Age-related cellular and microstructural changes in the rotator cuff enthesis
Abstract
Rotator cuff injuries increase with age. The enthesis is the most frequent site of rotator cuff injury and degeneration. Understanding age-related changes of the enthesis are essential to determine the mechanism of rotator cuff injuries, degeneration, and to guide mechanistically driven therapies. In this study, we explored age-related cellular changes of the rotator cuff enthesis in young, mature, and aged rats. Here we found that the aged enthesis is typified by an increased mineralized zone and decreased nonmineralized zone. Proliferation, migration, and colony-forming potential of rotator cuff derived cells (RCECs) was attenuated with aging. The tenogenic and chondrogenic potential were significantly reduced, while the osteogenic potential increased in aged RCECs. The adipogenic potential increased in RCECs with age. This study explores the cellular differences found between young, mature, and aged rotator cuff enthesis cells and highlights the importance of using age-appropriate models, as well as provides a basis for further delineation of mechanisms and potential therapeutics for rotator cuff injuries.
1 INTRODUCTION
The incidence of musculoskeletal system diseases increases with age, resulting in severe joint pain, dysfunction, and a large socioeconomic burden.1 With age musculoskeletal tissues are prone to injury, degeneration, and slow healing.
Shoulder rotator cuff tears are one of the most common musculoskeletal clinical disorders, and the incidence increases rapidly after age 50.1 There are 4.5 million shoulder injuries per year in the United States, of which 70% are due to rotator cuff tears.2 Rotator cuff tears result in chronic pain, tissue dysfunction, and reductions in mobility and activity, and thus impair healthy aging.2, 3
Rotator cuff injuries are often found at the tendon-bone interface (i.e., the enthesis). The enthesis is a composite tissue with a gradient of the tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone.1, 4, 5 This transitional zone from tendon to bone is typified by well-organized tendon fibers splayed out into thinner enthesis fibers, which are firmly attached to the bone.6-10 This structure exhibits unique functions in flexibility and reducing stress concentration between tendon (soft) and bone (hard) tissue, thus protecting the tendon from fatigue injury at its insertion during shoulder movement.11
It is well understood that tendon and bone undergo degenerative processes with age. However, it remains unclear how the microstructure and cellular functional properties of the enthesis changes with age. Rotator cuff tears can occur at any age but are increasingly evident with aging.1, 12 The repair and restoration of the functionality of the enthesis after injury and degeneration remain a significant clinical challenge.
In this study, we hypothesized that the cells from rotator cuffs of aged individuals would have reduced proliferation, migration, tenogenic, and osteogenic potential, as compared to cells from younger individuals. To explore this, we used a rat shoulder rotator cuff model. We isolated rotator cuff enthesis cells (RCECs) from young, mature, and aged rats. RCECs are a mixed population of cells that are found in the rotator cuff, including tenogenic, chondrogenic, and osteogenic cells. To our knowledge, this is the first time that primary cells isolated from young, mature, and aged rotator cuff entheses have been assessed for proliferation, migration, colony formation, and progenitor potential.
2 METHODS
2.1 Sample preparation
Supraspinatus-enthesis-bone complex (supraspinatus were used here for its particular vulnerability in aging process13) were dissected from male Sprague-Dawley rats immediately after sacrifice from other Institutional Animal Care and Uses Committee-approved studies. All samples were divided into three groups according to the rats' age: Young adult group (Y-group 8–12 weeks), Mature group (M-group 12–15 months), and Aged group (A-group 20–24 months).
2.2 Histology and image analysis
Five samples from each group were fixed in 4% paraformaldehyde and decalcified in decal solution for 10 days, then embedded in paraffin and cut into ~7 µm thick slide. H-E, Toluidine Blue and Picrosirius red staining were conducted following the manufacturer's protocols. Slides were observed with 40 times magnification under a light microscope and images were collected by a camera mounted on the microscope. All measurements were performed by two independent researchers. One hematoxylin and eosin (H&E) stained slide and one Toluidine blue stained slide from each sample were used for cell counting and area measurement. Briefly, the enthesis was divided into the mineralized and nonmineralized zone by the tidemark, the region between tidemark and subchondral bone was identified as the mineralized zone and the region distal to the tidemark was identified as the nonmineralized zone. Both regions were outlined according to these criteria. The number of cells in each region were manually counted using the Photoshop counting tool. Areas of mineralized/nonmineralized regions were quantified. For Picrosirius red staining, a polarized light scope was used to observe collagen fibers. Ten square regions were randomly selected from each slide and the grayscale value was measured using Image J software.
2.3 Rotator cuff enthesis derived cells isolation
Isolation of rotator cuff enthesis-derived cells (RCECs) was based on the method of Harada et al. with minor modifications.14, 15 Briefly RCECs were derived from four different individuals in each group. Immediately after sacrifice, the supraspinatus and infraspinatus tendons were cut along the tendon-bone conjunction. The conjunction tissue was cut into small fragments and cultured on 75 mm plates with Dulbecco's modified Eagle medium (Thermo Fisher Scientific) containing 10% fetal bovine serum (Sigma-Aldrich) and 1% antibiotics and incubated at 37°C in 5% humidified CO2 incubator. After 2 weeks of culture, tissue fragments were removed, and the culture medium was changed every 3 days and cells were passaged when they reach 80% confluent and assessed at passage 3 (P3).
2.4 Proliferation and migration assay
Proliferation and migration assays were completed. Briefly, RCECs (n = 4) were plated in triplicate on 12-well plates at 5 × 104 cells/well and cultured and monitored using an IncuCyte system (IncuCyte S3 Live-cell analysis) with automated image acquisition every 8 h until confluence. For the migration assay, a scratch was made using 100 µl tips and the migration of cells was recorded every 8 h for 2 days. Four locations were randomly selected to measure the migration area. The culture medium was changed every 3 days.
Proliferation was assessed using a Cell Counting Kit-8 (Dojindo) using the IncuCyte system. Briefly, RCECs were seeded in 96-well plates at a concentration of 1000 cells/well. Cells were stained with CCK-8 following manufacturer's protocols on days 1, 3, and 5.
2.5 Colony-formation unit (CFU) assay
The CFU assay was conducted to evaluate self-duplicate capacity. Briefly, RCECs (n = 4) were triple-plated on 60 mm plates in triplicate at 100 cells/dish with media half-changed every 3 days. After 10 days of culture, RCECs were fixed with 4% PFA for 15 min and stained with crystal violet for 10 min (Sigma-Aldrich) and counted using a light microscope.
2.6 Osteogenic differentiation
RCECs (n = 4) were triple-plated in 12-well plates at a concentration of 5 × 104 cells/well and cultured with osteogenic differentiation medium (StemPro® Osteogenesis Differentiation Kit; Gibco). The cells cultured in basal culture medium served as controls. The culture medium was changed every 3 days. After 21 days of culture, osteogenic differentiation was evaluated by Alizarin Red S, lineage-specific genes (Osteopontin (Spp1), RUNX Family Transcription Factor 2 (Runx2), Osteocalcin (Bglap), primers sequences are listed in Supporting Information Material 1) were evaluated by quantitative reverse transcription polymerase chain reaction (q-RT-PCR).
2.7 Adipogenic differentiation
RCECs (n = 4) were triple-plated in 12-well plates at a concentration of 5 × 104 cells/well and cultured with adipogenic differentiation medium (StemPro® Adipogenesis Differentiation Kit; Gibco). The cells cultured in basal culture medium served as controls. The culture medium was changed every 3 days. After 21 days of culture, adipogenic differentiation was evaluated by Oil red-O staining of the cellular accumulation of neutral lipid vacuoles. Lineage-specific genes (Peroxisome Proliferator Activated Receptor Gamma [Pparg], adipocyte protein 2 [Ap2], lipid transfer protein 1 [Lpl], and primers sequences are listed in Supporting Information Material 1) were evaluated by q-RT-PCR.
2.8 Chondrogenic differentiation
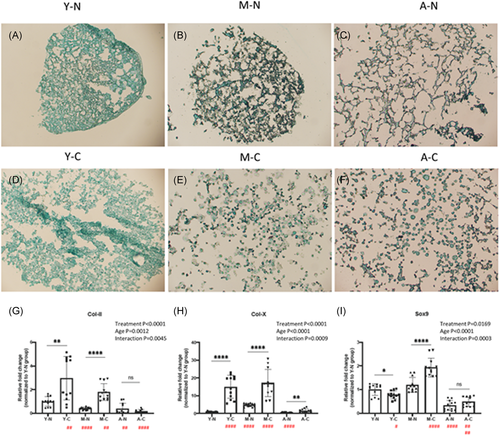
Chondrogenic potential was assessed using 8 × 105 RCECs (n = 4, three repeats) centrifuged at 1500 rmp for 3 min in a 15-ml polypropylene tube to form a pellet. Cell pellets were cultured with a chondrogenic differentiation medium (StemPro® Chondrogenesis Differentiation Kit; Gibco). The cells cultured in basal culture medium served as controls. The culture medium was changed every 3 days. After 21 days of culturing, chondrogenic differentiation was evaluated by toluidine blue staining, the pellets were embedded in OCT and cryo-sectioned for microscopy. Lineage-specific genes (collagen type II [Col-IIa1], SRY-Box Transcription Factor 9 [Sox9], collagen X [Col-X], and primer sequences are listed in Supporting Information Material 1) were analyzed by q-RT-PCR.
2.9 Tenogenic differentiation
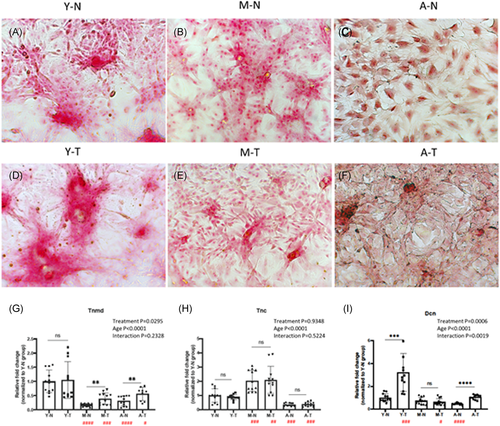
RCECs (n = 4) were triple-plated in 12-well plates at a concentration of 5 × 104 cells/well. and cultured with a 50 ng/ml BMP12 (catalog# 120-37; peproTech). The cells cultured in basal culture medium served as controls. The culture medium was changed every 3 days. After 14-day culturing, tenogenic differentiation was evaluated by Picrosirius red staining of collagen deposition. Lineage-specific genes (Tenomodulin [Tnmd], Tenascin [Tnc], Decorin [Dcn], and primer sequences are listed in Supporting Information Material 1) were evaluated by q-RT-PCR.
2.10 Gene expression
For gene expression analysis total RNA was isolated by Trizol (Invitrogen Life Technologies; Thermo Fisher Scientific), and the RNA was reverse-transcribed into cDNA using an iScriptTM cDNA Synthesis Kit (Bio-Rad Laboratories). Quantitative PCR was performed with RT2 SYBR Green qPCR Master Mix (Qiagen), using an AC1000 Touch™ Thermal Cycler (Bio-Rad Laboratories) under the following cycling conditions: 95°C for 10 min followed by 40 cycles of amplification (95°C for 15 s and 60°C for 1 min). Expression levels were geomean normalized to β-actin, Gapdh, and Tbp. Samples were compared using the ΔΔCt method.
2.11 Statistical analysis
Data are presented as mean ± SD and were tested for normality. Statistical analyses of the histological quantification were performed using the Kruskal-Wallis test. Two-way analysis of variance and multiple comparisons were used to compare the CCK8, proliferation, migration, CFU, and gene expression results among groups. Relative gene expression was normalized to the young nondifferentiated group. Differences between all treatments and between each pair of treatments (undifferentiated and differentiated) were examined by multiple comparisons. All statistical analyses were performed with SPSS software (version 8.2.1; SPSS Inc.); a p value <0.05 was considered significant.
3 RESULT
3.1 Histological age changes in rotator cuff
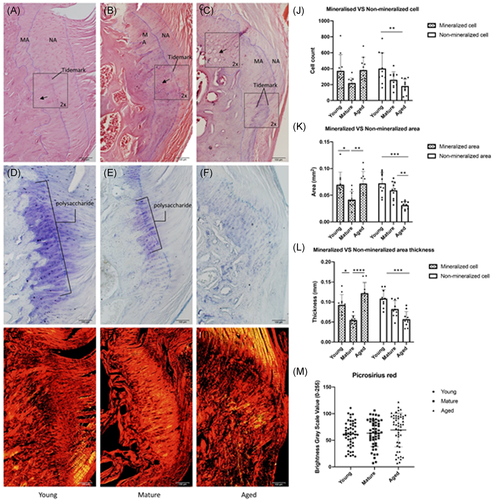
Histological assessment of enthesis morphology, cellular fraction, mineralization, and collagen alignment was assessed with H&E, Toluidine blue and Picrosirius staining. Samples were divided into three groups according to age (young group: 8–12 weeks, mature group: 12–15 months, aged group: 20–24 months). In the young group, the margins of the enthesis are well defined with small and well-aligned cells. The tide mark dividing the mineralized area and nonmineralized area is clear and sharp (Figure 1A), large defined polysaccharide regions were found in toluidine blue-stained sections (Figure 1D). In the mature group, the structure of enthesis remained intact, but with increased cellular disorder and decreased polysaccharide expression (Figure 1B,E). In the aged rotator cuff the cellular organization and tidemark were increasingly disorganized and the fibro-chondrocytes in mineralized area enlarged and disorganized. The polysaccharide region is substantially reduced (Figure 1C,F). Quantification of histological data is shown (Figure 1J). Data are reported as mean ± SD. Cell counts in the mineralized area are 372.7 ± 202.87, 220.4 ± 63.11, and 383.3 ± 159.06 in young, mature, and aged groups, respectively (p = 0.0362). In the nonmineralized area, the cell counts are 401 ± 197.14, 260 ± 100.88, and 182.5 ± 89.23 young, mature, and aged groups, respectively (p = 0.0094). Mineralized areas in young, mature, and aged groups are 0.069 ± 0.024 mm2, 0.042 ± 0.015 mm2, and 0.072 ± 0.023 mm2 (p = 0.0037) while nonmineralized areas are 0.072 ± 0.020 mm2, 0.059 ± 0.017 mm2, and 0.031 ± 0.007 mm2, respectively (p = 0.0001) (Figure 1K). The thickness of the enthesis was measured as the mean of three randomly selected locations. Mineralized thickness in these three groups are 0.092 ± 0.026 mm, 0.055 ± 0.011 mm, and 0.125 ± 0.027 mm (p < 0.0001) in the young, mature, and aged groups. Nonmineralized area, they are 0.109 ± 0.021 mm, 0.083 ± 0.021 mm, and 0.057 ± 0.019 mm (p = 0.0004), respectively (Figure 1L). Brightness grayscale values were measured using Image J software based on picrosirius red staining figures (Figure 1G–I), 61.51 ± 23.54, 63.41 ± 25.14, and 69.38 ± 31.71 for young, mature, and aged groups (p = 0.1554).
3.2 Proliferation and wound healing assay
Rotator cuff enthesis cells (RCECs) were isolated and cultured from young, mature, and aged rats. Cells were cultured and assessed using proliferation and migration assays. After 10–14 days of culture, radially clustered, spindle-shaped cells were observed, exhibiting a fibroblast-like morphology. This feature is clear in the young group (Figure 3A) but diminished with age. In the aged group, the RCECs are rounded andenlarged (Figure 3C).
Next, we assessed the proliferation potential of P3 RCECs. Here we found that young RCECs showed a significantly higher proliferation rate as compared to mature and aged RCECs as evaluated by CCK8 proliferation assay (Figure 2B) and fold change assessed by IncuCyte phase object confluence analysis (Figure 2C).
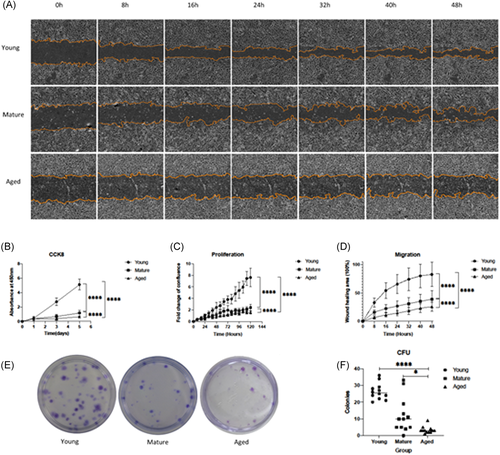
We further assessed RCECs migration potential. Our migration assay shows that young RCECs have a significantly increased migration rate as compared to mature and aged cells. After 48 h of culture, 7 out of 16 selected areas fully bridged the scratched region in young RCECs cultures, compared to the mature RCECs, which had a moderate migration of 59% healing area, while aged RCECs only had 40% migration (Figure 2A,D).
3.3 Colony-forming units
CFUs are another measure of proliferative potential. The proportion of cells with self-replicating potential was determined by counting the number of colonies, to evaluate the overall cell proliferation potential. Here we assessed the ability of RCECs to form CFU (Figure 2E). Young RCECs formed significantly more CFUs than mature and aged RCECs. The mean CFU for young RCECs was 26.33 ± 4.89 units per 100 cells, significantly higher than both the mature (12.17 ± 10.51 units per 100 cells, p < 0.01) and aged groups (3.33 ± 2.06 units per 100 cells, p < 0.001) (Figure 2F). Further, we noted that the size of colonies formed in the young group was larger than those in mature and aged RCECs.
3.4 Multidifferentiation potential
RCECs in all three age groups had the ability to differentiate into osteogenic, adipogenic, and chondrogenic lineages. However, mature RCECs and aged RCECs did not differentiate into tenogenic lineage in contrast to young RCECs.
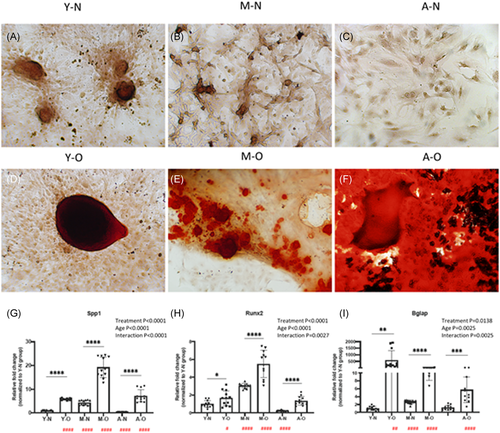
Calcium accumulation, demonstrated by Alizarin red staining, was formed in all ages of osteogenic RCECs (Figure 3D–F). Lineage-specific osteogenic genes (Spp1, Runx2, Bglap) were upregulated in all differentiated groups, interactions between age and treatment (osteogenesis) were significant (Figure 3G–I).
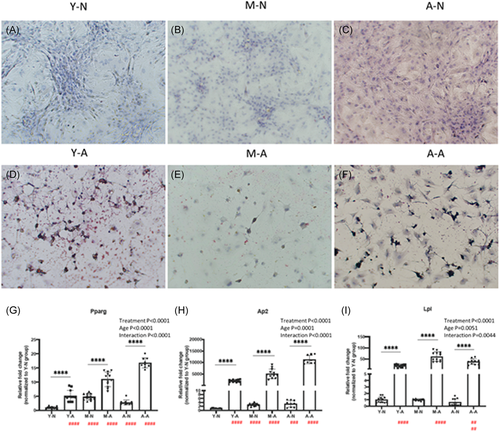
Adipogenesis was verified by Oil red staining, red-stained lipids were found in all ages of adipogenic RCECs (Figure 4D–F). Lineage-specific adipogenic genes (Pparg, Ap2, Lpl) were upregulated in all differentiated groups, interactions between age and treatment (adipogenesis) were significant (Figure 4G–I).
Chondrogenesis of RCECs was assessed by Alcian blue staining and mRNA expression of lineage-specific genes (collagen II, collagen X, Sox9). Before chondrogenic inducement, RCECs demonstrated a connected, reticular fibroblast-like morphology. After 21 days of chondrogenic treatment, RCECs exhibited rounded, granular chondrocyte-like phenotype in all ages of chondrogenic groups (Figure 5D–F). Lineage-specific chondrogenic genes were upregulated in young and mature chondrogenic RCECs. However, Col-II and Sox9 were not upregulated in aged chondrogenic RCECs, only COL-X was upregulated in aged chondrogenic RCECs. Interactions between age and chondrogenic treatment were significant (Figure 5G–I).
RCECs were treated with BMP12, a proposed tenogenic inducer, for 14 days.16-18 Picrosirius red collagen staining was performed to visualize the tenogenic differentiation. Young RCECs showed strong collagen staining, which was enhanced after BMP12 induction (Figure 6A,D). However, there was less staining in the mature and aged groups after BMP12 treatment. The aged RCECs displays enlarged, rounded, isolated morphology before treatment (Figure 6C), but became more spindle-shaped and clustered after BMP12 induction (Figure 6F). Lineage-specific gene, Tnmd, was expressed significantly higher in the young RCECs with or without linage induction (Figure 6G) and were upregulated in BMP12 induction in the mature and aged groups. Interestingly, for Tnmd, there were no significant interactions between age and treatment, while both separately were significant. Tnc, interactions between age and treatment were not significant (Figure 6H). The expression of Dcn was upregulated after BMP12 induction in young RCECs and aged RCECs. Expression of Dcn was much lower in mature and aged RCECs regardless of BMP12 induction. Interactions between age and treatment were significant (Figure 6I).
4 DISCUSSION
This study explored the age-related cellular and microstructural changes found in the rotator cuff enthesis using a rat model. The histological assessment showed the typical gradient structure of entheses was found in our models, including the fibrous structure of tendon, nonmineralized fibrocartilage, and mineralized fibrocartilage and bone. With age, well-aligned collagen fibers in the rotator cuff enthesis become increasingly disorganized. Further with age, this enthesis zone narrows, substantially decreasing the contact area between the tendon and bone.
There was a clear boundary between bone tissue and fibrocartilage in the enthesis, which is evident with toluidine blue staining. In fibrocartilage, the polysaccharide in the matrix stains blue, which is not found in the bone. Chondrocytes/fibro-chondrocytes synthesize polysaccharides. Polysaccharides allow the ECM to be permeable and absorb more water, which helps the tissue resist compressive loads. With age, the polysaccharide area diminishes, resulting in poor mechanical performance.
The fibrocartilage is further divided into mineralized and nonmineralized areas, separated by a tidemark.19 Cells in the mineralized fibrocartilage are more differentiated, rounded, and hypertrophic,20, 21 while in nonmineralized fibrocartilage, they are smaller and aligned in columns, similar to immature chondrocytes.22, 23 In our findings, the nonmineralized fibrocartilage and cellularity decrease with age. The nonmineralized fibrocartilage plays an important role in dissipating the stress upon loading,24-26 so a decrease in the size of this zone may result in a greater risk of injury. Further, the reduced number of cells in nonmineralized fibrocartilage may indicate reduced regeneration potential. The tide mark becomes increasing disorganized with age, which reflects the resultant tears and attenuated mechanical properties.27 Collectively, these structural changes weaken the elastic buffer effect of the enthesis, making it more fragile and prone to injury.
Structural changes are mitigated in large part by cellular changes that contribute to the rotator cuff injuries that are found with aging. There are three dominating aging cellular changes: (a) decreased proliferation rate, (b) loss of wound-healing/migration capability, and (c) shifted differentiation potential.
Uniquely in this study, we isolated primary cells from the rotator cuff enthesis, instead of focusing on specific cell types, as this more accurately represents the cellular diversity found in the enthesis. The cell types can include cells from the bone, cartilage, fibrocartilage, tendon, stromal progenitors, adipose, and immune system. Assessing the different lineages separately limits the ability to assess the overall impact of this composite tissue. The assessment of the primary enthesis cells provides a broader picture of enthesis as an integrated tissue, which is the functional unit of the tendon-bone interface. The primary RCECs were assessed for migration, proliferation, and differentiation potential. Here we found that RCECs derived from mature and aged rats have significantly decreased proliferative, colony-forming, and migrating potential as compared to the RCECs from young animals. In the young enthesis when micro-wounding occurs,28 the RCECs can quickly proliferate and repair the damage, so as to avoid further tears of the rotator cuff. However, in the aged enthesis, this ability is weakened, the damage cannot be repaired effectively in time, consequently, the rotator cuff tear can more readily propagate.
Assessment of RCECs in different age groups showed that each group retained their multilineage differentiation potentials; however, the potential for differentiation shifted. Significant interactions were found between treatment and age in these differentiation experiments. Mature and aged RCECs demonstrated high differentiative potential toward osteocyte and adipocyte, as shown in the lineage-specific gene expression and histological staining.
After chondrogenic differentiation, lineage-specific markers of RCECs increased significantly in young and middle-aged groups, but not in the aged group. Only Col-X was upregulated in aged chondrogenic RCECs. This type of collagen is synthesized by hypertrophic chondrocytes, which is evident in aged chondrocytes.20, 21, 29
Assessing the role of tenogenic lineage capacity is more challenging. To date, there is no established method for this tenogenic assessment. Some reports have suggested tenogenic induction using BMP12.16-18 Although mature and aged RCECs had some tenogenic potential, tenogenic markers were largely attenuated in mature and aged RCECs. It is important to note that among all tenogenic markers, Tnmd tends to be the most representative biomarker for stemness.30-33 The significant loss of Tnmd in mature and aged RCECs is an essential indicator of the loss of tenocyte-related gene expression and perhaps differentiation.34, 35 Further, the loss of Tnmd can cause premature tendon aging by dysregulating collagen fibrinogenesis and reducing cell proliferation.3637 Interestingly, Tnmd was not upregulated in young RCECs after BMP12 induction. It is possible that RCECs in the young group show a strong phenotype of tendon/tendon stem cells already. In previous studies, Tnc was used as a marker for tendon differentiation,38, 39 but Tnc is not solely expressed in the tendon, being highly expressed in the bone and cartilage as well.40, 41 Our results show that Tnc was not upregulated after BMP12 induction.
The results of the multidifferentiation assays showed that in the aging process, the RCECs gradually lose their chondrogenic and tenogenic potentials. This makes it difficult to form the typical enthesis structure with four distinct zones during repair and regeneration, especially in regard to the fibrocartilage structure.
Aging is the largest risk factor for many degenerative musculoskeletal diseases, including rotator cuff disorders.1, 42 The role of aging in tissue homeostasis in the rotator cuff enthesis has not been well characterized. This unique composite tissue represents a gradient of the tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone.1, 4, 5 As musculoskeletal tissues age, increased matrix degradation, fibrosis, and adipose infiltration are evident.43-45 The etiology of enthesis degeneration represents an important gap in knowledge, particularly the understanding of cellular mechanisms that are required for robust healing and for the development of evidence-based therapeutic strategies. Many biologic and engineering therapeutic strategies have been proposed for rotator cuff enthesis healing and regeneration;46 however, animal model research has largely been focused on juvenile and young animals rather than mature or aged animals, which is a substantial weakness given that age-associated changes are not present in these model systems. It is important to note that during aging systemic and local changes occur including increased accumulation of senescent cells within tissues and systemic increases in sterile inflammation, also termed inflammation, which compromise the ability of tissue to repair and regenerate.
Cellular senescence is a physiological response that limits the proliferation of damaged or aged cells.47, 48 Chronic age and disease-related cellular senescence result from a variety of cellular stresses, such as telomere shortening, reactive oxygen species, mechanical stress, DNA damage, and mitochondrial dysfunction.48-51 The subsequent accumulation of senescent cells has become increasingly implicated in age-related disease pathologies.48, 52 Senescent cells are resistant to apoptosis and exhibit an abundant secretory phenotype, termed senescence-associated secretory phenotype (SASP). Both the accumulation of the senescent cells and the SASP mediate many disease pathologies, including comorbidities, such as metabolic syndrome, which are negatively associated with healing outcomes.53, 54 Additionally another consequence of the increased accumulation of senescent cells is the depletion of stem and progenitor cells.55 The use of age-appropriate models must be considered when assessing age-associated enthesis pathology and therapeutic strategies. The age-related phenotypic changes of mixed cell populations at the enthesis have not been assessed in previous studies, to the best of our knowledge. A strength of this study is assessing the composite mixed population of cells rather than focusing on a specific cell type. This mixed population of primary cells more accurately reflects the enthesis and the changes found with aging. Understanding cellular changes that occur with age will aid in the development of novel targets and therapeutic approaches to improve tendon to bone healing that is essential for effective clinical repair of rotator cuff injuries. A possible strategy to attenuate the damage caused by aging would be the shifting of the aged osteogenic/adipogenic enthesis phenotype to a more fibrogenic phenotype. Approaches to change cellular phenotypes include cell fusion,56 nuclear transfer,57 and micro vesicles-based therapy.58 These cell-derived vesicles have been shown to alter cellular phenotypes in many different cells and tissue combinations.59-63 Ongoing work in our laboratories focuses on determining if promoting fibro-tenogenic differentiation will aid in the regeneration in aged rotator cuff enthesis, which may help reduce age-related injuries and improve native tissue regeneration after repair.
Future work assessing the cellular composition and changes with age in the rotator cuff enthesis, including single-cell RNA sequencing (scRNA-seq), cellular indexing of transcriptomes, and epitopes by sequencing (CITE-seq), as well as spatial transcriptomics techniques will be important tools to determine the cellular age-related changes and help guide therapeutic strategies to promote healing. The technical aspects of cellular isolation from composite tissues, such as the enthesis, is technically challenging, however, should be feasible. Further in vitro work such as primary culture assessments and techniques must reflect the population's changes found in vivo. It is likely that increased senescent cells accumulation within the rotator cuff in vivo will be an important challenge that must be considered while completing in vitro work, as senescent cells are nonreplicative and with passaging will not be present in primary cell culture. During aging the cells of the rotator cuff enthesis have reduced migration, proliferation, and colony-forming potential. Further, with age, the cells of the enthesis have increased adipogenic and osteogenic induction, whereas tendon markers are reduced. These results correspond to the increased fatty osteogenic infiltration found in the enthesis with aging. Taken together, these cellular shifts within the enthesis may be responsible for both the frequent rotator cuff injuries and the limited healing and regeneration that is found in patients as they age.
ACKNOWLEDGEMENTS
This study was funded by an NIH/NIAMS AR07381 grant, Mayo Clinic Career Development Award in Orthopedics Research, China Scholarship Council, the Mayo Clinic Foundation, and Indiana University School of Medicine, Department of Orthopaedic Surgery.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Zeling Long, Peter C. Amadio, Chunfeng Zhao, and Anne Gingery contributed to the conception, study design, and data interpretation. Zeling Long, Koichi Nakagawa, and Zhanwen Wang performed experimental assays. Zeling Long and Anne Gingery completed data analysis. Zeling Long and Anne Gingery wrote the manuscript. All authors contributed to the revision of the manuscript. All authors have read and approved this manuscript.




