Muscle activation for targeted elbow force production following surgical reconstruction in adults with brachial plexus injury
Abstract
Nerve transfer or grafting surgery to restore elbow flexion in peripheral nerve injuries has been an effective technique, but alters neuromuscular control compared with healthy individuals. This study compared neuromuscular control in the elbow flexors of 11 unimpaired control subjects and 11 adult patients with traumatic brachial plexus injury who underwent a nerve transfer or grafting procedure to the biceps motor branch to restore elbow flexion. The subjects performed a series of trials to generate a specific percentage of their maximum elbow torque. Each trial had an increasing and decreasing stairstep torque pattern that the subjects were asked to match. The amount of time that the subject's elbow torque was maintained within 5% of the target torque was calculated. The hypothesis was that there was a significant difference in the neuromuscular control between the two groups during elbow isometric torque generation. A secondary hypothesis was that a relationship existed between the neuromuscular control and the torque level for each group. The results demonstrated that neuromuscular control was different between the groups and there were significant differences in how torque levels are generated. The control group more easily modulated their myoelectric activation and achieved greater neuromuscular control variability with varying torque demand. The nerve transfer or grafting group could not modulate their myoelectric activation with changing torque demands. Further studies focusing on the improvement of neuromuscular control are needed to optimize functional outcomes in nerve injury patients.
1 INTRODUCTION
The brachial plexus (BP) is the complex of nerves supplied by the C5 to T1 nerve roots and provides the motor and sensory function of the upper extremity. Injury to any portion of the BP can result in the loss of motor function and sensation in the upper extremity. Traumatic brachial plexus injuries (BPI) in adults are most commonly seen in high-speed motor vehicle accidents.1 The increased incidence of adult traumatic BPI is related to the popularity of extreme sports and the rising number of vehicular accident survivors.2-4 Traumatic BPI often results in a life-altering injury with severe physical disability and psychological effects.5
Most traumatic BPI patients require surgery to restore elbow flexion. When feasible, primary nerve surgery should be performed within 6–8 months from the time of injury secondary to the time dependant degeneration of the motor end plates.6 Nerve grafting or nerve transfers can be performed to restore elbow flexion, and are typically directed to the biceps brachii muscle.1 Maximal recovery takes up to 2–3 years to determine the surgical outcome. Intuitive motor nerves (i.e., those that are synergistic to elbow flexion) are not typically available for transfer, thus other non elbow flexing motor nerves (spinal accessory nerve, intercostal motor nerves, portions of functional ulnar or median nerves, etc.) which are not synergistic and not intuitive for elbow flexion are used.
-
The neuromuscular control during isometric elbow torque generation differs between patients with a brachial plexus injury who have nerve reconstruction directed to the biceps motor branch and unimpaired control subjects (Null: The means for the groups are similar).
-
Each group can modulate their muscular activation based on the changing elbow torque demands. (Null: No relationship exists between muscular activation and amount of torque produced).
2 METHODS
2.1 Subject sampling
-
Data collection to occur at least 6 months postsurgery.
-
Subject above 18 years and under 65 years of age.
-
mBMRC Grade of 2 or greater.
The exclusion criteria were as follows:
-
Patient underwent a procedure other than a nerve transfer or graft to the biceps motor branch.
-
mBMRC grade of M5, as it is not feasible to have an M5 after any nerve reconstruction procedure.
-
Subject's refusal or inability to participate, secondary to inability to follow spoken instructions.
2.2 Experimental testing
The subject's forearm was secured to a test apparatus with hook and loop straps (Figure 1). The elbow joint axis was aligned with the axis of a torque transducer (TS11-20, Interface Inc.). The subject's arm was placed in 90° of elbow flexion with the palm facing upwards. Subjects with no control over their forearm pronation/supination were allowed to rest their forearm in its natural position. Elbow flexor muscle activation was collected at 500 Hz using a surface electromyography (sEMG) sensor (MA100, Motion Lab Systems). The sensor information and dimensions can be found on Motion Lab Systems website.10 The sensor was cleaned with alcohol prep pads. The electromyography (EMG) electrode connected to an EMG preamplifier (Z03, Motion Lab Systems) was placed on the skin over the mid-belly of the elbow flexor, in the direction of the muscle fibers. The electrode was secured in place using adhesive tape (3 M Tegaderm 1624W transparent film dressing frame style tape), and then held firmly in place with athletic prewrap (Cramer tape, SKU: 214546). The EMG signals were processed using an MA300-XVI data acquisition system (Motion Lab Systems) connected into a National Instruments data acquisition (DAQ) system. A custom DAQ program developed on LabVIEW 2019 was used to collect and display the data.
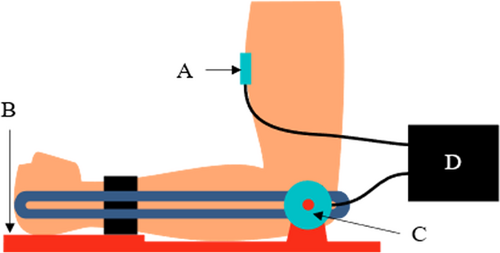
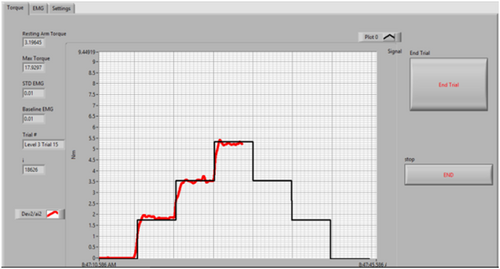
Isometric muscle force data was collected. Data collection included a quiescent trial, a maximum voluntary contraction (MVC) trial and eight stairstep trails. The subject was instructed to match their elbow torque to a target torque, which was a predefined percentage of their maximum torque. An on-screen biofeedback displayed the real-time trace of the target torque as well as the subject's output torque (Figure 2). No EMG data were displayed to the subject. The stairstep trails included varying levels of torque ranging from 10% to 40% with 5% increments (Table 1). The stairstep pattern challenged the subjects to maintain their torque output at each level for 5 s.
| Trial | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| 1 | 10 | 15 | 20 | 15 | 10 |
| 2 | 15 | 20 | 25 | 20 | 15 |
| 3 | 10 | 20 | 30 | 20 | 10 |
| 4 | 20 | 25 | 30 | 25 | 20 |
| 5 | 15 | 25 | 35 | 25 | 15 |
| 6 | 25 | 30 | 35 | 30 | 25 |
| 7 | 20 | 30 | 40 | 30 | 20 |
| 8 | 30 | 35 | 40 | 35 | 30 |
2.3 Data analysis
The time series data were analyzed using MATLAB 2020b (MathWorks). A resting sEMG signal was collected for 5 s to establish an average and SD of the quiescent sEMG signal. A signal >3 SD of the quiescent level was taken to be EMG threshold level.11 The sEMG data were filtered with a 4th order IIR bandpass Butterworth filter (15–200 Hz). An envelope for the sEMG data was calculated using a 5th order IIR lowpass Butterworth filter (10 Hz). The data filters followed the recommendations of the International Society of Electrophysiology and Kinesiology.12 The sEMG data were normalized using the highest sEMG envelope value recorded during the entire experiment.
The subject-generated torque data were compared with each trial's target. The time when the generated data was within ±5% of the target torque was determined. The sEMG envelope were averaged for each trial and defined as “Mean EMG.” The variation in muscular activation was assessed using the coefficient of variation (COV) of the sEMG data, which is the SD of the data normalized by the mean of the data. The Mean EMG and COV values were determined for each subject group and torque percentage (henceforth “Levels”).
2.4 Statistical analysis
The Mean EMG and COV were analyzed in R.13 A two-factor mixed analysis of variance (between Groups and within Levels) was performed. Mean EMG and COV were the dependent variables, and the Groups and Levels were independent variables. Significance level was set to 95% (p < 0.05). Post hoc tests (simple main effect test) were performed if a significant interaction was observed. Multiple comparisons usually incur Type I errors. Hence, Sture Holm's method14 was used to adjust the p values to avoid rejecting a true null hypothesis. Logistic regression analysis was performed on the data to assess the relationship between the categorical independent variables (Levels) and continuous dependent variables (Mean EMG, COV) within each group. The log odds of increase in the dependent variable and the statistical significance of the increase were calculated.
3 RESULTS
The subjects included in the repair and Control groups were similar in age, height, and weight (Table 2).
| Repair group (mean ± Std.) | Control group (mean ± Std.) | Statistical difference | |
|---|---|---|---|
| No. of subjects | 11 | 11 | – |
| Height (m) | 1.76 ± 0.09 | 1.74 ± 0.09 | p = 0.633 |
| Weight (kg) | 89.03 ± 20.35 | 78.79 ± 16.28 | p = 0.208 |
| Age (years) | 38.18 ± 14.57 | 38 ± 12.98 | p = 0.976 |
| Time since surgery (Months) | 18.86 ± 10.30 | NA | NA |
| mBMRC grade (N) | 2(5); 3(2); 4(4) | NA | NA |
| Type of procedure (N) | Ulnar nerve transfer (7); | NA | NA |
| Sural graft (2); | |||
| Spinal accessory nerve transfer (2); |
- Abbreviations: ANOVA, analysis of variance; mBMRC, modified British Medical Research Council.
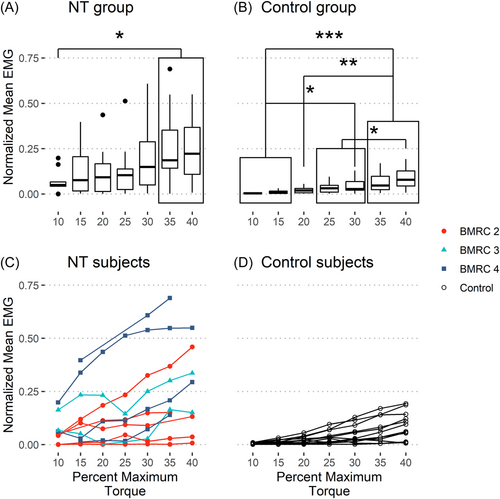
To evaluate the difference between the Control and the Repair group's neuromuscular control during isometric torque generation at the elbow, the normalized mean EMG and COV data were analyzed. The normalized mean EMG was different between the groups (p = 0.028) and levels (p < 0.001) with no interaction between the groups and levels (p = 0.08). Levels (p = 0.042) and the interaction between groups and levels (p = 0.042) were significant for the COV data, while Groups were not significant (p = 0.304). Hence, all the subjects exhibited significantly different neuromuscular control depending on the demanded elbow torque. The repair group had an average neuromuscular activity 4.25 times higher than the Control group, but their variability in neuromuscular activity was similar.
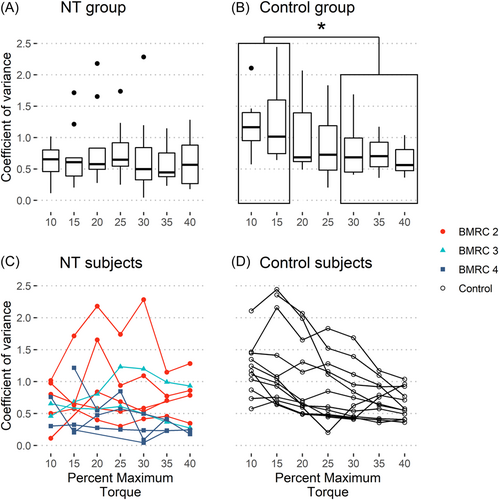
Additional analysis was done to determine if the groups could modulate their muscular activation based on the demanded elbow torque. The Mean EMG for the Repair group showed a weak positive relationship with the demanded torque (Figure 3A), with no such relationship for the COV data (Figure 4A). The Control group showed a strong positive relationship for mean EMG and a moderate negative relationship for COV with respect to the demanded torque (Figures 3B and 4B, with individual subject data plotted in Figures 3D and 4D). The data for the Repair group did not show any trend when subdivided by BMRC grades (Figures 3C and 4C). Hence, the Control group could better modulate their muscular activation, increasing average muscular activation, and decreasing variability with demanded torque.
4 DISCUSSION
This study demonstrated a difference in motor control between patients with a BPI who had either nerve grafting or transfer to their bicep brachialis muscle and healthy individuals. The Control group increased their myoelectric drive as the torque demand increased, while the subjects with a nerve transfer or grafting only generated a weak increase in the myoelectric drive with increased demand.
Variability of the myoelectrical drive decreased as the torque demand increased for the Control group to maintain the elbow flexion torque output level. Such a decrease in variability was not observed in the Repair group. Fallentin et al. found that at lower levels of activation (10% MVC) the fine wire EMG data for the brachial biceps muscle spiked at higher amplitudes than at higher levels of activation (40% MVC).15 This is similar to the trend in the COV data seen in the Control group. Fallentin et al. stated that the higher amplitude spikes were due to “motor unit rotation” which was absent at 40% MVC. Hence, due to the nature of reconstruction performed on the Repair group's nerves, the motor unit rotation in their brachial biceps muscle was flawed.
The Repair group had a muscle activation pattern that weakly mimicked the Control group's pattern. The average muscular activation for the Repair group showed higher odds of increasing compared with the Control group as torque demand increased, indicating the Repair group had to exert more effort to achieve similar results. The reason for this difference could be attributed to the surgical procedure as there is often donor nerves that intuitively flex the elbow that are available to use. There are typically not enough donor nerves to transfer to both the biceps and brachialis motor nerve branches during nerve transfer surgery. Thus, the biceps motor branch is preferentially selected. Even if both the biceps and brachialis motor branches are reinnervated, motor outcomes are not significantly different.16-18 It has been documented that 83% of the subjects with nerve transfer recover following the surgical procedure and are satisfied with the surgical outcome.19 Six patients in a study conducted by Mackinnon et al. with a postsurgery follow-up range of 13–43 months showed a mean time nerve reinnervation to be 5.5 months with a BMRC Grade of 4 at an average of 20.5 months.20 The subjects in the Repair group of this study were, on average, 18 months postsurgery. Therefore, it can be surmised that the subjects were close to maximal recovery at the time of data collection. It is unlikely that a longer recovery period would improve the Repair group's response and sEMG variability based on the clinical course of nerve reinnervation and functional outcomes we have observed over the past 21 years.
The subjects recruited for the Repair group were strong enough for the experiment, based on the senior surgeon's assessment. The traditional means of accessing a patient's motor function is the BMRC's grade scale or its numerous modifications. Brown et al. showed that the process of muscle function recovery is multifaceted and the BMRC grade excludes multiple factors that play into the functional outcome of BPI surgeries.21 A study accessing nerve regeneration in monkeys showed that the time between nerve lesion and reinnervation was an important factor in recovery.22 Such a relationship between successful outcomes and time was also noted in humans.1, 23 It is worthwhile to note that there was no apparent difference in the neuromuscular control between the different BMRC grades (Figures 3C and 4C). Hence, there is a need for a quantitative measurement of motor recovery.
The limitations of this study also need to be recognized. The number of subjects recruited for the study was small. The nerve transfer/grafting of brachial plexus injuries is a relatively rare procedure and a sample size of eleven patients is a relatively large number at a single center. It is common for studies on BPI to have a small sample size. Brown et al. had a sample size of six,21 Tsai et al. had a sample size of nine per group,24 and Estrella's study consisted of just nine nerve transfer subjects.25 Studies with a higher subject population have been retrospective over multiple years.19, 26 Also, none of these other studies examined neuromuscular control at the elbow joint. The convenience sampling technique applied for recruitment of subjects was another limitation. The groups could have been further subdivided into the type of nerve/procedure used, time since surgery, and so forth. However, a bigger subject population would be needed. The Repair group was not subdivided into single versus double nerve transfer procedures, since Carlsen et al. did not find any significant differences between the outcomes of the two procedures.16 In the future, it would be worthwhile to evaluate the neuromuscular control aspect of these two types of nerve transfers, however, it is also important to recognize the nuances that patients with less severe BPI often have more nerve transfer options than patients with severe BPI. It is also recommended that the relationship between BMRC and objective neuromuscular control be further studied, since no apparent difference was observed in the current study.
The subjects in the Repair group achieved similar, although weakly correlated, elbow torque profiles, as normal individuals, using unconventional means of muscle recruitment. Physical therapy and rehabilitation approaches for surgical patients after a BPI play an important role in determining their motor control strategies. Patients with BPI undergo extensive physical therapy to reduce muscle atrophy and secondary deformities.27 These rehabilitative activities can also be performed using robotic rehabilitation devices. Orthotic devices for elbow rehabilitation have been applied in stroke patients.28-30 Application of similar devices for BPI are rarely discussed due to the variable nature of the surgical outcomes. Quantitative neuromuscular control studies on patients with BPI will aid in the development of such orthotic devices. The findings in this study demonstrates that the patient with a nerve transfer has a different neuromuscular control compared with normal individuals. To gauge the efficacy of a purpose-built orthosis, it needs to be tested on a representative patient population. Testing such a device on normal individuals (which is the norm for preliminary device testing) may not be accurate. Hence, a targeted approach toward development of an orthotic rehabilitative device for BPI is recommended.
5 CONCLUSION
The study explored the differences between the elbow neuromuscular control in unimpaired subjects compared to patients with a BPI. The results demonstrated that the patients with a BPI had a different muscular activation strategy when compared to control subjects. The average amount of muscle activation magnitude was disorganized for varying torque demands for patients with a BPI while the control subjects showed a definite positive relationship between their activation magnitudes and torque output. Further, the unimpaired subjects exhibited decreasing variability as torque demand increased, indicating improved neuromuscular control with increasing torque output. This same reduction was not detected in patient with BPI who had received nerve transfer or graft surgery. Further research into the motor control capability of patients with BPI would be useful for their rehabilitation.
AUTHOR CONTRIBUTIONS
Sandesh Bhat collected and analyzed the data. Emily Miller collected the data. Alexander Shin and Kenton Kaufman guided the study design and analysis. All the authors were involved in writing the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Griffin Mess for his contributions in the data acquisition program; Mr. Eric Noonan for his contributions in data collection; Mr. Paul Kane for his efforts in designing and building the test setup; Mrs. Michelle Loosbrock at the Brachial Plexus Clinic, Mayo Clinic, Rochester, MN, USA. Research funding for this study was provided by Department of Defense Award Number W81XWH-20-1-0923, the W. Hall Wendel Jr Musculoskeletal Research Professorship, and a generous Mayo Clinic benefactor who wishes to remain anonymous. The sponsors were not involved in the planning, execution, or analysis of this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.