The effect of mechanical stress on enthesis homeostasis in a rat Achilles enthesis organ culture model
Abstract
Tendons and ligaments are jointed to bones via an enthesis that is essential to the proper function of the muscular and skeletal structures. The aim of the study is to investigate the effect of mechanical stress on the enthesis. We used ex vivo models in organ cultures of rat Achilles tendons with calcaneus including the enthesis. The organ was attached to a mechanical stretching apparatus that can conduct cyclic tensile strain. We made the models of 1-mm elongation (0.5 Hz, 3% elongation), 2-mm elongation (0.5 Hz, 5% elongation), and no stress. Histological evaluation by Safranin O staining and Toluidin Blue and Picro Sirius red staining was conducted. Expression of sex-determining region Y-box 9 (Sox9), scleraxis (Scx), Runt-related transcription factor 2 (Runx2), and matrix metalloproteinase 13 (Mmp13) were examined by real-time polymerase chain reaction and immunocytochemistry. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling and live/dead staining and was conducted for evaluation of the apoptosis and cell viability. The structure of the enthesis was most maintained in the model of 1-mm elongation. The electronic microscope showed that the enthesis of the no stress model had ill-defined borders between fibrocartilage and mineralized fibrocartilage, and that calcification of mineralized fibrocartilage occurred in the model of 2-mm elongation. Sox9 and Scx was upregulated by 1-mm elongation, whereas Runx2 and Mmp13 were upregulated by 2-mm elongation. Apoptosis was inhibited by low stress. The results of this study suggested that 1-mm elongation can maintain the structure of the enthesis, while 2-mm elongation promotes degenerative changes.
1 INTRODUCTION
Connective tissues including tendons and ligaments are joined to bones through a specialized interface defined as an enthesis.1 The enthesis is vital for the proper function of the respective muscular and skeletal structures.1 A fibrocartilaginous enthesis, such as the Achilles tendons or rotator cuff, has four distinct zones consisting of bone, mineralized fibrocartilage, unmineralized fibrocartilage, and fibrous connective tissue.2
All cell types found at the enthesis are mechanoresponsive. Several reports have demonstrated that the formation of a graded transition between tendon and bone requires physiologic mechanical stress.3, 4 It has been revealed that the postnatal reduction in muscle loading inhibited enthesis development using a paralyzed rotator cuff muscle model induced by injection of botulinum toxin A.3 The reduction of loading also impaired mineral deposition, fibrocartilage formation, and fiber distribution. Although scleraxis (Scx) and SRY-box containing gene 9 (Sox9) are known as tenogenic and chondrogenic transcription factors, respectively,5, 6 these factors are also required for enthesis formation.7, 8
In contrast, several studies have reported that tensile overload can be a factor that leads to degenerative changes and the formation of bony spurs at the enthesis.9-12 Degradative changes in the enthesis caused by overuse were identified in the lateral epicondylitis.12 Bony spur formation can occur by endochondral ossification of enthesis fibrocartilage.10, 13 In endochondral ossification, cells continue to generate a mineralized matrix, and further enhance expression of the maturation makers, including collagen type 10 (Col10), Runt-related transcription factor 2 (Runx2), and matrix metalloproteinase 13 (Mmp13) that regulate chondrocyte proliferation and differentiation.14
Although the relationship between overload and degenerative changes has been reported, the pathogenic mechanisms of enthesis remain unclear. In addition, the role of mechanical stress in the homeostasis of the enthesis is poorly understood. Therefore, the degeneration and homeostasis of the enthesis is becoming a popular topic in the field of orthopedic sports medicine. The aim of this study is to reveal the effects of mechanical stress on the structure of the enthesis in an original Achilles tendon model.
2 MATERIALS AND METHODS
2.1 Animals
All animal experiments were conducted in compliance with a protocol reviewed by the Institutional Animal Care and Use Committee and approved by the President of Okayama University. Specific-pathogen-free female Sprague–Dawley rats (CLEA Japan Inc.) were used in all experiments. Rats were housed in polycarbonate cages (area: 1120 cm2; height: 20 cm) in a temperature- (22–24°C) and humidity- (40%–60%) controlled room. Food and water were provided ad libitum in a 12 h light/dark cycle. We anesthetized and euthanized the rats using 100% CO2.
2.2 Ex vivo model
In vitro, it is difficult to make the graded fibrocartilaginous transition of the enthesis on two- or three-dimensional culture. Whereas, in vivo, it is difficult to arrange mechanical stress on the enthesis using a stretching apparatus, although the graded transition can be kept. We, therefore, used ex vivo models in organ cultures of rats. The organs, including the calcaneus, the enthesis, Achilles tendon, and a part of the gastrocnemius, were taken from 8-week-old rats weighting about 200 g. Subsequently, 3-0 nylon threads were passed through the calcaneus and the gastrocnemius and tied to set in the chamber (Figure 1A,B).
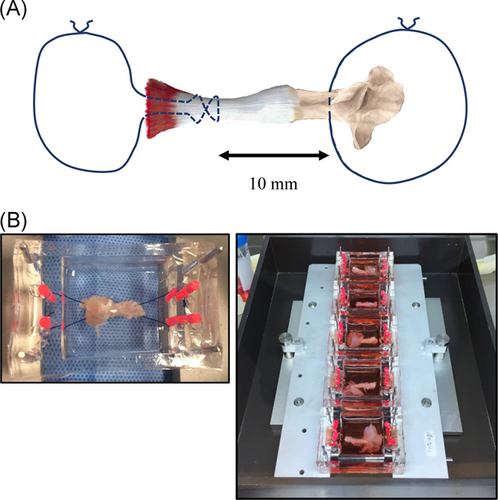
Organs were cultured in 8 ml of alpha minimal essential medium (αMEM; Wako Pure Chemical Industries Ltd.) containing 10% fetal bovine serum (FBS; Hyclone), 100 U/ml penicillin, and 100 mg/ml streptomycin (Nacalai Tesque Inc.) at 37°C in an incubator. The medium was changed every day.
2.3 Cyclic tensile strain on ex vivo model
We prepared an original silicone chamber in which the organ with the Achilles tendon and calcaneus could be set (Figure S1). Mechanical stresses were applied using the ST-140-10 mechanical stretch system (STREX) that can conduct cyclic tensile strain (CTS). To apply mechanical stress, the chamber with the organ was firmly attached with no slack to the stretching apparatus (Figure 1B), which has one fixed side opposite a movable side (Figures 1C and S2). The movable frame was linked to a driven shaft whose amplitude and frequency of stretch were controlled by a programmable microcomputer. We prepared models of 1-mm elongation (0.5 Hz), 2-mm elongation (0.5 Hz), and no stress using the original ex vivo mechanical stress models. We made six organ models for each group in all experiments. CTS was applied continuously in an atmosphere of 5% CO2 and 95% air at 37°C for 4 h every day and continued for a week, while the organs with no stress, which were used as controls, were cultured in dishes. No significant adverse events, such as fracture of the bone, tendon rupture, infection of the organ, and mechanical trouble were observed during CTS.
2.4 Histologic analysis
Organs were decalcified in 0.3 M ethylenediaminetetraacetic acid (pH 7.5) for a week. After decalcification, samples were divided into two blocks along the sagittal plane, dehydrated in a graded ethanol series, and embedded in paraffin. Standard sagittal sections measuring 4.5 μm were prepared and stained with Safranin O. Sections were deparaffinized in xylene and hydrated in a graded series of ethanol solutions in distilled water. They were stained with Weigert's ion hematoxylin working solution (Muto Pure Chemicals Co., Ltd.) for 3 min, and then stained with fast green solution (Nacalai Tesque Inc.) for 15 min after washing. Sections were quickly rinsed with acetic acid and stained in 0.1% Safranin-O solution (Waldeck GmbH & Co., KG, Division Chroma) for 3 min. Thereafter, they were dehydrated and cleaned first with ethyl alcohol (99.5%).
Sections were also stained with Toluidin Blue and Picro Sirius Red to examine enthesis and collagen organization. By evaluating the collagen birefringence and cartilage formation under polarized light microscopy, differences in collagen and cartilage deposition at the enthesis could be detected. Microscope stage rotation was configured at the same angle for obtaining all images, because stage rotation may change the color of the collagen fibers. In this method, thinner and more disturbed fibers were greenish representing collagen type III (Col3), while thicker fibers are shown stained in orange-yellow representing collagen type I (Col1).15 The cartilage was represented as orange-red. In the Toluidin Blue and Picro Sirius Red protocol, sections were stained with 0.05% Toluidin Blue (Muto Pure Chemicals Co., Ltd.) for 30 min after deparaffinization and hydration, and then stained with Van Gieson solution: 100.0 ml of saturated Picric acid solution (Nacalai Tesque, Inc.) and 3.0 ml of 1% Sirius Red solution (Muto Pure Chemicals Co., Ltd.) were mixed for 10 min. Finally, polarized histological images were observed with a light microscope (Olympus System Microscope Model BX53 LED; Olympus Co.) after sections were rinsed and dehydrated.
We also performed transmission electron microscopy (TEM) to examine precisely calcification and cell apoptosis at the enthesis. Before small enthesis fragments were excised under a microscope, we used hematoxylin and eosin staining of the slide made from a part of the organ for TEM to identify the enthesis area. The fragments were fixed with 2.5% glutaraldehyde solution, postfixed in 1% osmium tetroxide, and embedded in EPON epoxy resins. Ultra-thin sections were observed using an H-7650 transmission electron microscope (Hitachi).
2.5 Real-time polymerase chain reaction analysis
To test the effects of CTS on anabolic or catabolic gene expression, we examined the expression of Sox9, Scx, Runx2, and Mmp13 in enthesis by real-time polymerase chain reaction (PCR) after CTS for 1 week.
After CTS, the enthesis of organs was picked up under a microscope and washed with phosphate-buffered saline (PBS). Total RNA was extracted using a QIAGEN RNeasy Mini Kit according to the manufacturer's protocol and reverse-transcribed to complementary DNA (cDNA) using Primescript RT master mix (Takara Bio). The purity and concentration of total RNA were assayed by NanoDrop spectrophotometer (Thermo Fisher Scientific).
Real-time PCR was performed using the Mx3000P QPCR System (Agilent Technologies) with TaqMan Gene Expression Assays for rat. Amplification of Gapdh, a housekeeping gene, was applied to normalize the efficiency of cDNA synthesis and the amount of RNA. Final expression levels were obtained by dividing the expression levels of Sox9, Scx, Runx2, and Mmp13 by the expression level of Gapdh. To clarify the effects of mechanical stress, each value calculated for no stress models was set at one for normalization to the no stress condition. Primers were as follows for TaqMan® Gene Expression Assays (Applied Biosystems): Gapdh (Rn01775763_g1), Sox9 (Rn01751070_m1), Scx (Rn01504576_m1), Runx2 (Rn01512298_m1), and Mmp13 (Rn01448194_m).
2.6 Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling staining
Deparaffinized and dehydrated sections were incubated with bovine testicular hyaluronidase (1 mg/ml, 60 min at 37°C; Sigma-Aldrich) in 0.1 M sodium acetate buffer. Endogenous peroxidase was blocked by immersing samples in 3% H2O2 in PBS at room temperature for 10 min. DNA fragmentation at the enthesis was detected by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) biotin nick end-labeling (TUNEL) method. Sections were labeled with biotin-labeled 16-dUTP (Sigma-Aldrich) using terminal transferase (Sigma-Aldrich) and incubated at 37°C for 60 min. After washing with PBS, sections were subjected to the avidin-biotin-peroxidase complex (ABC) using VECTASTAIN® ABC Kits (VECTOR Laboratories Inc.) at room temperature for 45 min, and reacted with diaminobenzidin (DAB; Histofine simple stain DAB; Nichirei Biosciences Inc.), resulting in a brown color. Sections were counterstained with hematoxylin (VECTOR Laboratories Inc.).
2.7 Live/dead staining
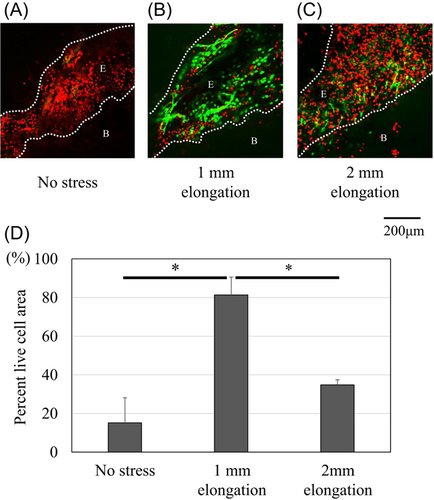
Organs were assessed for cell viability using live/dead staining. After CTS for 7 days, whole organs were incubated for 20 min in 1X PBS containing fluorescein diacetate (8 μg/ml; Sigma-Aldrich) and propidium iodide (35 μg/ml; Sigma-Aldrich) for viable and nonviable cells, respectively. The enthesis of organs was imaged using an LSM780 confocal laser scanning microscope (Carl-Zeiss-Promenade 1007745 Jena). Extra soft tissue around the enthesis was eliminated to ensure penetration into the interior of explants before imaging. The percent live-cell area was quantified using ImageJ software for measurement of the fluorescence intensity ratio (FIR) of live/dead staining. FIRs were measured in four different areas of the enthesis and the values were averaged.
2.8 Immunocytochemistry
Immunostaining for Scx, Sox9, Runx2, Mmp13, and Col10 was performed using the paraffin sections of the enthesis tissue obtained from the models of control, 1-mm elongation, and 2-mm elongation. For immunohistochemistry, deparaffinized and dehydrated sections were pretreated by boiling in 0.1 M of citrate buffer (pH 6.0) at 95°C for 5 min, and allowed to slowly cool to room temperature. Endogenous peroxidase was blocked by immersing samples in 3% H2O2 in PBS at room temperature for 10 min. Rabbit polyclonal anti-Sox9 antibody (5 μg/ml; EMD Millipore Corporation), and anti-Col10 antibody (10 μg/ml; Thermo Fisher Scientific) were used as the primary antibodies, and were incubated with samples at 4°C overnight and washed with PBS. They were incubated with Histofine simple stain rat MAX PO(R) (Nichirei Biosciences Inc.) at room temperature for 30 min. Tissue sections were visualized by DAB (Nichirei Biosciences Inc.), resulting in a brown color. Stained sections made from fresh uncultured organs and those incubated without primary antibodies were used as positive and negative controls, respectively (Figure S4).
Immunopositive cells at mineralized and unmineralized fibrocartilage zones were counted individually in four different areas under a microscope, and the number of cells was averaged. Two observers who were blinded with regard to the experimental group (T. H. and S. N.) conducted the cell counting. The threshold of positive cells was determined interactively by the two observers and then held constant for all viewing sections. The percentage of immunopositive cells was determined by dividing the number of positive cells by the total number of cells within the field.
2.9 Cytokine analysis
To examine whether inflammatory cytokines were released from musculoskeletal cells at the tendon, muscle, bone, and enthesis to the culture medium, we collected the medium after CTS at Days 1, 4, and 7 in culture. The concentrations of tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) in the medium were measured using TNF-α and IL-1β Enzyme-Linked Immunosorbent Assay (ELISA) Kits (Legend Max Rat TNF-α ELISA Kit; Biolegend Inc. and Rat IL-1β Assay Kit; IBL Co., Ltd.) according to the manufacturer's protocols.
2.10 Statistical analysis
All data are expressed as means ± 95% confidence intervals (CIs) and repeated at least six times and similar results were acquired. Differences among groups were analyzed using a one-way analysis of variance (ANOVA) with Bonferroni post-hoc test by R for windows (WWW.r-project.org).
3 RESULTS
3.1 Stress of 1-mm elongation maintained the structure of a graded transition and homeostasis at the enthesis
Figure 2 shows histological sections at the enthesis with Safranin O staining (Figure 2B–E), and Toluidin Blue and Picro Sirius Red staining (Figure 2F–I). Safranin O staining revealed that the chondrocytes at the enthesis were arranged in a columnar pattern perpendicular to the subchondral bone plate in the models of 1-mm elongation (Figure 2D), whereas the organs in no stress models showed no transitional fibrocartilage (Figure 2C). In the models of 2-mm elongation, the transitional structures at the enthesis were poorly organized, although the fibrocartilage was maintained (Figure 2E). Toluidine Blue and Picro Sirius Red staining show the interdigitation of the fibers under polarized light with Col1 stained in yellow, Col3 stained in green, and fibrocartilage stained in orange-red. The graduated structure was maintained in the models of 1-mm elongation (Figure 2H). On the other hand, the models of 2-mm elongation showed clear tide marks that separated unmineralized and mineralized fibrocartilage (Figure 2I). No stress models showed the periosteal fibrocartilage clearly disappearing, or rumpled collagen fibers (Figure 2G), while fresh models showed that the fibrocartilage was identified, and that tendons were arranged in parallel in an organized fashion towards the bone (Figure 2B,F). The contrasting predominance of Col1 was seen in fresh models and the models of 1-mm elongation, and of Col3 was seen in the models of no stress and 2-mm elongation.
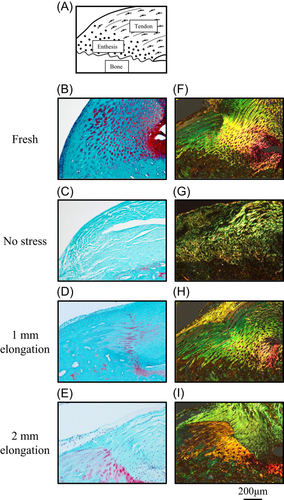
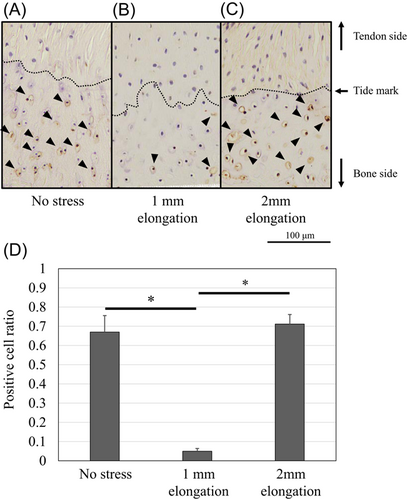
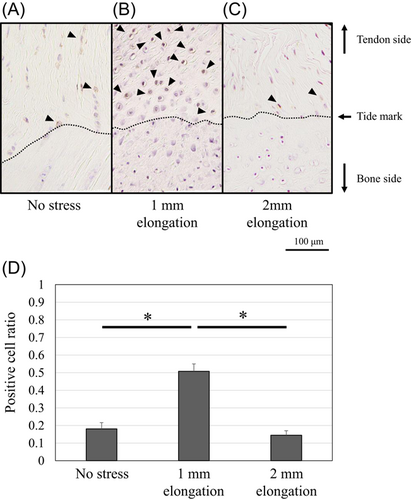
Figure 3 shows that the chondrocytes at the mineralized fibrocartilage zone contained a large number of apoptotic cells in the models of no stress and 2-mm elongation (TUNEL-positive ratio, 0.67 [95% CI: 0.59–0.75] and 0.71 [95% CI: 0.66–0.76], respectively), while chondrocytes rarely showed apoptotic cells in the models of 1-mm-elongation (TUNEL-positive ratio, 0.05 [95% CI: 0.04–0.06]). Electron microscopy also revealed nuclear changes, including chromatin condensation and shrunken nucleus, which indicate apoptosis in the no stress model (Figure 4D). Live/dead imaging showed that the viability of the enthesis was mostly maintained in the 1-mm-elongation model (Figure 5). The percent live-cell area at the enthesis of the 1-mm-elongation model was significantly higher than those of other models (Figure 5D).
3.2 The stress of 2-mm elongation increased calcification of mineralized fibrocartilage
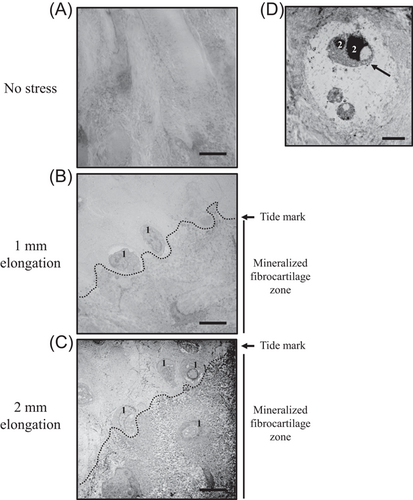
Electron microscopy showed that the enthesis in the no stress model had ill-defined borders between unmineralized and mineralized fibrocartilage (Figure 4A), and that calcification of mineralized fibrocartilage in the models of 2-mm elongation occurred more readily than those in other models (Figure 4C).
We also examined the injury response of sutured tissues using hematoxylin and eosin stain. There were no inflammatory and injury responses, such as accumulation of inflammatory cells or fracture at the tendon around the sutured area and the calcaneus around the bone tunnel through which the thread passed in all groups (Figure S3A,B).
3.3 Changes in mechanical stress have an effect on gene expression at the enthesis
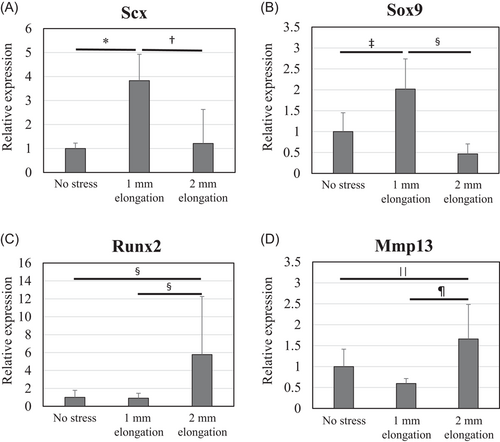
The results of real-time PCR (n = 6 per experimental group) showed that messenger RNA expression of Scx was upregulated fourfold by mild mechanical stress when compared with unstretched controls (Figure 6A). Mild mechanical stress also upregulated the expression of Sox9 to twice the levels seen in controls (Figure 6B). In contrast, expression of Runx2 and Mmp13 was upregulated by about 6 times and 1.5 times the levels in controls, respectively (Figure 6C,D).
3.4 Changes in mechanical stress affected protein expression at the enthesis
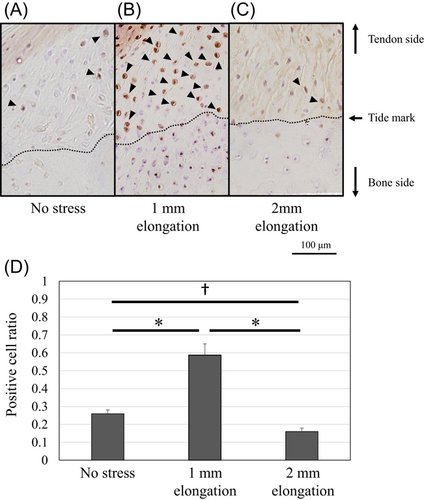
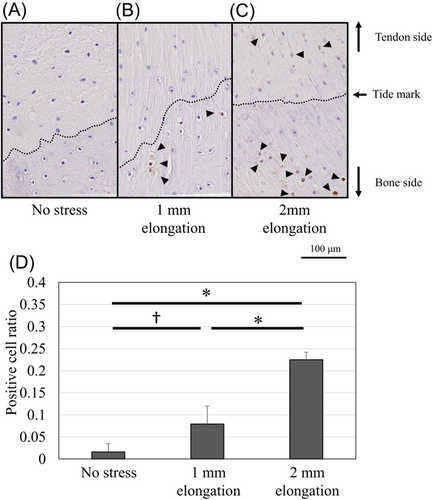
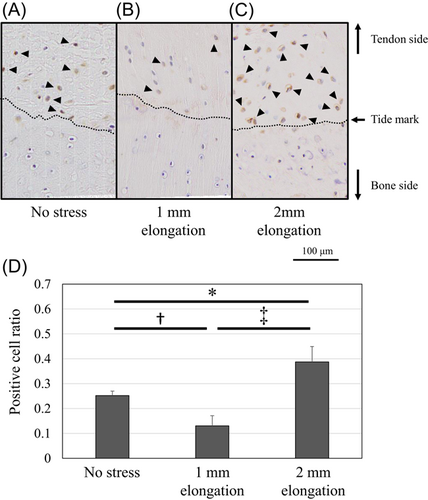
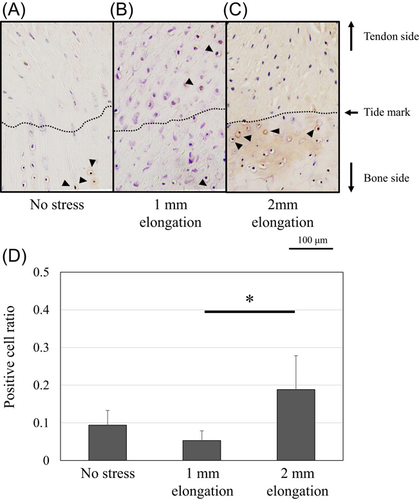
Protein expression of Scx and Sox9 was upregulated and localized in the unmineralized fibrocartilage zone following 1-mm elongation (Figures 7 and 8). Positive cell ratios for Scx and Sox9 in the models of 1-mm elongation were high (0.53 [95% CI: 0.47–0.59] and 0.61 [95% CI: 0.53–0.69], respectively). However, expression was not upregulated following 2-mm elongation (positive cell ratios in the models of 2-mm-elongation; 0.15 [95% CI: 0.11–0.19] and 0.16 [95% CI: 0.14–0.18], respectively). In contrast, Runx2 and Mmp13 were upregulated following 2-mm elongation (Figures 9 and 10). Positive cell ratios for Runx2 and Mmp13 in the 2-mm-elongation models were high (0.23 [95% CI: 0.20–0.26] and 0.40 [95% CI: 0.34–0.46], respectively). Expression of Runx2 and Mmp13 was mainly localized in the mineralized and unmineralized fibrocartilage zone, respectively. Col10 was also upregulated and localized in the mineralized fibrocartilage zone following 2-mm elongation (Figure 11); the positive cell ratio in the 2-mm-elongation models was 0.20 [95% CI: 0.11–0.29].
It was also revealed that inflammatory cytokines in the medium were below the detection limit of the kit in all groups at Days 1, 4, and 7 (data not shown).
4 DISCUSSION
This study revealed that 1-mm elongation of the enthesis maintained the structure and upregulated the expression of Sox9 and Scx. Apoptosis was also inhibited by the 1-mm elongation at the enthesis. On the other hand, the stress of 2-mm elongation induced fibrocartilage mineralization, collagen fibril fragmentation, and expression of Runx2, Mmp13, and Col10 at the enthesis.
Mechanical stress affects the mature enthesis. In this study, the no stress models showed disappearance of hierarchical structures, including no transitional fibrocartilage and rumpled collagen fibers, while 1-mm elongation prevented apoptosis and maintained the hierarchical structure and viability in the enthesis. It was reported that the cells in a mouse rotator cuff explant model showed apoptosis after 3 days in culture.16 This present study also showed that cell apoptosis was identified in the model of no stress and 2-mm elongation. In addition, live/dead imaging revealed that cell viability in no stress models was almost lost at Day 7 in culture. This result coincides with previous findings.16 It was demonstrated that unloading of the enthesis resulted in decreased strength and toughness of the attachment by investigating the shoulders of adult mice paralyzed via botulinum toxin A infection.17 Unloading of disrupted hierarchical structures leads to enthesis failure by avulsion of mineralized tissue from the attachment site.17 On the other hand, viability was maintained in the models of 1-mm elongation. These investigations indicated that mechanical loading is required to maintain enthesis homeostasis. Mechanobiology also plays an essential role in the development of the enthesis.3, 4, 18, 19 The volume of supraspinatus muscles injected with botulinum toxin from birth was decreased by 66% when compared to the contralateral saline-injected muscle.3 The botulinum toxin-injected shoulders also showed no fibrocartilage between the tendon and bone by 14 days postnatally and poor fibrocartilage development by 56 days.
The expression of Scx and Sox9 was regulated by mechanical stress in this study. Specifically, this expression was upregulated by 1-mm elongation. Cyclic loading maintained Scx expression while the expression decreased without mechanical stress.20 Mechanical stress on healing tendons during late-stage remodeling can also upregulate Scx expression.21 Sox9 expression is increased via a decrease in ERK phosphorylation after application of 5 Mp hydrostatic pressure.22 These previous studies were consistent with the outcomes of the present study. Scx and Sox9 are essential factors to develop tendon enthesis.5, 6 Scx + /Sox9 progenitors functionally help to establish the junction between hyaline cartilage and tendon/ligament.8 Mice with conditional knockout for Scx were smaller, with altered bone shape and impaired enthesis morphology, morphometry, and organization.23 The structural alteration reduced strength and stiffness.23 In addition, conditionally knocking out Sox9 in Scx-expressing cells also leads to defective enthesis formation in tendons.1, 8 Considering these results, the expression of Sox9 and Scx may help to maintain the enthesis homeostasis.
This study revealed that the expression of Sox9 and Scx is localized in the unmineralized fibrocartilage zone by 1-mm elongation. It was already revealed that Sox9 is not expressed in the mineralized portion of the growth plate, and that heterozygous Sox9 mutant mice are characterized by hypoplasia of all bones composed of endochondral ossification of all cartilages,24 therefore, premature mineralization is likely the result of allele insufficiency existing in cells that express Sox9. A previous study also showed that Scx-expressing cells were located in the enthesis with unmineralized fibrocartilage.23 The localized upregulation and spatial variations of these factors would assist in maintaining the graded enthesis.
Degenerative changes, including calcification and collagen fibril changes, were detected in the present models of 2-mm elongation. Several reports demonstrated that repetitive trauma or overload can lead to degenerative changes and the development of bony spurs at the enthesis.9, 10, 12, 25, 26 The degenerative changes, such as the calcifying process, collagen fibril size variability, and collagen fragmentation, was observed in lateral epicondylitis.26 Bony spurs can be generated by endochondral ossification of fibrocartilage at the enthesis.10 Endochondral ossification is the process by which chondrocytes proliferate, undergo hypertrophy and apoptosis. The cartilage extracellular matrix becomes mineralized and breaks down, allowing entry of the invading blood vessels, osteoclasts, osteoblasts, and bone marrow cells during endochondral ossification.27 These sequential changes are regulated by transcription factors, including Sox9 and Runx2.28, 29 Sox9 stimulates the proliferation of chondrocytes, while it was revealed that overexpression of Sox9 inhibits chondrocyte hypertrophy and mineralization via the upregulation of PTHrP.30 Runx2 is well known for the essential role during the differentiation of mineralizing cell types.29, 31 Global deletion of Runx2 gene results in interrupted osteoblast differentiation and compete loss of cartilage mineralization.32, 33 In this study, 1-mm elongation upregulated Sox9 expression and preventing apoptosis, while 2-mm elongation promoted Runx2 expression, and mineralization at the enthesis. These sequence changes are consistent with previous reports. It was also revealed that Runx2 expression is needed to promote chondrocyte hypertrophy.34, 35 Terminally differentiated hypertrophic chondrocytes express Mmp13 and Col10.14 Mmp13 is induced by Runx2 and regulates cartilage matrix and a process that leads to mineralization.36 This study also showed upregulation of Mmp13 and Col10 expression in 2-mm-elongation models. The results suggest that 2-mm elongation may induce endochondral ossification.
Mechanical stress affected collagen composition in this study. It was elucidated that collagen has a natural birefringence that is attributed to the array of fibers.37 Picro Sirius Red staining under polarized light microscopy is a good method to examine collagen alignment. because it can consistently stain collagen fibers without fading. The fibrils of Col1 are resistant structures that provide strength and mechanical durability, whereas Col3 is constituted by thinner fibrils and provides reduced resistance to mechanical stress.38 It was demonstrated that Col1 decreased more than Col3 in the elderly rats, leading to a predominance of Col3 and predisposition of spontaneous tendon rupture.39 This study revealed that Col3 was predominant in the models of no stress and 2-mm elongation. It may reduce resistance to mechanical stress.
There are some limitations in our organ culture model. First, we used the αMEM containing FBS and antibacterial drugs. The type of medium used for organ culture may affect the outcomes. Second, some cytokines were released from musculoskeletal cells at the tendon, muscle, bone, and enthesis by mechanical loading. These cytokines also may affect the outcomes. However, we changed the medium daily and the inflammatory cytokines that can affect the outcomes were not increased in the medium. The values of inflammatory cytokines were very low and under the sensitivity of ELISA Kits. The accuracy could not be proved though ELISA revealed that there was a very little inflammatory cytokine in the medium. Therefore, it appears that these cytokines did not have much influence in this model. As chondrocytes undergo apoptosis and degrade, the degraded cartilage becomes vascularized during endochondral ossification. In organ culture models, the effect of vascularization in enthesis cannot be assessed. However, this study revealed that only mechanical stress stimuli without blood flow could affect the structure of the enthesis. This implies that mechanical stress itself has a strong influence on changes in the enthesis structure.
This study revealed that varying degrees of mechanical stress caused different changes in the enthesis. Several reviews have analyzed the anabolism and catabolism that occur in cartilage under various mechanical stress situations.40-42 A predominant hypothesis predicts that the chondrocyte response to moderate mechanical stress is essential for normal cartilage homeostasis.41 It was reported that loading within the physiological range maintained the structural integrity for 6 days in the tendon ex vivo.43 On the other hand, mechanical overloading of the tendon induced a negative effect on homeostasis, such as increased apoptosis and degenerative changes.43, 44 Based on these previous studies, it appears that 1-mm elongation is moderate stress, and that 2-mm elongation is overload stress in this study model.
Injuries to the enthesis are common in the field of orthopedic medicine; however, high failure rates are often related with their repair.45, 46 Enthesopathy involving degenerative changes and the presence of bony spurs caused by tensile overload can lead to pain and gait disorders.25 To treat these disorders, it is important to understand the effects of mechanical stress on the structure of the enthesis. However, it remains unclear how the differences in stress induce anabolic or catabolic effects. Thus, further studies are needed to clarify the mechanism.
ACKNOWLEDGMENTS
This study was supported by the following grants: The Japan Society for the Promotion of Science (Grant No. 18K16662). This funding sources had no further role in the study design, data collection, analysis and interpretation of data, the writing of the manuscript; the decision to publish. The authors would like to thank FORTE (https://www.forte-science.co.jp/) for English language editing.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conception and research design: Taichi Saito, Ryo Nakamichi, Keiichiro Nishida. Data acquisition: Taichi Saito, Takaaki Hiranaka, Yuki Okazaki, Satoshi Nezu. Data analysis and interpretation: Taichi Saito, Minami Matsuhashi, Takayuki Furumatsu. Statistical analyses: Aki Yoshida, Yasunori Shimamura. Drafting of the article: Taichi Saito, Toshifumi Ozaki. All other authors contributed to writing and revising the manuscript for scientific content and approved the final version before submission.




