig. 1a), but not with the clay content (R2 = 0.0) or the specific surface area (SSA; R2 = 0.0; data not shown). The adsorption isotherms of three clays 04S, 01S and 06B clearly followed saturation functions, whereas clay 39W showed a more or less continuous increase in P adsorption and was not yet saturated at the highest P level, which was also applied in the batch experiment (Fig. 1b). None of the clays showed considerable P desorption and the equilibrium concentration, at which neither desorption nor adsorption occurred, Pe-0, was almost the same. The clays 01S and 04S had only gently inclined curves and reached the maximum P adsorption (Pmax) at much lower P concentrations in the solution than the clay 06B. The adsorption isotherm of the pure peat sample was very similar to the one of clay 04S at low solution concentrations, but its adsorption capacity was clearly higher (Pmax = 130 mg kg−1; data not shown). The maximum adsorption capacity could be calculated by fitting the data observed to the Langmuir equation. It was in the same range as the adsorption observed in the batch experiment (Pnew; Fig. 1a). Only for clay 39W the calculated adsorption capacity was much higher than the Pnew observed in the batch experiment.
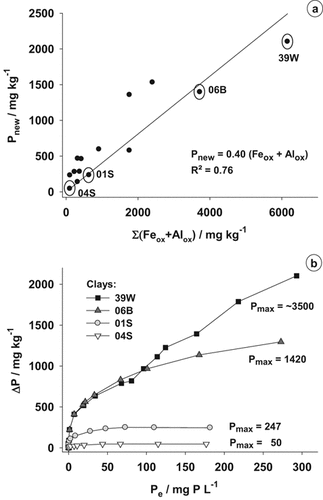
(a) Relationship between the content of oxalate soluble Fe and Al (ΣFeox + Alox) and the P adsorption (Pnew) of 14 clays in a batch experiment. The clays marked were used for detailed analysis of P dynamics and in the plant experiment. (b) Adsorption isotherms of P for the four substrate clays used in the plant experiment (ΔP = the amount adsorbed, Pe = the equilibrium concentration, Pmax = the adsorption capacity calculated in mg kg−1).
The P release of the four clays pre-supplied with 750–1500 mg P kg−1 clay to adjust a Pe of ≈ 65 mg P L−1 solution differed clearly and could be well described by logarithmic functions (Fig. 2a). The clay 04S showed a very low absolute P desorption followed by clay 01S. Pronounced P release in both clays did not start until Pe decreased below 5 mg P L−1. The two other clays (06B and 39W) released clearly more P than 01S and 04S, already starting at equilibrium concentrations of about 40–30 mg P L−1. A decrease in Pe from 3 to 2 mg P L−1, for example, caused a P release of 32 mg P kg−1 in clay 06B, whereas the desorption from clays 39W, 01S and 04S was only about 70, 20 and 6% of this value, respectively. On average, the clays released between 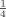 and
and 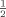 of the Pnew adsorbed at the beginning of the experiment, but the rate of P desorption (P amount released per 24 h) strongly decreased with decreasing equilibrium concentrations (Fig. 2b).
of the Pnew adsorbed at the beginning of the experiment, but the rate of P desorption (P amount released per 24 h) strongly decreased with decreasing equilibrium concentrations (Fig. 2b).
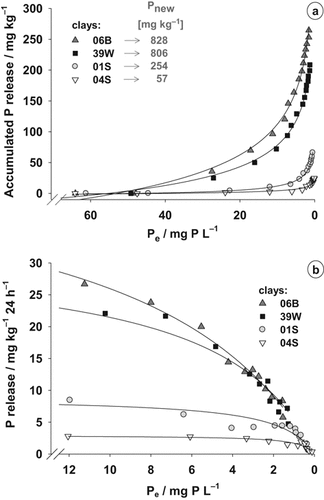
(a) Accumulated P release of the four selected clays with decreasing P concentrations (Pe) in the equilibrium solution. Prior to the desorption experiment, the clays were loaded with P in order to reach a Pe of ≈ 65 mg P L−1. The P amounts adsorbed at the beginning of the desorption experiment (Pnew) are given. Curves were fitted to the function y = y0 + a × ln(x); and (b) P release per 24 h of the four clays with decreasing Pe derived from the desorption experiment. Curves were fitted to the function y = a × (1 – e-b×x). Mean values of three replications.
3.2 Plant-available P in substrates
At the beginning of the plant experiment, the mixed substrates contained the adjusted CAT extractable P concentration (PCAT) of 20–25 mg L−1 substrate. Furthermore, the P concentration in the substrate solution (Psol) was determined and the buffer power (b) was calculated (Fig. 3a). As Psol decreased with the increasing adsorption capacity of the substrates, b was increased. Peat–clay blend 39W had a ≈ 4–5 times higher buffer power than peat and peat–clay blend 04S. In the substrates peat, peat + clay 04S and peat + clay 01S, the buffer power calculated was < 1 as the solution P concentrations were very high.
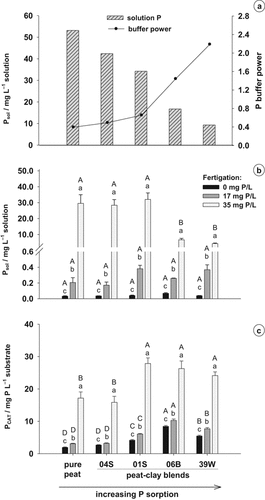
(a) P concentration in the substrate solution (Psol) of peat and peat–clay mixtures and the resulting P buffer power at the beginning of plant culture, (b) Psol and (c) CAT-extractable P concentrations (PCAT) of peat and peat-clay mixtures at the end of the experiment at different P concentrations in the fertigation solution. Error bars represent standard errors. Different capital letters indicate significant differences between substrates at the same P fertigation level and different small letters indicate significant differences between fertigation treatments within the same substrate (P < 5%).
At the end of plant culture, the substrate solution of all substrates was strongly depleted of P (Fig. 3b). Considerably high P concentrations were only found at 35 mg P L−1 fertigation solution, and these were decreased compared to the beginning of cultivation only in peat and peat–clay blend 04S. The same pattern was observed in the CAT-extractable P content of the different substrates at the end of plant cultivation (Fig. 3c). The initial P concentration of about 20–25 mg L−1 substrate could only be maintained in the highest P fertigation level (35 mg P L−1) in the stronger-buffered blends 01S, 06B and 39W compared to peat and peat + clay 04S. At medium (17 mg P L−1) or without P fertigation, PCAT was clearly decreased in all substrates. PCAT after plant cultivation at all fertigation levels was significantly lower in peat and in peat–clay blend 04S than in the blends with other clays, where a clear order was observed: clay 01S < clay 39W < clay 06B.
3.3 Plant growth and availability of non-CAT-extractable P
After 10 weeks of cultivation the varied P fertigation resulted in clear differences in plant growth and plant quality among the substrates (Fig. 4). Plants grown without P fertigation in pure peat and in the blend with clay 04S showed clear symptoms of P deficiency such as leaf-shedding and stunted habitus. Weak symptoms of P deficiency were also observed in the blend with clay 01S. The internode length was reduced in these substrates with a lower P-binding capacity compared to peat–clay blends containing clays 06B and 39W, and fertigation of P increased the internode length (Fig. 4a). The same trend was observed for plant diameter and plant height (data not shown). The P fertigation enhanced the dry matter yield of plants in all five substrates (Fig. 4b). Without P fertigation, the yield of plants grown in peat and in the blend with clay 04S was lower than in the other three peat–clay blends. No differences between the substrates were observed at medium and high P supply.
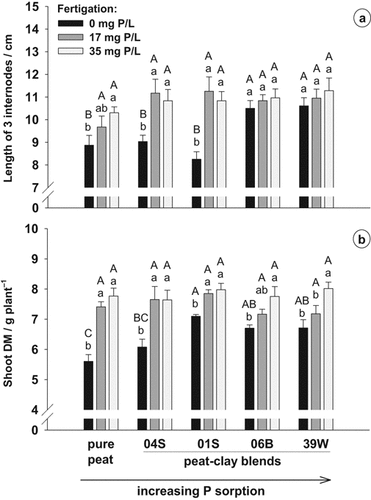
(a) Internode length and (b) shoot dry matter of Impatiens walleriana plants grown in peat and different peat-clay mixtures at different P concentrations in the fertigation solution. Error bars represent standard errors. Different capital letters indicate significant differences between substrates at the same P fertigation level and different small letters indicate significant differences between fertigation treatments within the same substrate (P < 5%).
The increasing P supply via fertigation resulted in increased P concentrations of plants in all substrates (Fig. 5a). Without P fertigation and at 17 mg P L−1, the P concentrations of shoot matter were the lowest in peat and in the peat–clay blend 04S, and the highest in the blend 06B. The differences in shoot P concentrations between substrates were no longer observed at the highest P fertigation level of 35 mg P L−1 solution. The P concentrations in plant matter necessary for optimum growth ranged between 5 and 7 mg g−1 dry matter at 17 mg P L−1 fertigation solution.
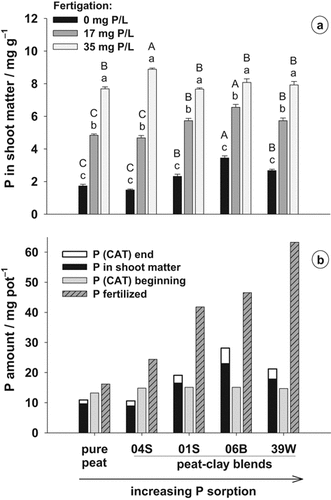
(a) P concentration of shoot dry matter of Impatiens walleriana plants grown in peat and different peat-clay mixtures at different P concentrations in the fertigation solution, and (b) comparison between the amounts of fertilized P and CAT-extractable P (PCAT) at the beginning of plant culture, and P in shoots of plants grown without P fertigation and PCAT left in the pots at the end of plant culture. Error bars represent standard errors. Different capital letters indicate significant differences between substrates at the same P fertigation level and different small letters indicate significant differences between fertigation treatments within the same substrate (P < 5%).
The P amount contained in shoot DM was calculated for the treatments without P fertigation to characterize the capacity of different clays to supply P to plants (Fig. 5b). The substrates with amendment of clays 01S, 06B and 39W supplied considerably more P to the plants than pure peat and the blend with clay 04S. In the latter substrates, plants did not contain the P amount determined by CAT extraction before planting, whereas in the other substrates, especially the one with clay 06B, even more P was taken up than quantified as plant-available by the CAT extraction. However, more P was fertilized in all substrates including pure peat than could be extracted by CAT or was taken up by the plants. This was generally more pronounced in the blends with clays having a high binding capacity for P (01S, 06B and 39W).
4 Discussion
4.1 Growth and P uptake of I. walleriana
Without P fertigation, the buffering capacity of the clay amended to the substrate influenced the growth of I. walleriana (Fig. 4). The higher buffering capacity, especially of the substrates with clays 06B and 39W, but also 01S, resulted in an enhanced growth of the plants compared to the more weakly buffered substrates with clay 04S. P deficiency led to chlorosis and leaf-shedding in peat and the blend with clay 04S. Fredeen et al. (1989) also described a significant decrease in shoot growth, leaf expansion and leaf surface area with low P supply, which can be explained in terms of an impaired extension of plant cells. The number of leaves can also be decreased under P deficiency mainly through effects on the number of nodes and branching (Lynch et al., 1991). Hansen and Nielsen (2001) recommended a reduced P availability as a method for the non-chemical growth regulation of ornamental plants. Under P deficiency, we observed a reduction of internode length and compaction of plants in the substrates peat and peat + clay 04S. At the same time, the DM production was decreased and leaf-shedding was induced showing that such a strict shortage of P was not a successful strategy for forming plants.
The plant P concentrations necessary for optimum growth were in the same range as reported by Khandan-Mirkohi and Schenk (2009) for poinsettia and marigold plants and were achieved with a 17 mg P L−1 fertigation solution (Figs. 4b and 5a). However, the P fertilization was high enough only at 35 mg P L−1 to approximately maintain the initial PSol and PCAT during cultivation (Fig. 3b, c).
4.2 P adsorption capacity of clays
Our results clearly indicate that P in the clays is mainly bound to Fe and Al oxides because the P adsorption (Pnew) observed correlated positively with ΣFeox + Alox (Fig. 1a). Many authors described a similar relationship (Borggaard et al., 1990; Börling et al., 2001; Wang et al., 2012), and it is a common practice in the Netherlands and some other countries to determine the P adsorption capacity of soils indirectly by the ammonium oxalate-extractable amounts of Fe and Al (Börling et al., 2004). Freese et al. (1992) observed an even closer correlation of ΣFeox + Alox with the total P adsorption capacity (Ptot), taking the P initially adsorbed onto the Fe- and Al-oxides (Pinit) into account, for different soils from eastern Germany. This was not the case in our experiment, because the maximum adsorption capacity (Pmax) was not achieved with clay 39W (Fig. 1b).
Information about the process of adsorption were obtained with the adsorption isotherms (Fig. 1b). The calculated Pmax of the peat and the clays 04S, 01S and 06B confirmed the results of the batch experiment (Fig. 1a). Bache and Williams (1971) and Wang et al. (2012) observed a high correlation between the P amounts adsorbed determined in batch experiments and the adsorption maxima calculated from adsorption isotherms using the Langmuir equation for samples of different acid and alkaline soils. The pure peat showed a considerable P adsorption capacity, which was even higher than the one of clay 04S. This was in line with the higher contents of oxalate-extractable Fe and Al in the peat sample.
The isotherm of the clay 39W showed a more or less continuous increase and a maximal adsorption capacity could only be calculated with uncertainties, but it was about 50% higher than the Pnew observed in the batch experiment. Thus, the total adsorption capacity of this clay could not be reliably determined in the batch experiment because the P concentration used in the shaking solution was not high enough to reach saturation. However, it has to be considered, that at such high P concentrations in the solution, which were used in the batch experiment, a secondary mechanism of P adsorption can occur, which seems to be the case for clay 39W.
The simple Langmuir function was developed for simple one-site adsorbents and in multi-site adsorbent systems such as clays, peat or soil it can lead to an incorrect, often underestimated calculated adsorption maximum (Holford, 1997). In these systems, a “two-surface” or “double” Langmuir equation is often used, considering the existence of two populations of adsorbing sites with different adsorption strengths. However, in our study the simple Langmuir function was used as a simplification. The absolute Pmax of the substrate clays was only of minor importance because in mixed substrates generally lower P levels are adjusted than necessary for reaching Pmax.
The order in P adsorption of the clays determined, 39W > 06B > 01S > 04S, was also observed in the substrates blended with the clays, in which the fertilized P amounts to obtain a PCAT of 25 mg L−1 substrate were ranked in this order. However, the differences in the peat–clay substrates were not as pronounced as the differences of Pmax between the pure clay samples, because the equilibrium concentrations in the substrates were clearly lower (Psol in Fig. 3a). Furthermore, it has to be considered that the clays react with peat and solute compounds in the substrate, thereby changing their surface properties. Hence, their behavior can be different than expected from adsorption isotherms or batch experiments. A change in pH value, high concentrations of dissolved organic matter released from the peat and the presence of other cations and anions, especially Ca2+, in the solution from fertilization and liming may influence the adsorption of P (Hua et al., 2008; Weng et al., 2012). Antelo et al. (2007) could show a reduction of the phosphate adsorption to goethite with increasing concentrations of a soil humic acid up to 45% under certain pH conditions.
In summary, the batch experiment performed with a single high dose of P is an easy and quick method for the estimation of the P adsorption capacity of a clay, even if Pmax is not achieved, because the equilibrium concentrations in mixed substrates are generally lower than required for Pmax. An indication of the adsorption behavior to be expected can already be obtained by the determination of Feox+Alox contents.
4.3 Plant-available P in substrates
The five substrates used for the plant experiment had similar CAT extractable P concentrations, but the Psol decreased with the increasing adsorption capacity of the clays (Fig. 3a). The amount of PCAT in peat was equivalent to the amount of P found in Psol, whereas the Psol in blend 39W was only 20% of PCAT. Psol in all five substrates was very high and the P adsorption and b were very low compared to mineral soils (Barber, 1995; Jungk and Claassen, 1997). Khandan-Mirkohi and Schenk (2008) reported similar Psol values for black peat and peat mixed with mineral components and b ranged from 1 to 17. Thus, the P mobility in substrates is high and plants are able to exhaust the whole substrate volume since the RLD is also high (Khandan-Mirkohi and Schenk, 2008).
In substrates with a higher buffer power Psol is held constantly at a low level, which reduces the risk of P leaching in outdoor cultivation and possible P toxicity, while guaranteeing an adequate P supply of the plants by replenishing the substrate solution (Marconi and Nelson, 1984; Williams and Nelson, 2000; McDowell et al., 2001; Ogutu et al., 2009). Owen et al. (2007) showed a reduction of P loss by leaching of 34% in a pine-park substrate amended with a commercial bentonite compared to a control treatment without mineral amendment. Phosphorus toxicity in Helleborus niger was diminished by growing the plants at a Psol of < 15 mg P L−1 in a peat–clay substrate (Dombrowski, 2008; data unpublished).
Impatiens walleriana plants strongly depleted the Psol in the lower P fertigation levels, 0 and 17 mg P L−1 (Fig. 3b), during cultivation, resulting in a release of previously adsorbed P, as indicated by the reduction of PCAT (Fig. 3c). Fertigation with 17 mg P L−1 did not lead to a considerably higher PCAT compared to 0 mg P L−1, but Psol was clearly enhanced in all substrates. Obviously, the rate of P dissolution was not sufficient in the 0-fertigation treatment to replenish the Psol. The same observation was made for the highly buffered blends with clays 06B and 39W, which had PCAT contents twice as high as pure peat and the blend with clay 04S. This is in agreement with the results of the desorption experiment (Fig. 2), displaying only a low P release at the respective Psol of < 1 mg L−1. The insufficient P supply for compensating the P uptake of the plants resulted in low shoot P concentrations and growth depressions in the 0-fertigation treatment (Figs. 4 and 5a). Only at the fertigation with 35 mg P L−1 was the P fertilization high enough to match the plant uptake as far as possible, as indicated by a more or less constant Psol and PCAT during the cultivation (Fig. 3b, c).
The P content of a substrate extracted with CAT is considered available to plants (Alt and Peters, 1992). Thus, substrates with the same PCAT are thought to supply plants with similar amounts of P. Surprisingly, the plants grown in the weakly buffered substrates pure peat and peat + clay 04S did not take up as much P as was determined to be available with CAT (Fig. 5b). This may be due to an underestimation of P uptake, since P was lost by leaf-shedding in these substrates. Furthermore, P contained in roots was not considered. On the other hand, the plants grown in the highly buffered substrates amended with clays 01S, 06B and 39W were able to take up even more P than was characterized as available to plants with the CAT method at the beginning of the experiment. The proportion of this non-CAT-extractable P in P uptake was highest in the substrate with clay 06B, which also showed the highest absolute P release in the desorption experiment (Fig. 2a). Nevertheless, as stated before, the rate of P release from the formerly non-CAT-extractable P pool was not high enough to enable optimal plant growth (Fig. 4b). The proportion of PCAT in the fertilized P amount (0.57, 0.35, 0.32, and 0.23 for peat–clay substrates 04S, 01S, 06B, and 39W, respectively; Fig. 5b) agreed well with the proportion of released P in Pnew of the desorption experiment (0.49, 0.26, 0.32, and 0.26 for peat–clay substrates 04S, 01S, 06B, and 39W, respectively; Fig. 2a). Thus, the amount of easily desorbable P was reliably determined with the CAT extraction. However, the whole amount of fertilized P could not be used by the plants in all peat–clay substrates. The discrepancy between the fertilized P and the taken up P generally increased with the increasing adsorption capacity of the clays (Fig. 5b). Only in substrate 06B more than 50% of the P initially fertilized was taken up by the plants.
The high P release of clay 06B may be explained in terms of its high degree of P saturation (DPS) and its high P stock, composed of the fertilized P and the high content of Pinit, compared to the clays 01S and 39W (Table 1, Fig. 5b). This is in line with the findings of Börling et al. (2004) and Casson et al. (2006), who observed a higher risk of P losses by leaching with increasing DPS.
5 Conclusions
The P adsorption capacity of the clays was strongly correlated with their oxalate-extractable Fe and Al content and could be quickly determined with a simple single-point adsorption experiment. A higher P buffer capacity of the substrate resulted in a decreased Psol, although PCAT was the same in all substrates amended with clay, reducing the risk of P leaching and P toxicity. The CAT extraction described the P amount in a substrate which was easily available to plants. Nevertheless, plants were able to take up not only the P amount that was characterized as available according to the CAT extraction, but also part of the non-CAT-extractable P. Clays with a high P adsorption capacity and a high DPS generally release more P than those with a low adsorption capacity and a low DPS. Thus, the non-CAT-extractable P contributes to the reliability of plant cultivation, but its rate of release may be too low to ensure optimal plant growth.
Acknowledgements
We thank Eva Schmidt and Dr. Matthias Schellhorn from Stephan Schmidt KG and Gerald Schmilewski from Klasmann-Deilmann GmbH for the provision of substrate raw materials and the helpful discussions. The team in the laboratory is gratefully acknowledged for their excellent technical assistance. This work was supported by the Bundesministerium für Bildung und Forschung (BMBF) in the frame of the GEOTECHNOLOGIEN programme “Mineral Surfaces – From Atomic Processes to Industrial Application” (grant 03G0722A).




